Abstract
Nine chaetoglobosins (1–9) including five previously undescribed ones (1–5) were obtained from the culture broth of an endophytic fungus (Chaetomium sp. UJN-EF006) isolated from the leaves of Vaccinium bracteatum. The structures of these fungal metabolites were elucidated by spectroscopic methods including mass spectroscopy, nuclear magnetic resonance, single crystal X-ray crystallography, and electronic circular dichroism. To accelerate the development of novel fungicides, all of the isolated chaetoglobosins were evaluated for their antifungal activity against two crop pathogens, Botrytis cinerea and Sclerotinia sclerotiorum. The assay results revealed that chaetoglobosins 2, 6, 7, and 9 possessed a significant fungicidal effect against B. cinerea, with EC50 values all below 10 μg/mL. Particularly, the most potent compound, 7, was 175- and 96-fold as active as the commercially available fungicides carbendazim (EC50 70.11 μg/mL) and azoxystrobin (EC50 39.02 μg/mL), respectively. A further observation under scanning electron microscope indicated that compound 2 could markedly impair the fungal hyphae of B. cinerea. The study demonstrates that the chaetoglobosins had excellent in vitro antifungal activities against B. cinerea.
1. Introduction
Endophytic fungi (or fungal endophytes) from living plants have received tremendous attention from the scientific community due to their special symbiotic relationship with their hosts [1,2] and are particularly well-known in the literature for their capability to produce diverse bioactive small-molecule natural products [3,4]. These specialized metabolites are believed to possess huge application potential in agriculture to combat crop pests and pathogens [5,6,7] as well as in the pharmaceutical industry to fight against various human diseases [8,9,10]. Therefore, studies on endophytic fungi have emerged as frontline research topics in recent years and are currently on a fast development track.
Vaccinium bracteatum Thunb., a native Chinese plant species, is a member of the famous Vaccinium genus that has a number of berry trees (e.g., blueberry) with important economic value [11,12]. Our previous investigations into the bioactive constituents of this plant have afforded a large number of compounds including lignans, iridoids, and flavones with various bioactivities [13,14]. V. bracteatum in its original habitat is rarely affected by plant pathogens except for Rhizoctonia solani, and its leaf extract has been used by the local residents as a food preservative. Chaetomium globosum (family Chaetomiaceae, order Sordariales) is a ubiquitous filamentous fungus renowned as both a prolific source of structurally complex secondary metabolites and an effective biocontrol agent against phytopathogens such as mediating antagonism against plant pathogens through secreted chaetoglobosins and underpinning its potential in sustainable agriculture [15]. Initially characterized by its distinctive globose ascomata and coiled peridial hairs, this species has been isolated from diverse ecological niches, including soil, plant material, and crucially as an endophyte in medicinal plants such as V. bracteatum (Ericaceae) [16,17], while chaetoglobosins A and B were originally isolated from Chaetomium globosum in 1973 by Natori and coworkers [18]. The most important cytochalasan-producing fungi are members of the genera Chaetomium and Aspergillus, and nearly 200 cytochalasans reported to date (accounting for about 40%) have been isolated from these two genera [19].
Cytochalasans represent a class of structurally diverse fungal metabolites featuring a perhydroisoindolone moiety fused to a macrocyclic ring. There are altogether six groups (about 500 compounds) of cytochalasans in terms of the different amino acids incorporated into the polyketide skeleton. The chaetoglobosins are a group of cytochalasans with a tryptophan unit in the core structure, and 133 chaetoglobosins are included in this section [19]. These chaetoglobosins demonstrate broad-spectrum bioactivities due to their complex molecular architectures, which have attracted significant attention from the chemical and pharmacological scientific communities, and have already been reviewed in recent years from different vantage points such as their chemistry and biology [20], biosynthesis [21], and total synthesis [22]. Plant pathogenic fungi that often cause adverse effects in agricultural production mainly include Fusarium, Alternaria, Phytophthora, etc. Botrytis cinerea, a necrotrophic fungal pathogen of global significance, has a wide range of hosts and can cause disease in many economically important crop species such as grapes, strawberries, tomatoes, and ornamental plants [23]. Chemical fungicides currently serve as the dominant approach for plant disease management due to their cost-effectiveness and potent efficacy. Nevertheless, pervasive fungicide overuse has precipitated widespread resistance in phytopathogenic fungi, diminishing the effectiveness of conventional agents. This necessitates the development of novel, high-performance fungicides to resist resistance and mitigate agricultural disease burdens. In this study, we conducted an investigation into the inhibitory effects of chaetoglobosins on Botrytis cinerea, aiming to offer a reliable and safe natural agent for effectively managing postharvest decay in tomatoes.
2. Materials and Methods
2.1. General Experimental Procedures
Specific optical rotation data were measured on a Rudolph VI polarimeter (Rudolph Research Analytical, Hackettstown, NJ, USA). UV and ECD (electronic circular dichroism) spectra were acquired on a Chirascan circular dichroism spectrometer (Applied Photophysics, Surrey, UK). NMR experiments were performed on a Bruker Avance DRX600 spectrometer (Bruker BioSpin AG, Fallanden, Switzerland) and referenced to residual solvent peaks (CD3OD: δH 3.31, δC 49.00; CDCl3: δH 7.26, δC 77.16). HR-ESIMS spectra were recorded on an Agilent 6545 Q-TOF mass spectrometer (Agilent Technologies Inc., Waldbronn, Germany). ESIMS analyses were performed on an Agilent 1260-6460 Triple Quad LC-MS instrument (Agilent Technologies Inc., Waldbronn, Germany). HPLC analyses and separations were conducted on an Agilent 1260 series LC instrument (Agilent Technologies Inc., Waldbronn, Germany) coupled to a SilGreen-C18 column (10 mm × 250 mm, Greenherbs Science and Technology, Beijing, China). Conventional column chromatography (CC) was performed on D101-macroporous absorption resin (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China), reversed-phase C18 silica gel (Merck KGaA, Darmstadt, Germany), Sephadex LH-20 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), and silica gel (300–400 mesh; Qingdao Marine Chemical Co. Ltd., Qingdao, China). All solvents used for CC were of analytical grade (Tianjin Fuyu Fine Chemical Co. Ltd., Tianjin, China), and solvents used for HPLC were of HPLC grade (Oceanpak Alexative Chemical Ltd., Goteborg, Sweden). Pre-coated silica gel GF254 plates (Qingdao Marine Chemical Co. Ltd., Qingdao, China) were used for TLC monitoring. All mixed solvent systems used in the experiments were in the form of v/v unless otherwise specified. A Tecan Spark10M microplate reader (Salzburg, Austria) and scanning electron microscope (Zeiss, Gemini300, Jena, Germany) were used in the biological tests.
2.2. Fungal Source and Culture Conditions
The fungal species was isolated from the fresh leaves of Vaccinium bracteatum. Fresh plant leaves were collected and surface-sprayed with 75% ethanol for sterilization, which were cut using a sterilized scalpel. Then, the incised leaves were placed onto pre-prepared PDA plates. The plates were incubated at 28 °C, and the colonies were monitored and transferred to fresh PDA plates for fungal isolates. The ITS sequencing information and phylogenetic tree analysis of UJN-EF006, shown in the Supplementary Materials (Figure S45), indicated a 97.00% similarity to Chaetomium subglobosum. The fermentation and extraction of Chaetomium sp. UJN-EF006 was the same as that previously described [17].
The plant pathogens Botrytis cinerea and Sclerotinia sclerotiorum (Lib.) de Bary were acquired from Shandong Academy of Pesticide Science, and the pure cultures with 20% (v/v) glycerol were stored at −20 °C as spores and mycelial fragments. The two fungal pathogens were cultured on potato dextrose agar (PDA: potato extract 20 g/L, dextrose 20 g/L, agar 15 g/L, and pH 7.2) at 25 °C in the dark.
2.3. Extraction and Isolation
The EtOAc partition (700 g) generated from the EtOH extract of the fungal materials was acquired as recorded in a recent report [17] and then fractionated by D101 macroporous resin CC (EtOH-H2O, 30%, 50%, 80% and 90%) to obtain four fractions. The 50% EtOH eluate (150 g) was subsequently separated on a silica gel column eluted with step-gradient CH2Cl2–MeOH (100:0 to 50:1) to produce eight subfractions (Fr.1–Fr.8). Fr.2 was chromatographed on RP-C18 silica gel CC (MeOH-HO, 65% to 90%) to produce five subfractions, and the fifth one was further purified by semi-preparative HPLC (65% MeCN-H2O) to afford 4 (1.6 mg, tR = 14.0 min). Both Fr.4 and Fr.7 were separated by Sephadex LH-20 CC (in CH2Cl2–MeOH) to afford three respective subfractions, Fr.4-1 to Fr.4-3 and Fr.7-1 to Fr.7-3. Fr.4-1 was separated by silica gel CC (CH2Cl2-acetone, 50:1 to 10:1) to yield three subfractions, and the second one was further purified by semi-preparative HPLC (55% MeCN-H2O) to afford 2 (45.0 mg, tR = 12.3 min), while the third one was purified by semi-preparative HPLC (45% MeCN-H2O) to afford 9 (15.0 mg, tR = 27.0 min). Fr.7-2 was fractionated by silica gel CC (CH2Cl2–MeOH, 200:1 to 20:1) to afford four subfractions, and the second one was further purified by semi-preparative HPLC (45% MeCN-H2O) to afford 1 (12.6 mg, tR = 17.0 min). Fr.5 was separated by silica gel CC (CH2Cl2–MeOH, 200:1 to 30:1) to yield six subfractions (Fr.5-1 to Fr.5-6), and Fr.5-4 was separated by Sephadex LH-20 CC (in CH2Cl2–MeOH) to afford two further subfractions (Fr.5-4-1 and Fr.5-4-2). Fr.5-4-1 was purified by semi-preparative HPLC (52% MeCN-H2O) to afford 7 (12.5 mg, tR = 21.5 min). Fr.6 was separated by silica gel CC (CH2Cl2–MeOH, 400:1 to 15:1) to yield four subfractions (Fr.6-1 to Fr.6-4), and Fr.6-3 and Fr.6-4 were separated by Sephadex LH-20 CC (in CH2Cl2–MeOH) to afford four respective subfractions Fr.6-3-1 to Fr.6-3-4 and Fr.6-4-1 to Fr.6-4-4. Fr.6-3-2 was chromatographed on a RP-C18 silica gel column (MeOH-H2O, 40% to 60%) to produce three subfractions (Fr.6-3-2-1 to Fr.6-3-2-3), and Fr.6-3-2-2 was further purified by semi-preparative HPLC (45% MeCN-H2O) to afford 5 (1.5 mg, tR = 28.5 min). Fr.6-4-2 was separated by silica gel CC (CH2Cl2–acetone, 15:1 to 10:1) and semi-preparative HPLC (50% MeCN-H2O) to give 3 (2.1 mg, tR = 40.0 min) and 6 (27.4 mg, tR = 44.0 min). Fr.6-4-4 was chromatographed on a RP-C18 silica gel column (MeOH-H2O, 35% to 50%) to produce three subfractions (Fr.6-4-4-1 to Fr.6-4-4-3), and Fr.6-4-4-2 was further purified by semi-preparative HPLC (50% MeCN-H2O) to afford 8 (12.2 mg, tR = 29.0 min).The isolation procedures are displayed in flowchart in the Supplementary Materials (Figure S44).
19-Oxo-deoxaphomin B (Compound 1): white solid; [α]D25 +80 (c 1.0, MeOH); UV (MeOH) λmax (log ε) 225 (4.40) nm; ECD (0.13 mg/mL, MeOH) λmax (Δε) 240 (−2.95), 232 (−0.64), 200 (+55.34) nm; 1H and 13C NMR data (in CD3OD) see Table 1; (+)-ESIMS m/z 508.3 [M + H]+; (+)-HR-ESIMS m/z 508.2694 [M + H]+ (C30H38NO6, calcd. 508.2694).

Table 1.
1H and 13C NMR data of compounds 1–5.
19-O-acetyl-dehydrochaetoglobosin F (Compound 2): light yellow solid; [α]D25 +36 (c 0.50, MeOH); UV (MeOH) λmax (log ε) 220 (4.96) nm; ECD (0.13 mg/mL, MeOH) λmax (Δε) 325 (+4.80), 260 (−53.35), 220 (+59.38), 205 (+25.0) nm; 1H and 13C NMR data (in CDCl3) see Table 1; (−)-ESIMS m/z 605.3 [M + Cl]−; (+)-HR-ESIMS m/z 571.2798 [M + H]+ (C34H39N2O6, calcd. 571.2803).
19-O-acetyl-21,22-dihydro-22-methoxychaetoglobosin A (Compound 3): light yellow solid; [α]D25 −40 (c 0.10, MeOH); UV (MeOH) λmax (log ε) 222 (4.16) nm; ECD (0.13 mg/mL, MeOH) λmax (Δε) 295 (−4.69), 205 (+6.33) nm; 1H and 13C NMR data (in CDCl3) see Table 1; (+)-ESIMS m/z 603.1 [M + H]+; (+)-HR-ESIMS m/z 603.3060 [M + H]+ (C35H43N2O7, calcd. 603.3065).
19-O-acetyl-21,22-dihydro-22-ethoxychaetoglobosin A (Compound 4): yellow solid: [α]D25 −93 (c 0.10, MeOH); UV (MeOH) λmax (log ε) 222 (4.59) nm; ECD (0.15 mg/mL, MeOH) λmax (Δε) 295 (−8.91), 210 (−0.20) nm; 1H and 13C NMR data (in CDCl3) see Table 1; (+)-ESIMS m/z 617.3; (+)-HR-ESIMS m/z 617.3222 [M + H]+ (C36H44N2O7, calcd. 617.3221).
19-O-acetyl-21,22-dihydro-21-methoxychaetoglobosin A (Compound 5): light yellow solid; [α]D25 −140 (c 0.75, MeOH); UV (MeOH) λmax (log ε) 220 (4.82) nm; ECD (0.19 mg/mL, MeOH) λmax (Δε) 305 (−15.63), 215 (+22.0) nm; 1H and 13C NMR data (in CDCl3) see Table 1; (−)-ESIMS m/z 637.2 [M + Cl]−; (+)-HR-ESIMS m/z 620.3334 [M + NH4]+ (C35H46N3O7, calcd. 620.3330).
2.4. X-Ray Crystallographic Analysis
The crystallographic data for compound 1 have been deposited at the Cambridge Crystallographic Data Center (CCDC) as a supplementary publication (registration no. CCDC 2339221), and copies of the data can be obtained free of charge from the CCDC (12 Union Road, Cambridge CB2 1EZ, UK (Fax: Int. + 44(0) (1223) 336 033; email: deposit@ccdc.cam.ac.uk).
Crystallographic data for 1: C30H36NO6, M = 506.60, a = 78930 (2) Å, b = 13.8373 (3) Å, c = 12.5689 (2) Å, β = 97.9440 (10) Å, V = 1359.57 (5) Å3, T = 150 K, space group P21, Z = 2, μ (Cu Kα) = 0.694 mm−1, 8807 reflections measured, 4650 independent reflections (Rint = 0.0268). The final R1 values were 0.0668 (I > 2σ (I)). The final R1 values were 0.0671 (all data). The final wR (F2) values were 0.1525 (I > 2σ (I)). The final wR (F2) values were 0.1531 (all data). The goodness of fit on F2 was 1.097. Flack parameter = 0.08 (7) [24].
2.5. ECD Calculation
The absolute configuration of compound 2 was first subjected to random conformational analysis with the MMFF force field, and determined by time-dependent density functional theory (TD-DFT) based electronic circular dichroism calculation with Gaussian 09 software at the CAM-B3LYP/6-311G (d, p) level in vacuo. The ECD spectrum of each conformer was simulated by SpecDis 1.71 with a half-bandwidth of 0.3 eV, and the final ECD spectra of the target molecules were obtained according to the Boltzmann calculated contribution of each conformer with UV wavelength correction [13].
2.6. In Vitro Antifungal Evaluation
The preliminary anti-phytopathogenic effects of all of the isolated chaetoglobosins against B. cinerea and S. sclerotiorum at the concentration of 10 μg/mL were screened according to a mycelial growth inhibition method [25]. Briefly, each tested compound was dissolved in DMSO and then thoroughly mixed at about 55 °C in PDA medium sterilized to obtain a series of concentrations (0, 0.5, 2.5, 5.0 μg/mL), while azoxystrobin and carbendazim were used as a positive control. After solidification of the PDA medium, the mycelial dishes (5.00 mm) of phytopathogenic fungi were inoculated on the center of the PDA plates and incubated at 25 °C in the dark. Three replicates were performed for each parallel experiment. The diameters (mm) of the inhibition zones were measured by the cross-bracketing method after three days. The growth inhibition rates were calculated when the mycelia in the blank control group grew to the edge of the Petri dish according to the following formula: mycelial growth inhibition (%) = [(dc − dt)/(dc − 5 mm)] × 100%, where dc and dt are the average diameters of the fungal colony in the black control and treatment groups, respectively. Standard deviation (SD) values were calculated based on the inhibition data obtained from triplicate experiments for each assay.
Chaetoglobosins with good inhibitory activity were further evaluated to determine their median effective concentration (EC50) values using established procedures [26]. The values were calculated from the toxicity regression equation (y = ax + b) using Microsoft Excel software by the least-squares method, with the logarithmic values of concentrations as the independent variable (x) and the probit values of the inhibition rates as the dependent variable (y).
2.7. Scanning Electron Microscopy (SEM)
The effect of the new compound 2 on the morphology of B. cinerea was observed via SEM. The fresh mycelia of B. cinerea were inoculated into the potato dextrose broth medium and cultured at 25 °C for 12 h. The stock solution of compound 2 was added to the medium to achieve concentrations of 2.5 and 5 μg/mL and then cultured at 25 °C for 48 h. All of the samples were fixed with 2.5% glutaraldehyde stationary liquid at 4 °C for 12 h and then washed thrice with 0.1 M phosphate buffer (pH 7.2) for 5 min each time. After that, each sample was dehydrated in an ethanol gradient of 30%, 50%, 70%, 90%, 100% for 15 min each, and finally washed with 100% t-butanol twice [27]. Finally, the sample was freeze-dried, coated with gold at 2.0 kv, and visualized under a scanning electron microscope (Zeiss, Gemini300, Germany).
3. Results and Discussion
3.1. Structure Characterization of the Isolated Compounds
An endophytic fungal strain was isolated from the surface-sterilized fresh leaves of the host plant V. bracteatum (Ericaceae). Based on morphological and molecular characterization (ITS sequencing information is presented in the supporting material), this strain was identified as Chaetomium globosum. Due to its rich metabolite profile, according to HPLC analysis and a subsequent intensive fractionation on the EtOH extract of this fungus, this resulted in the separation and structural characterization of an array of chaetoglobosins (1–9) including five new ones (1–5) (Figure 1). Of particular note, compounds 2, 6, 7, and 9 showed significant inhibitory activity against a common crop pathogen Botrytis cinerea, with a much better effect than the positive controls azoxystrobin and carbendazim. Details of the isolation, structure characterization, and anti-phytopathogenic evaluation of these interesting fungal metabolites are presented in the current paper.
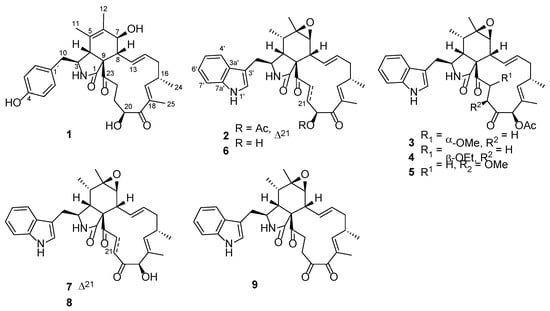
Figure 1.
Chemical structures of compounds 1–9.
Compound 1 was isolated as a white solid and showed a protonated molecular ion in the HR-ESIMS analysis at m/z 508.2694 (calcd. 508.2694), corresponding to the molecular formula C30H37NO6. Inspection of the NMR data (in CD3OD, Table 1) for 1 revealed the presence of three carbonyls (δC 177.1, 205.5, 211.2), a 4-hydroxyphenyl unit (δC 129.1, 131.4 (2C), 116.4 (2C), 157.4; δH 6.74, 6.93 (both d, J = 8.6 Hz, 2H)), a tetra-substituted double bond (δC 127.7, 134.4), a tri-substituted double bond (δC 150.5, 137.0; δH 6.31 (m)), a di-substituted trans double bond (δC 129.4, 136.1; δH 6.28 (ddd, J = 15.3, 10.0, 2.0 Hz), 5.23 (ddd, J = 15.3, 11.0, 2.9 Hz)), a sp3 quaternary carbon (δC 63.8), six sp3 methines [δC 34.6, 50.0, 52.6, 60.5, 70.0, 71.9; δH 2.13 (dd, J = 10.0, 9.7 Hz), 2.82 (m), 2.94 (brs), 3.33 (dd, J = 9.8, 5.5 Hz), 3.81 (brd, J = 9.7 Hz), 4.91 (dd, J = 7.8, 4.3 Hz)], four sp3 methylenes [δC 32.0, 38.2, 42.0, 42.2; δH 1.85 (m), 1.93 (m), 2.08 (ddd, J = 13.6, 12.1, 11.0 Hz), 2.18 (dd, J = 13.4, 9.8 Hz), 2.48 (m), 2.66 (dd, J = 13.4, 5.5 Hz), 2.91 (ddd, J = 19.6, 9.1, 5.4 Hz), 3.03 (dt, J = 19.6, 5.7 Hz)], and four methyls [δC 12.4, 14.8, 17.4, 20.2; δH 1.07 (d, J = 6.7 Hz), 1.28 (brs), 1.64 (d, J = 1.6 Hz), 1.83 (d, J = 1.3 Hz)]. These NMR spectroscopic features resembled those for the chaetoglobosins, which are a well-known class of fungal metabolites in the literature [28]. Further examination of the 2D NMR data (Figure 2) confirmed the aforementioned deduction, revealing key 1H-1H COSY correlations of H2-10/H-3/H-4, H-7/H-8/H-13/H-14/H2-15/H-16(H3-24)/H-17, and H-20/H2-21/H2-22 as well as diagnostic HMBC signals from H-3 and H-4 to C-1, H3-11 to C-4, C-5, and C-6, H3-12 to C-5, C-6, and C-7, H-8 to C-1, C-4, C-9, and C-23, H3-25 to C-17, C-18, and C-19, H-20 and H-21 to C-19, and H2-22 to C-23, which corroborated the chaetoglobosin core of 1 as shown (Figure 2). In addition, the connection between the 4-hydroxyphenyl group and the tricyclic chaetoglobosin core was confirmed by the HMBC correlations from H2-10 to C-1′ and C-2′(6′). Finally, the remaining unassigned elements and the chemical shits for C-7 (δC 70.0) and C-20 (δC 71.9) supported the locations of 7-OH and 20-OH. The planar structure of 1 was thus unambiguously established, and it is interesting to note that most formerly reported chaetoglobosins incorporate an indole fragment while compound 1 bears a benzene unit.
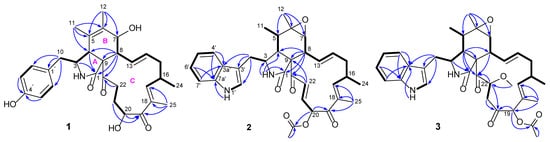
Figure 2.
Key 1H-1H COSY (bold lines) and HMBC (arrows) correlations of compounds 1–3.
The relative configuration of 1 was assigned on the basis of analyzing NOESY data (Figure 3) and proton couplings. The correlation of H-4/H-8 indicated that they were in a quasi 1,3-diaxial relationship and thus coplanar in ring B, and the correlations of H-4 with H2-10 and H-22 (δH 3.03) suggested that the CH2-10 and –COCH2– (C-23/C-22) groups were co-facial with H-4 in ring A. Then, H-7 was assigned to be on the opposite side of H-8 in ring B according to the large J7,8 value (9.7 Hz, quasi 1,2-diaxial relationship). Afterward, the correlation cascades of H-8/H-14/H-16/H3-25 and H-13/H-15 (δH 2.08)/H-18/H-20 established the geometries of double bonds (Δ13 and Δ17) and configurations of chiral centers (C-16 and C-20), as shown in Figure 3. To corroborate the structure of 1, we tried several solvent systems to culture suitable single crystals for X-ray crystallographic analysis, and this effort was eventually rewarded by the successful acquisition of a high-quality crystal in MeOH–H2O (10:1). The X-ray crystallographic structure of 1 is shown in Figure 4, and its absolute stereochemistry was also unequivocally assigned as 3S,4R,7S,8R,9R,13E,16S,17E,20S (flack parameter = 0.08 (7)).
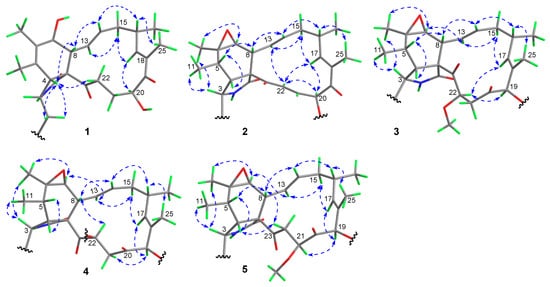
Figure 3.
Key NOESY correlations (double-headed dashed arrows) of compounds 1–5.
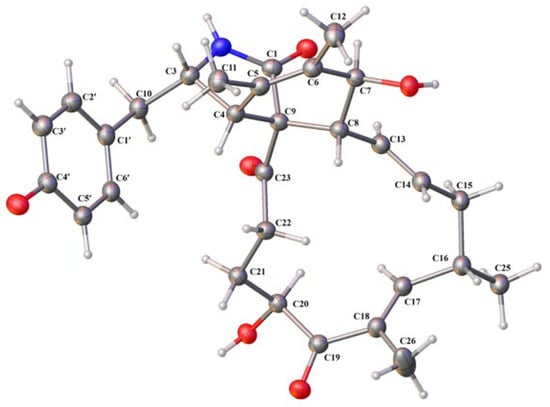
Figure 4.
X-ray crystallographic structure of compound 1.
Compound 2 was assigned the molecular formula C34H38N2O6 based on its HR-ESIMS ion at m/z 571.2798 ([M + H]+, calcd. 571.2803). Analysis of the NMR data (in CDCl3, Table 1) for 2 revealed a high similarity to those for its cometabolite chaetoglobosin F (6) [29], and the major difference was attributed to the appearance of signals for an acetoxy group (δC 169.9, 19.8; δH 2.21 (s)) and a E-double bond (δC 132.0, 136.8; δH 6.50 (dd, J = 15.3, 10.7 Hz), 7.44 (d, J = 15.3 Hz)) in 2 instead of those for two methylenes in 6. Further interpretation of the 2D NMR 1H-1H COSY and HMBC data (Figure 2) confirmed the classical chaetoglobosin framework for 2 as drawn, with key COSY correlations of H2-10/H-3, H-4/H-5/H3-11, H-7/H-8/H-13/H-14/H2-15/H-16(H3-24)/H-17, H-20/H-21/H-22, H-1′/H-2′, and H-4′/H-5′/H-6′/H-7′ as well as pivotal HMBC signals from H-2 to C-1, C-3 and C-4, H3-12 to C-5, C-6, and C-7, H-8 to C-1, C-4, C-9, and C-23, and H3-25 to C-17, C-18, and C-19. Particularly, the HMBC correlations from H-22 to C-9 and C-23 corroborated the presence of Δ21, and those from H-20 to C-19 and the acetoxy carbonyl carbon, together with the markedly downfield shifted H-20 signal (6.38 ppm, compared with that of 6), strongly supported the structural evolution from 6 to 2. The relative configuration of 2 was finally established to be identical with that of 6 based on a careful NMR comparison (especially analysis of proton couplings) and inspection of the NOESY data (Figure 3). The critical NOESY cross-peaks included H-3/H3-11, H-3/H3-12, and H3-12/H-7, and the correlation cascades comprised H-4/H-5/H-8/H-14/H-16/H3-25 and H-13/H-15 (δH 2.19)/H-17/H-20/H-22. Moreover, the NOESY signals of H-22 with H-13 and H-17 were also observed. Compound 2 was thus characterized as 19-O-acetyl-dehydrochaetoglobosin F.
Compound 3 was assigned the molecular formula C35H42N2O7 by HR-ESIMS analysis at m/z 603.3060 ([M + H]+, calcd. 603.3065), with 32 mass units (CH4O) more than 19-O-acetylchaetoglobosin A [29], indicative of a methanol adduct of the latter. Inspection of the NMR data (in CDCl3, Table 1) for 3 confirmed this hypothesis, revealing extra resonances (δC 42.7, 59.0, 80.8; δH 2.65 (dd, J = 15.5, 10.1 Hz), 2.94 (dd, J = 15.5, 1.7 Hz), δH 3.48 (s), 4.77 (dd, J = 10.1, 1.7 Hz)) for a –CH2CH(OMe)– fragment rather than those for the Δ21 double bond in 19-O-acetylchaetoglobosin A, which was further evidenced by the diagnostic 1H-1H COSY correlations of H2-21/H-22 and the HMBC correlation from the methoxy protons to C-22 (Figure 2). The location of this fragment was supported by the HMBC correlations from H-22 to C-23 and from H2-21 to C-20. The planar structure of 3 was further secured by careful examination of the full 2D NMR data (Figure 2). Finally, the relative configuration of 3 was established by analysis of the NOESY data (Figure 3). To be specific, most NOESY cross-peaks of 3 were consistent with those of 2, indicating common stereochemistries at most chiral centers (C-3, C-5, C-6, C-7, C-8, C-9, and C-16), and the correlations of H-17/H-22 and H-22/H-19 demonstrated the relative configurations at C-19 and C-22 as shown (Figure 3). Compound 3 was thus identified to be 19-O-acetyl-21,22-dihydro-22-methoxychaetoglobosin A.
Compound 4 was assigned the molecular formula C36H44N2O7 by HR-ESIMS analysis at m/z 617.3222 ([M + H]+, calcd. 617.3221), with 14 mass units (CH2) more than 3 indicative of a methylated analogue of the latter. Examination of the NMR data (in CDCl3, Table 1) for 4 confirmed this hypothesis, with resonances (δC 15.5, 64.5; δH 1.27 (t, J = 7.0 Hz), 3.56 (m), 3.67 (m)) for an ethoxy group replacing those for the 22-OMe in 3, which was further supported by the diagnostic HMBC correlations from the ethoxy methylene protons to C-22 (Figure S26, Supplementary Materials). Careful inspection of the full 2D NMR data (Figures S24–S26, Supplementary Materials) secured the planar structure of 4 as shown. However, the relative configuration of 4 seemed to be different from that of 3, as implied by the remarkable NMR variations around the C-22 chiral center. The most severe change occurred to the coupling pattern between H-22 and H2-21 (J21,22 = 5.8, 4.0 Hz in 4 vs. that (10.1, 1.7 Hz) in 3), indicating a likely inverted C-22 configuration. Finally, the relative configuration of 4 was confirmed by interpretation of the NOESY data (Figure 3) to be consistent with that of 3 except at C-22, which was supported by the key correlation of H-8 with H-22. Compound 4 could be derived from 3 via a SN2 nucleophilic substitution of the ethoxy group, leading to the configurational inversion of C-22. Compound 4 was thereby characterized as 19-O-acetyl-21,22-dihydro-22-ethoxychaetoglobosin A.
Compound 5 was assigned the same molecular formula as 3 based on its HR-ESIMS ion at m/z 620.3334 ([M + NH4]+, calcd. 620.3330), suggestive of an isomer of the latter. Analysis of the NMR data (in CDCl3, Table 1) for 5 confirmed this hypothesis, with all its spectroscopic features being consistent with those in 3. Further examination of the full 2D NMR data (Figures S31–S34, Supplementary Materials) demonstrated that compound 5 was a regio-isomer of 3, with the methoxy group located at C-21 as evidenced by the HMBC correlation from the methoxy protons to C-21. The relative configurations of all chiral centers except for C-21 in 5 were assigned to be consistent with those of their counterparts in 3 by careful inspection of the NOESY data (Figure 3), and the C-21 configuration was established as drawn via the pivotal correlation of H-19/H-21. Compound 5 was hence identified to be 19-O-acetyl-21,22-dihydro-21-methoxychaetoglobosin A.
The absolute configuration of compound 2 was established by comparing its experimental ECD (electronic circular dichroism) spectrum with the calculated ones for its two enantiomers. As shown in Figure 5, the measured ECD curve of 2 showed a good match with the computed curve for the (3S,4R,5S,6R,7S,8R,9R,16S,20S)-isomer. Based on a biogenetic correlation with 2, the absolute configurations of chaetoglobosins 3–5 were assigned as drawn.
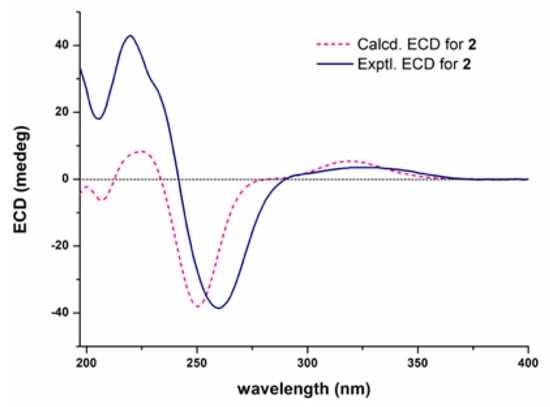
Figure 5.
Experimental and calculated ECD spectra for 2.
Four known chaetoglobosins, namely chaetoglobosin F (6) [29], chaetoglobosin A (7) [29], penochalasin F (8) [30], and chaetoglobosin C (9) [29], were also isolated in the present work. These were identified by detailed spectroscopic analyses and comparison with the reported data in the literature.
3.2. Anti-Phytopathogenic Evaluation
The preliminary antifungal activity of the isolated chaetoglobosins against two pathogenic fungi was first evaluated at 10 μg/mL, and the results in Table S1 (Supplementary Materials) showed that these chaetoglobosins, except 1, all exhibited various degrees of fungicidal effect. The EC50 values of the active chaetoglobosins toward B. cinerea were further measured, and the results are presented in Table 2. Most of these chaetoglobosins exhibited significant fungicidal activity against B. cinerea and were more potent than the commercial fungicides azoxystrobin and carbendazim, with EC50 values for 2, 6, 7, and 9 of 2.19, 8.25, 0.40, and 5.83 μg/mL, respectively. The new compound 2 was chosen for the subsequent in-depth biological assessment against B. cinerea, given its sufficient sample supply and good fungicidal activity.

Table 2.
Toxicity regression equations and EC50 values of compounds against B. cinerea.
The fungicidal effect of 2 on B. cinerea was further evaluated in a time and dose-dependent manner. As shown in Figure 6, compound 2 could effectively inhibit the growth of the pathogen at a concentration down to 0.5 μg/mL (a dose of about 1/5 of the EC50 value), and the inhibitory effect became more apparent as time went on. Even on the fourth day, the fungal colony diameter of the treatment group administrated with 5.0 μg/mL (about 2-fold of the EC50 value) 2 was still smaller than that of the control group on the second day. These observations demonstrate that compound 2 shows good suppressing activity toward B. cinerea and could be used as a protective agent against pathogenic infection.
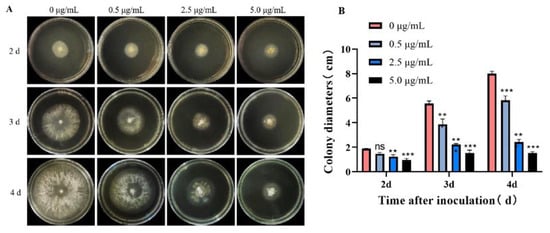
Figure 6.
Antifungal evaluation of 2 against B. cinerea. (A,B) The morphology and colony diameter of B. cinerea on PDA plate after treatment; data are presented as the mean ± standard deviation. ns: not statistically significant; * p < 0.05, ** p < 0.01 and *** p < 0.001 compared with the control group (0 μg/mL).
Next, the morphological changes of B. cinerea upon the treatment of 2 were observed under a scanning electron microscope. As shown in Figure 7 (amplified images in supporting material Figure S46), the hyphae of B. cinerea in the control group (treated with only 1% DMSO) looked normal, and the columnar surface was relatively smooth, intact, and uniform, while in the azoxystrobin-treated group (positive control), the hyphae surface became rough with many creases. In contrast, in the two groups treated with 2.5 and 5.0 μg/mL of 2, the morphology of the fungus was severely impaired with distorted and shrunken hyphae. These findings indicate that compound 2 could exert antifungal activity by disrupting the normal physiological function of fungal hyphae, with a much better effect that the reference drug azoxystrobin.
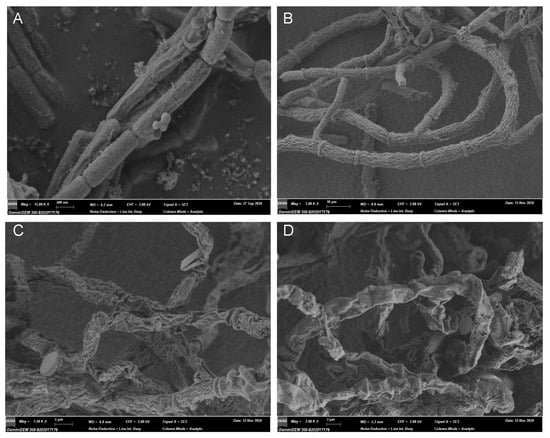
Figure 7.
Representative pictures of the hypha morphology of B. cinerea observed under SEM. (A) Blank control with 1% DMSO (15.00 k×, 0.5 μm); (B) positive control group treated with azoxystrobin at 39.0 μg/mL (1.00 k×, 10 μm); (C) treated group with compound 2 at 2.5 μg/mL (1.50 k×, 5 μm); (D) treated with 2 at 5.0 μg/mL (2.00 k×, 3 μm).
4. Conclusions
Currently, extensive studies have established the potent antitumor activities of chaetoglobosin derivatives across diverse cancer cell lines [20]. In our preliminary assays using the A549 and MDA-MB-231 cell lines, the isolated chaetoglobosins exhibited moderate activity (IC50 >20 μM) and did not surpass the efficacy of the reported derivatives. Given that our primary objective was to explore the antimicrobial activity of understudied chaetoglobosins, we prioritized the further investigation of their antimicrobial potential based on ecological relevance.
In this study, our chemical investigation into the culture fermentation of Chaetomium sp. UJN-EF006 derived from the leaves of V. bracteatum afforded nine chaetoglobosins including five previously undescribed ones (1–5). Most of these chaetoglobosins exhibited excellent antifungal activity against two phytopathogenic fungi. The new and major compound 2 was found to possess a strong fungicidal effect against B. cinerea with an EC50 value of 2.19 µg/mL, being much better than that (39.02 µg/mL) of the control drug azoxystrobin. A preliminary mechanistical study revealed that compound 2 could exert its antifungal activity by suppressing the normal growth and formation of fungal hyphae. To sum up, our current study demonstrates that Chaetomium sp. UJN-EF006 could be used as a biocontrol species, and its secondary metabolites could be developed into fungicides for the prevention and control of agricultural pathogens.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof11070511/s1, Table S1: Fungicidal activities of isolated compounds at 10 μg/mL; Figure S1: 1H NMR (600 MHz, CD3OD) spectrum of 1; Figure S2: 13C and DEPT NMR (150 MHz, CD3OD) spectra of 1; Figures S3–S6: 2D NMR spectra of 1; Figure S7: The (+)-HR-ESIMS spectrum of 1; Figure S8: 1H NMR (600 MHz, CDCl3) spectrum of 2; Figure S9: 13C and DEPT NMR (150 MHz, CDCl3) spectra of 2; Figures S10–S13: 2D NMR spectra of 2; Figure S14: The (+)-HR-ESIMS spectrum of 2; Figure S15: 1H NMR (600 MHz, CDCl3) spectrum of 3; Figure S16: 13C and DEPT NMR (150 MHz, CDCl3) spectra of 3; Figures S17–S20: 2D NMR spectra of 3; Figure S21: The (+)-HR-ESIMS spectrum of 3; Figure S22: 1H NMR (600 MHz, CDCl3) spectrum of 4; Figure S23: 13C and DEPT NMR (150 MHz, CDCl3) spectra of 4; Figures S24–S27: 2D NMR spectra of 4; Figure S28: The (+)-HR-ESIMS spectrum of 4; Figure S29: 1H NMR (600 MHz, CDCl3) spectrum of 5; Figure S30: 13C and DEPT NMR (150 MHz, CDCl3) spectra of 5; Figures S31–S34: 2D NMR spectra of 5; Figure S35: The (+)-HR-ESIMS spectrum of 5; Figure S36: 1H NMR (600 MHz, CDCl3) spectrum of 6; Figure S37: 13C and DEPT NMR (150 MHz, CDCl3) spectra of 6; Figure S38: 1H NMR (600 MHz, CDCl3) spectrum of 7; Figure S39: 13C and DEPT NMR (150 MHz, CDCl3) spectra of 7; Figure S40: 1H NMR (600 MHz, CDCl3) spectrum of 8; Figure S41: 13C and DEPT NMR (150 MHz, CDCl3) spectra of 8; Figure S42: 1H NMR (600 MHz, CDCl3) spectrum of 9; Figure S43: 13C and DEPT NMR (150 MHz, CDCl3) spectra of 9; Figure S44: Schematic Diagram of Compound Separation Process; Figure S45: ITS sequencing information of Chaetomium globosum and Phylogenetic tree analysis; Figure S46: The amplified representative pictures of the hypha morphology of B. cinerea observed under SEM.
Author Contributions
Conceptualization, J.B. and H.Z.; Methodology, Z.-Y.M., L.-J.W., X.-L.W., K.-L.W. and Y.-Y.W.; Validation, Y.-Y.W.; Formal analysis, Y.-Y.W.; Investigation, Z.-Y.M., L.-J.W., X.-L.W., K.-L.W., T.Z., R.-Y.H., J.-J.L. and Y.-Y.W.; Resources, H.Z.; Data curation, Z.-Y.M., L.-J.W., J.-J.L. and Y.-Y.W.; Writing—original draft preparation, Y.-Y.W.; Writing—review and editing, J.B. and H.Z.; Visualization, Y.-Y.W. and L.-J.W.; Supervision, J.B. and H.Z.; Project administration, Y.-Y.W.; Funding acquisition, J.B. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The funding support from the University of Shandong Province Higher Educational Youth Innovation Science and Technology Program (No. 2022KJ096) is greatly acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available in the Supplementary Materials attached to this article.
Acknowledgments
The authors thank the Shandong Academy of Agricultural Sciences for the spectra measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ji, X.; Xia, Y.; Zhang, H.; Cui, J.L. The Microscopic Mechanism between Endophytic Fungi and Host Plants: From Recognition to Building Stable Mutually Beneficial Relationships. Microbiol. Res. 2022, 261, 127056. [Google Scholar] [CrossRef] [PubMed]
- Collinge, D.B.; Jensen, B.; Jorgensen, H.J. Fungal Endophytes in Plants and Their Relationship to Plant Disease. Curr. Opin. Microbiol. 2022, 69, 102177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Wang, L.; Pan, Y.P.; Zheng, X.; Liang, X.; Sheng, L.; Zhang, D.; Sun, Q.; Wang, Q. Research Advances on Endophytic Fungi and Their Bioactive Metabolites. Bioprocess. Biosyst. Eng. 2023, 46, 165–170. [Google Scholar] [CrossRef]
- Settu, S.; Arunachalam, S. Fungal Endophytes: A Blooming Reservoir for Future Products. Int. J. Pharm. Sci. Rev. Res. 2020, 65, 169–178. [Google Scholar] [CrossRef]
- Manathunga, K.K.; Gunasekara, N.W.; Meegahakumbura, M.K.; Faraj, T.K.; Wanasinghe, D.N. Exploring Endophytic Fungi as Natural Antagonists against Fungal Pathogens of Food Crops. J. Fungi 2024, 10, 606. [Google Scholar] [CrossRef]
- Akram, S.; Ahmed, A.; He, P.; Liu, Y.; Wu, Y.; Munir, S.; He, Y. Uniting the Role of Endophytic Fungi against Plant Pathogens and Their Interaction. J. Fungi 2023, 9, 72. [Google Scholar] [CrossRef]
- Grabka, R.; Entremont, T.W.; Adams, S.J.; Walker, A.K.; Tanney, J.B.; Abbasi, P.A.; Ali, S. Fungal Endophytes and Their Role in Agricultural Plant Protection against Pests and Pathogens. Plants 2022, 11, 384. [Google Scholar] [CrossRef]
- Pokhriyal, A.; Kapoor, N.; Negi, S.; Sharma, G.; Chandra, S.; Gambhir, L.; Douglas Melo Coutinho, H. Endophytic Fungi: Cellular Factories of Novel Medicinal Chemistries. Bioorg. Chem. 2024, 150, 107576. [Google Scholar] [CrossRef]
- Saxena, S.; Dufosse, L.; Deshmukh, S.K.; Chhipa, H.; Gupta, M.K. Endophytic Fungi: A Treasure Trove of Antifungal Metabolites. Microorganisms 2024, 12, 1903. [Google Scholar] [CrossRef]
- Varghese, S.; Jisha, M.S.; Rajeshkumar, K.C.; Gajbhiye, V.; Alrefaei, A.F.; Jeewon, R. Endophytic fungi: A future prospect for breast cancer therapeutics and drug development. Heliyon 2024, 10, e33995. [Google Scholar] [CrossRef]
- Fan, M.C.; Li, T.T.; Li, Y.; Qian, H.; Zhang, H.; Rao, Z.; Wang, L. Vaccinium bracteatum Thunb. as a Promising Resource of Bioactive Compounds with Health Benefits: An Updated Review. Food Chem. 2021, 356, 129738. [Google Scholar] [CrossRef]
- Flora of China Editorial Committee of Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 1991; Volume 57, p. 75. [Google Scholar]
- Wang, Y.Y.; Zhang, J.S.; Wang, X.X.; Tian, L.L.; Li, Y.P.; Wang, C.; Ma, R.F.; Yin, Y.K.; Bao, J.; Zhang, H. Identification of Small-Molecule Bioactive Constituents from the Leaves of Vaccinium bracteatum Confirms It as a Potential Functional Food with Health Benefits. Foods 2023, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Li, J.J.; Wei, Q.H.; Wang, X.X.; Zhang, J.S.; Yan, J.J.; Zhang, H. Bioactive Neolignan, Iridoid and Flavonoid Glycosides from the Leaves of Vaccinium bracteatum. J. Asian Nat. Prod. Res. 2024, 26, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, V.; Ceci, A.; Giovannini, R.; Sciubba, F.; Persiani, A.M. The good fight: Minimedusa polyspora and Chaetomium globosum effectively antagonize phytopathogenic fungi in vitro conditions. Mycologia 2025, 117, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zou, J.M.; Li, L.; Bao, K.; Zhao, Y.; Miao, C.P.; Zhao, L.X.; Chen, Y.W.; Li, Y.Q. Cyclic peptide secondary metabolites with antifungal activity against root-rotpathogens of Panax Notoginseng produced by Streptomyces yatensis. Chem. Nat. Compd. 2021, 57, 1181–1183. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Pan, Y.B.; Wang, Z.Y.; Li, J.J.; Bao, j.; Zhang, J.S.; Zhang, H. Anti-inflammatory Polyketides from an Endophytic Fungus Chaetomium sp. UJN-EF006 of Vaccinium bracteatum. Chem. Biodivers. 2024, 21, e202400002. [Google Scholar] [CrossRef]
- Sekita, S.; Yoshihira, K.; Natori, S.; Kuwano, H. Structures of chaetoglobosin A and B, cytotoxic metabolites of Chaetomium globosum. Tetrahedron Lett. 1973, 14, 2109–2112. [Google Scholar] [CrossRef]
- Zhu, H.C.; Chen, C.M.; Tong, Q.Y.; Zhou, Y.; Ye, Y.; Gu, L.H.; Zhang, Y.H. Progress in the Chemistry of Cytochalasans. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A.D., Heinz Falk, H., Gibbons, S., Kobayashi, J., Asakawa, Y., Liu, J.K., Eds.; Springer: Cham, Switzerland, 2021; Volume 114, pp. 1–134. [Google Scholar]
- Tian, C.; Feng, Y.Y.; Zhang, H.; Mao, X.Y.; Zhu, X.Y.; Wang, X.; Hou, C.; Xiaoyang Han, X.Y.; Yang, H.X.; Liu, J.K. Discovery of highly oxygenated cytochalasans with antiproliferative activity from an endophytic fungus Boeremia exigua. Bioorg. Chem. 2025, 156, 108198. [Google Scholar] [CrossRef]
- Zhang, H.L.; Hantke, V.; Bruhnke, P.; Skellam, E.J.; Cox, J.R. Chemical and Genetic Studies on the Formation of Pyrrolones During the Biosynthesis of Cytochalasans. Chem. Eur. J. 2021, 27, 3106–3113. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Long, X.W.; Wu, H.; Deng, J. Recent advances in the total synthesis of cytochalasan natural products using bioinspired strategies. Org. Chem. Front. 2022, 9, 6979–6998. [Google Scholar] [CrossRef]
- Cui, X.M.; Ma, D.Y.; Liu, X.Y.; Zhang, Z.Q.; Li, B.Q.; Xu, Y.; Chen, T.; Tian, S.P. Magnolol inhibits gray mold on postharvest fruit by inducing autophagic activity of Botrytis cinerea. Postharvest Biol. Technol. 2021, 180, 111596. [Google Scholar] [CrossRef]
- Wei, Q.H.; Cao, X.X.; Xu, D.F.; Wang, S.T.; Zhang, J.S.; Zhang, H. Anti-inflammatory labdane diterpenoids from the aerial parts of Leonurus japonicus. Phytochemistry 2023, 210, 113646. [Google Scholar] [CrossRef]
- Zhu, J.K.; Gao, J.M.; Yang, C.J.; Shang, X.F.; Zhao, Z.M.; Lawoe, R.K.; Zhou, R.; Sun, Y.; Yin, X.D.; Liu, Y.Q. Design, Synthesis, and Antifungal Evaluation of Neocryptolepine Derivatives against Phytopathogenic Fungi. J. Agric. Food Chem. 2020, 68, 2306–2315. [Google Scholar] [CrossRef]
- Wang, D.L.; Yuan, C.X.; Li, Y.P.; Bai, S.; Feng, J.; Wang, Y.; Fang, Y.; Zhang, Z. Chelation of the Optimal Antifungal Pogostone Analogue with Copper (II) to Explore the Dual Antifungal and Antibacterial Agent. J. Agric. Food Chem. 2024, 72, 3894–3903. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.F.; Wu, T.L.; Du, S.S.; Wu, Z.R.; Hu, Y.M.; Zhang, Z.J.; Zhao, W.B.; Yang, C.J.; Liu, Y.Q. The Antifungal Mechanism of Isoxanthohumol from Humulus lupulus Linn. Int. J. Mol. Sci. 2021, 22, 10853. [Google Scholar] [CrossRef]
- Chen, J.H.; Zhang, W.Z.; Guo, Q.; Yu, W.; Zhang, Y.; He, B. Bioactivities and Future Perspectives of Chaetoglobosins. Evid. Based Complement. Altern. Med. 2020, 2020, 8574084. [Google Scholar] [CrossRef] [PubMed]
- Sekita, S.; Yoshihira, K.; Natori, S.; Harumitsu, K. Chaetoglobosins, Cytotoxic 10-(Indol-3-yl)-[13] cytochalasans from Chaetomium spp. III. Structures of Chaetoglobosins C, E, F, G, and J. Chem. Pharm. Bull. 1982, 30, 1629–1638. [Google Scholar] [CrossRef]
- Iwamoto, C.; Yamada, T.; Ito, Y.; Katsuhiko, M.; Atsushi, N. Cytotoxic Cytochalasans from a Penicillium Species Separated from a Marine Alga. Tetrahedron 2001, 57, 2997–3004. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).