Comparative Untargeted Metabolomic Analysis of Fruiting Bodies from Three Sanghuangporus Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Information
2.2. Dry Sample Extraction
2.3. UPLC-MS/MS Analysis
2.4. Data Processing and Metabolite Identification
2.5. Data Analysis
3. Result
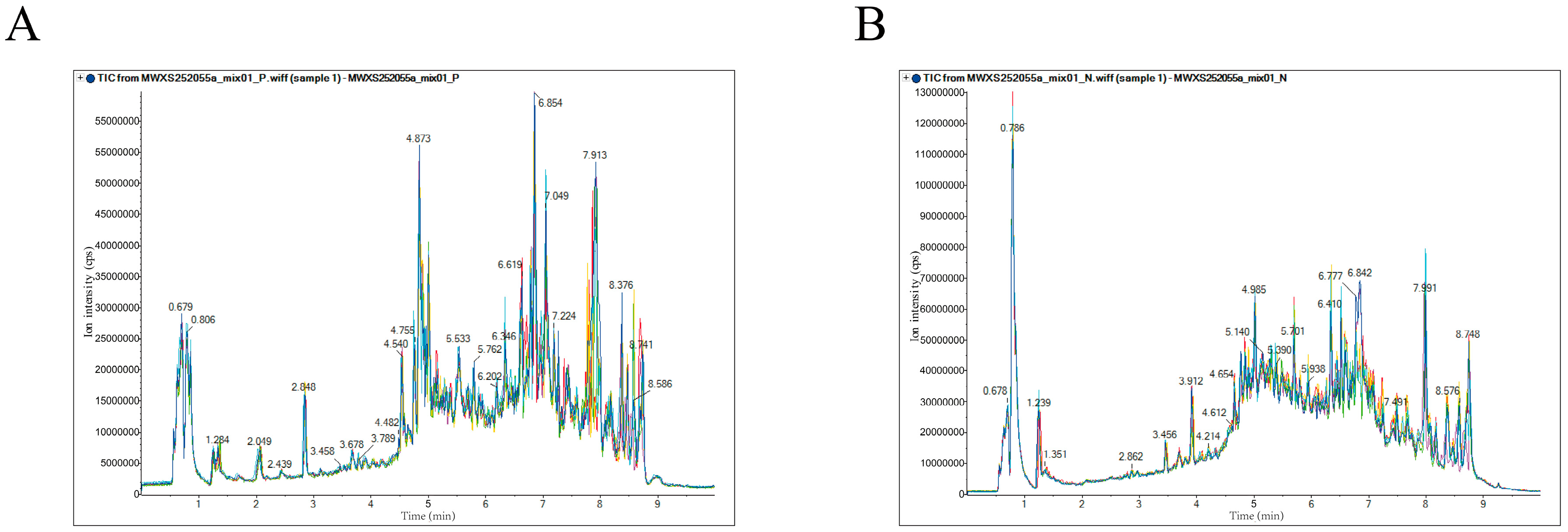
3.1. Total Ion Chromatogram Analysis
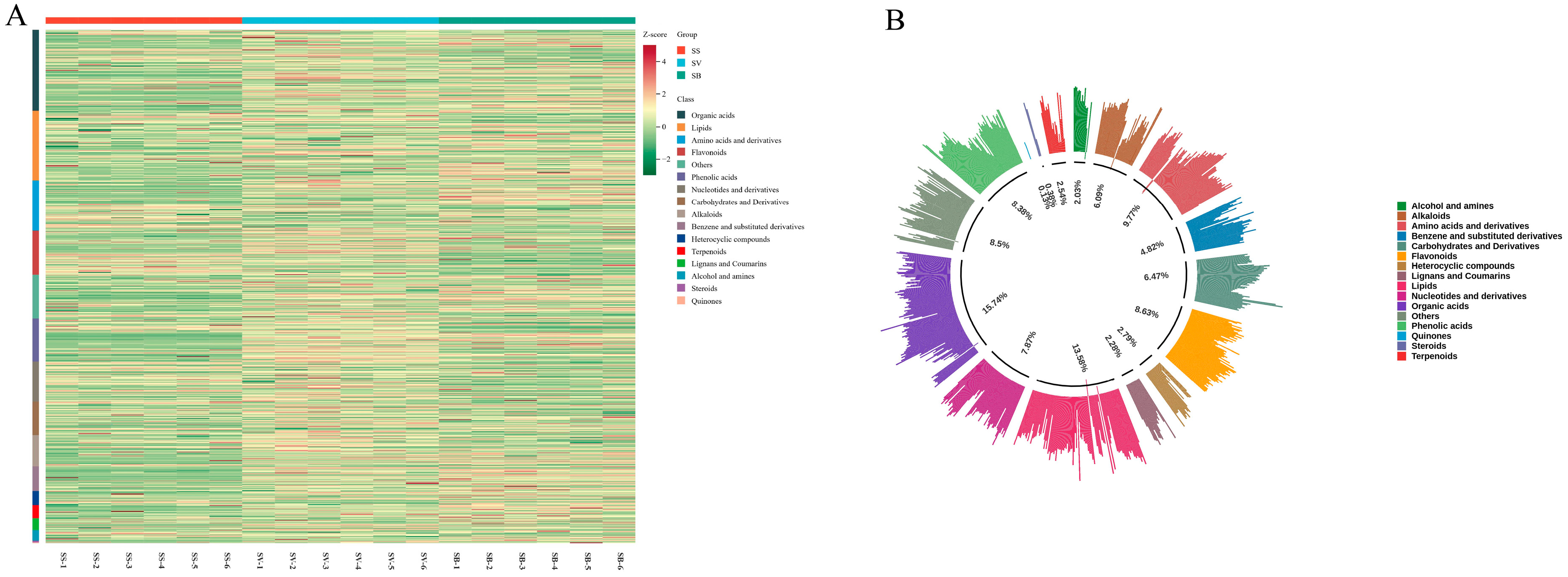
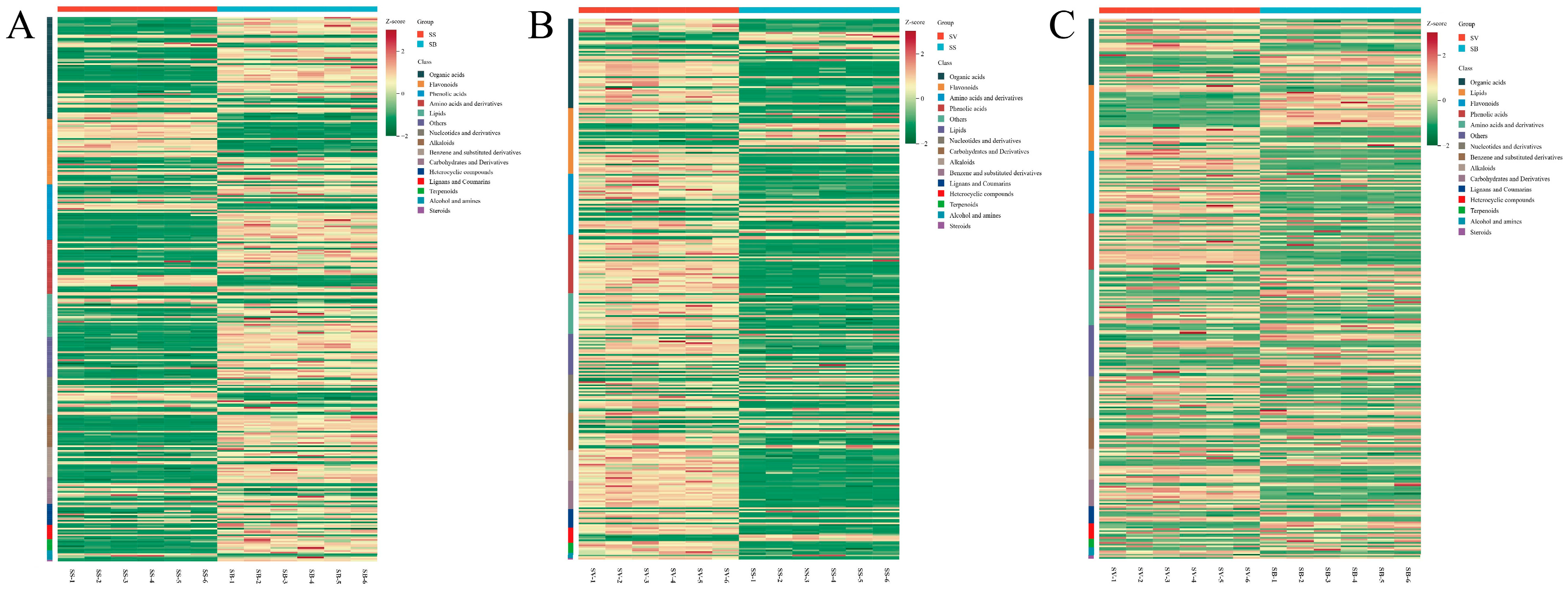
3.2. Classification Statistics of Metabolites in Different Sanghuangporus Species
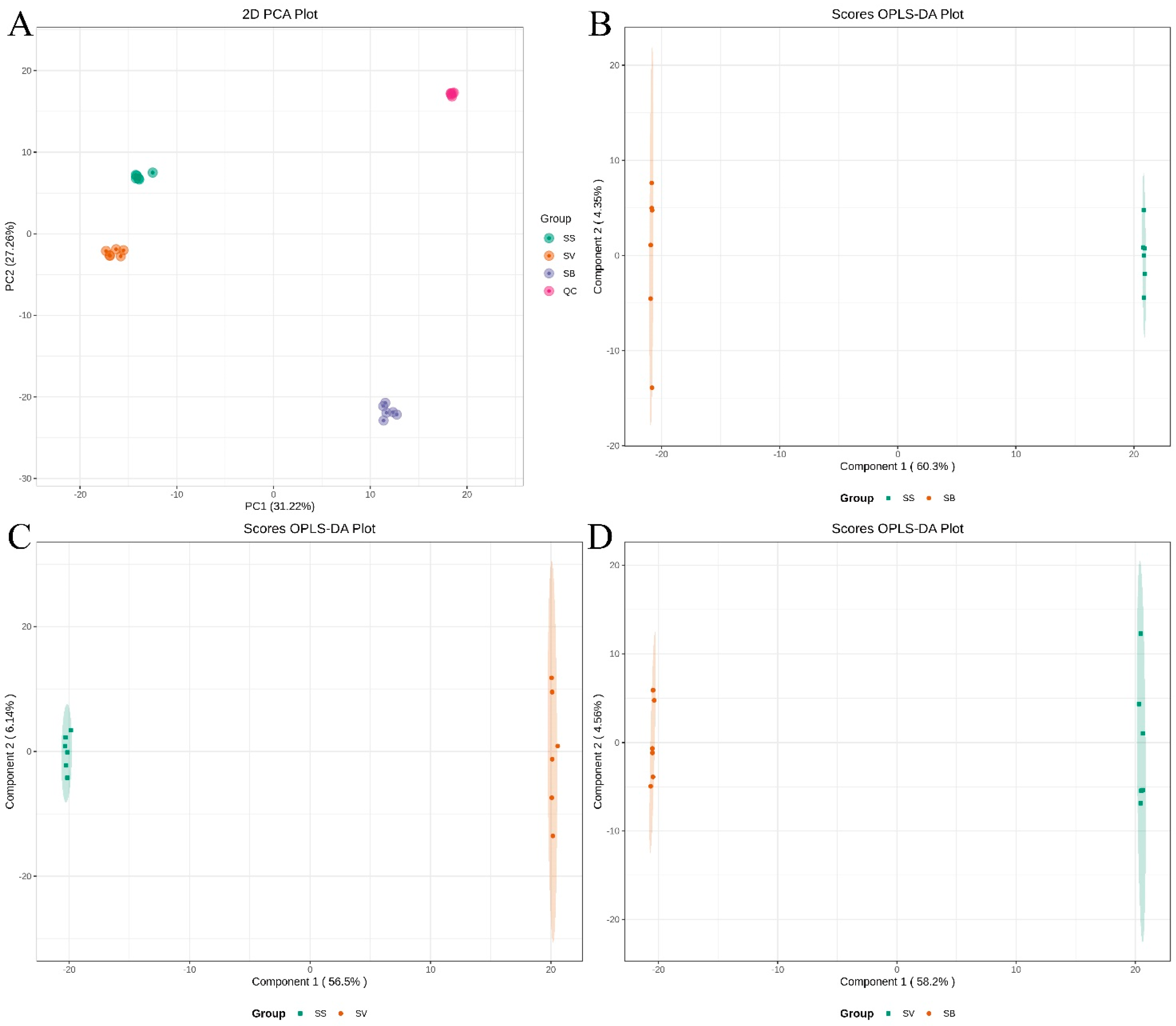
3.3. PCA and OPLS-DA
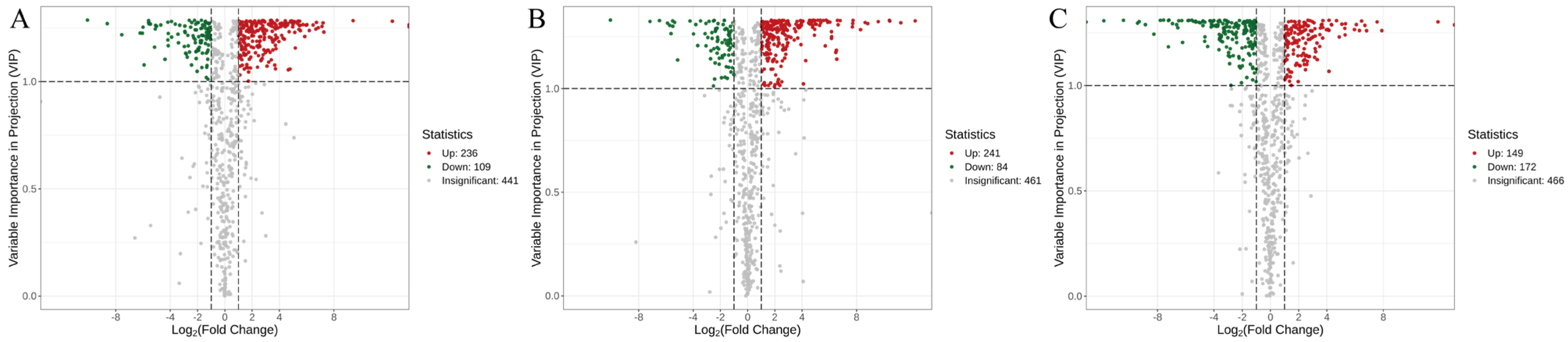
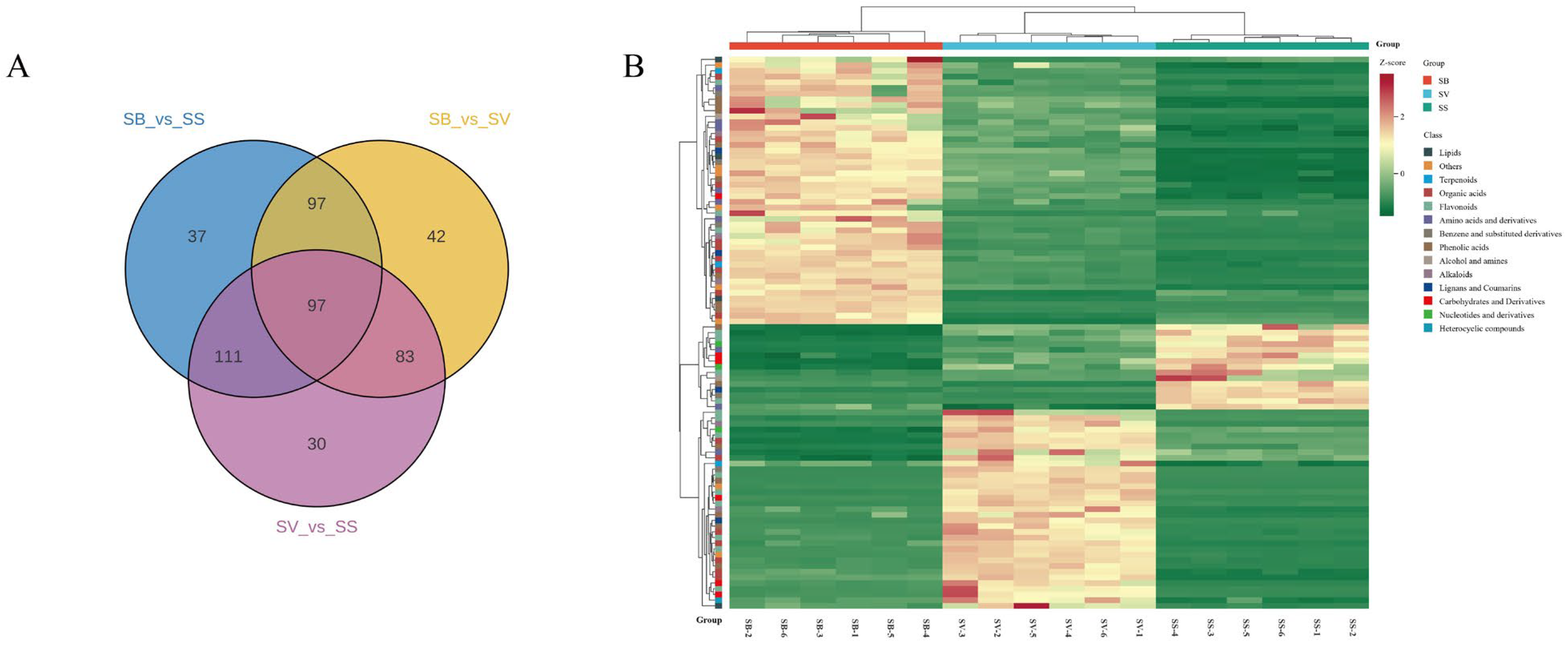
3.4. Screening of Differential Metabolites Among the Three Sanghuangporus Species
3.5. Analysis of Shared Differential Metabolites
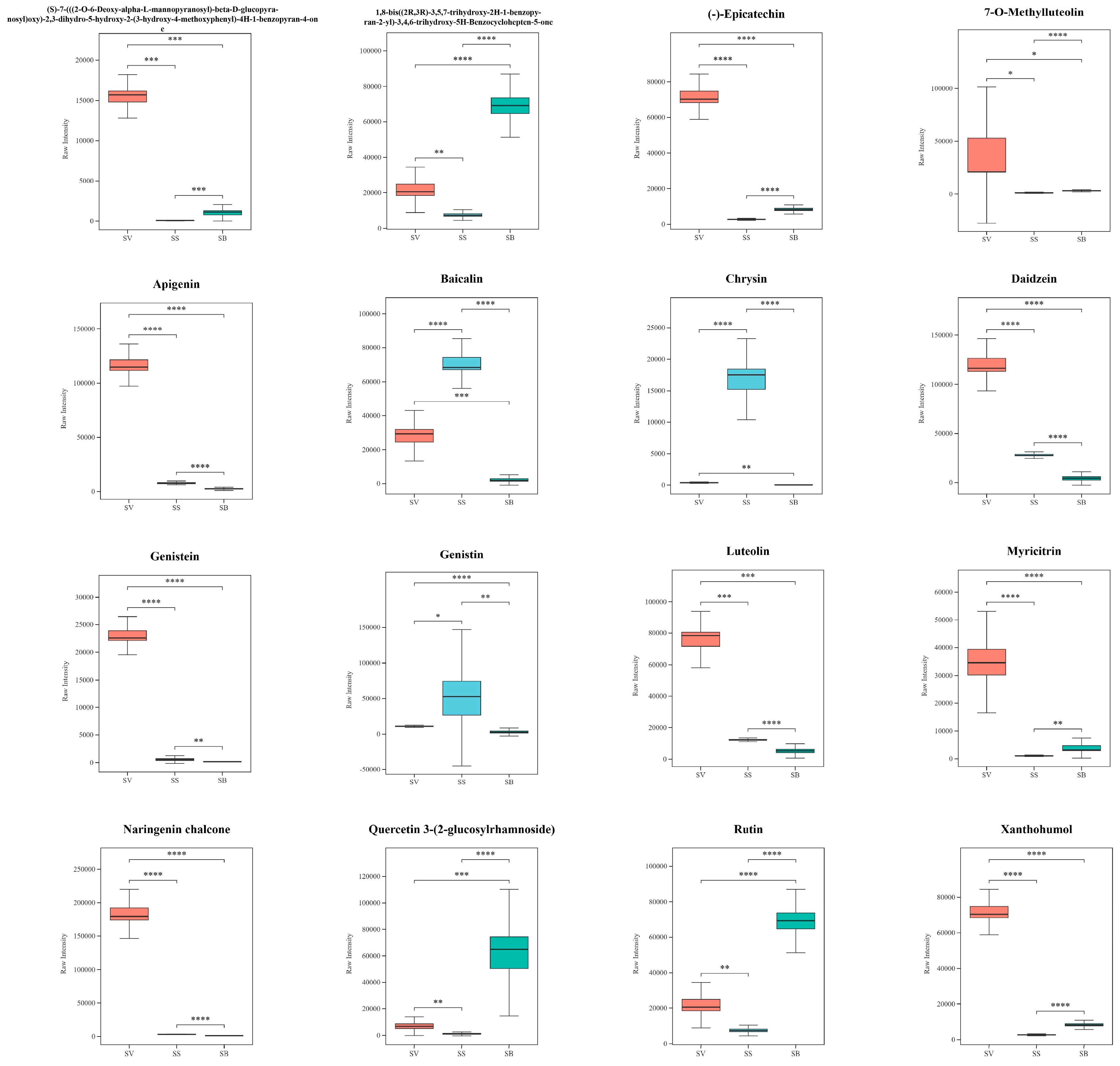
3.6. Analysis of Flavonoids in the Three Sanghuangporus Species
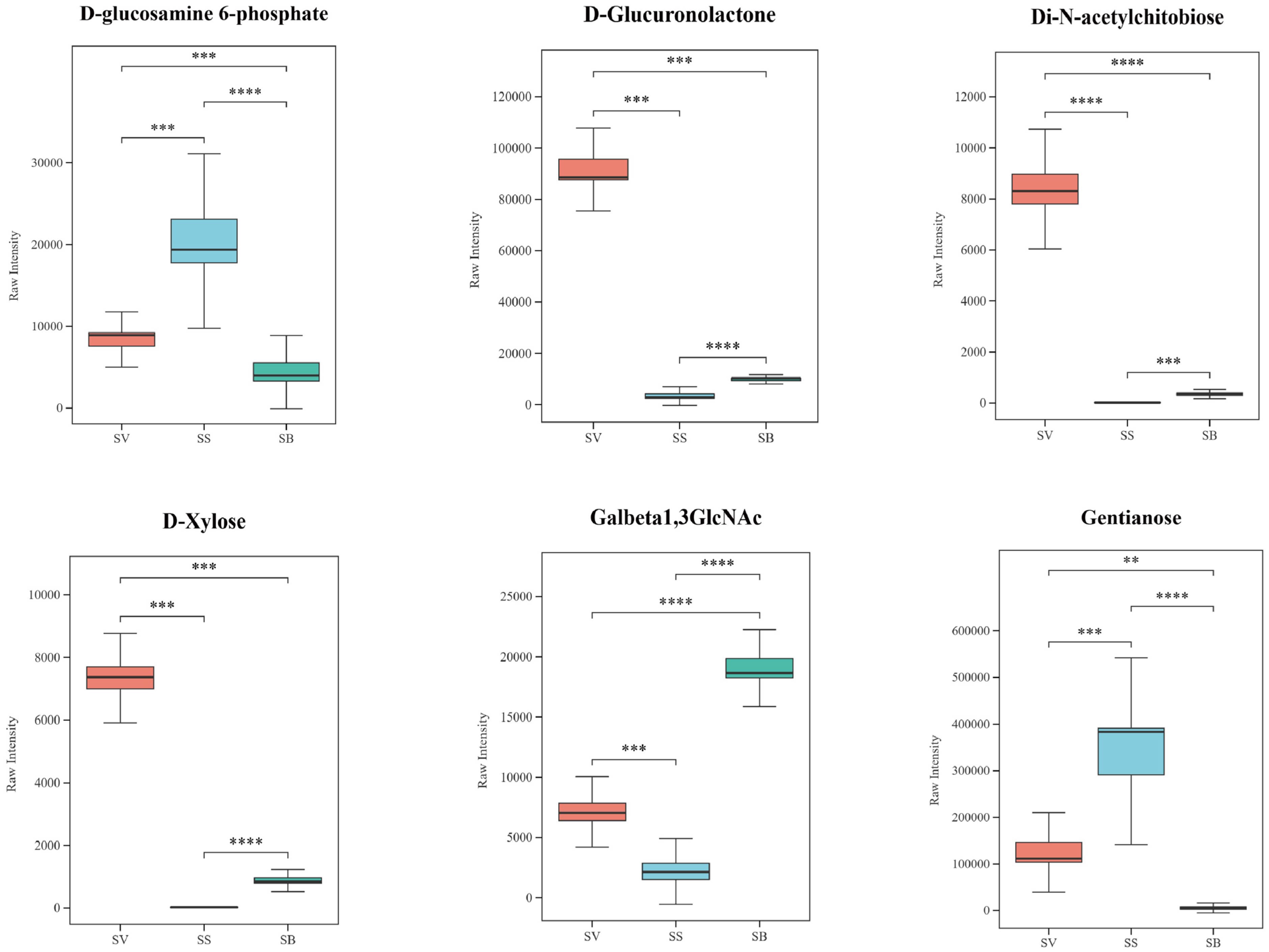
3.7. Analysis of Polysaccharides in the Three Sanghuangporus Species
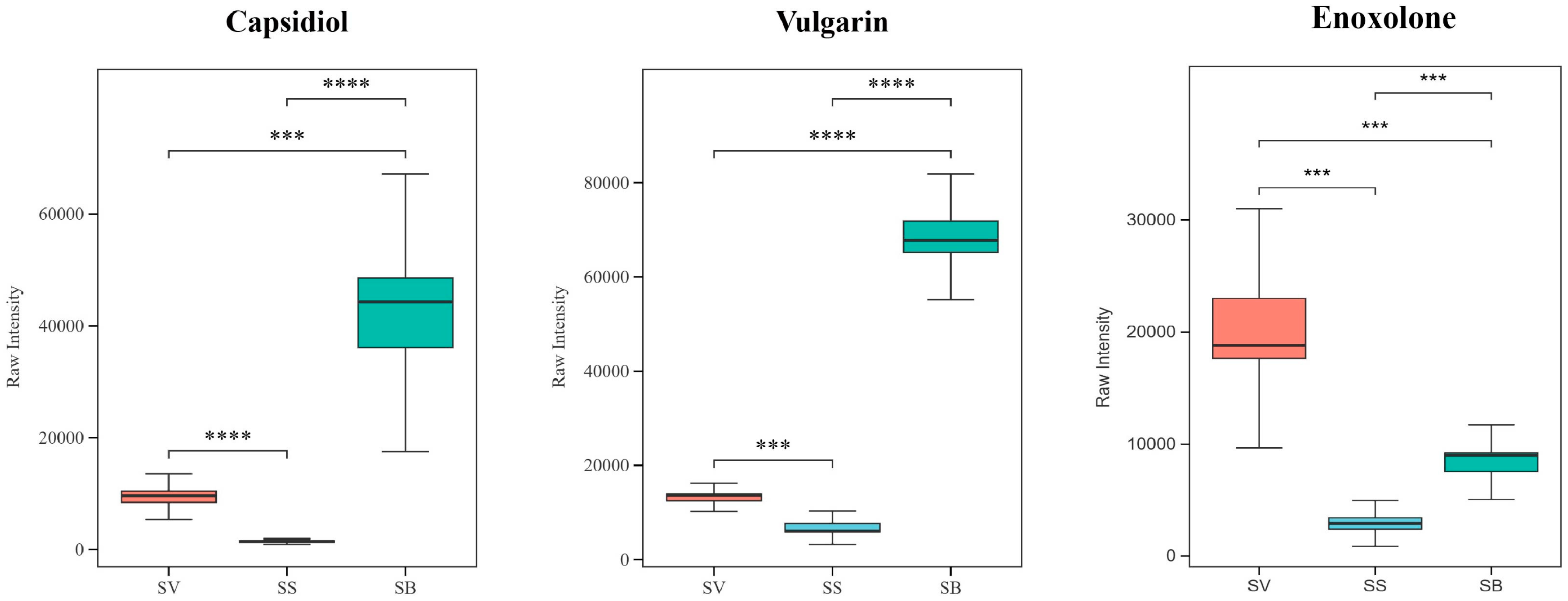
3.8. Analysis of Terpenoids in the Three Sanghuangporus Species
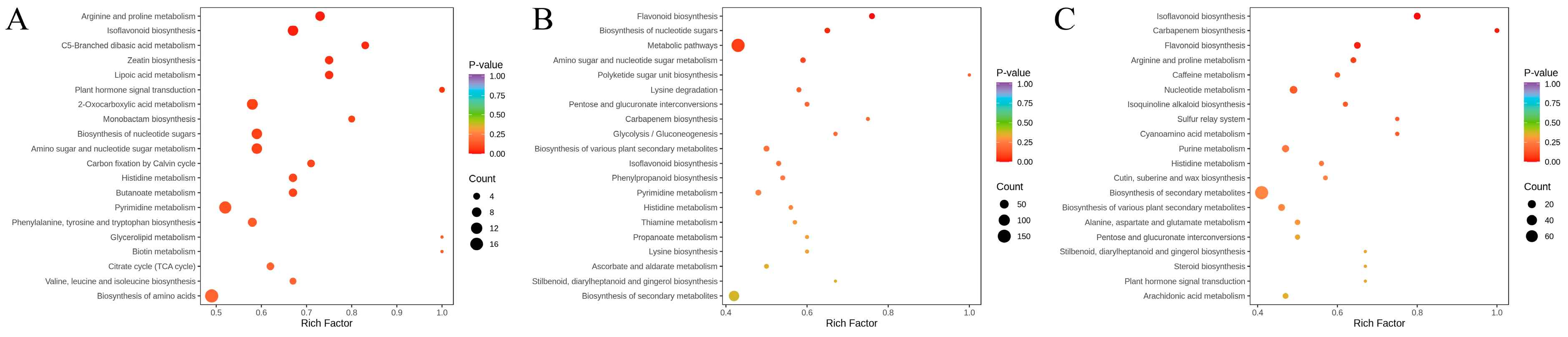
3.9. KEGG Pathway Enrichment Analysis of Differential Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Z.; Liang, S.; Zou, X.; Teng, Y.; Wang, W.; Fu, L. Determination of Phenolic Acids Using Ultra-High-Performance Liquid Chromatography Coupled with Triple Quadrupole (UHPLC-QqQ) in Fruiting Bodies of Sanghuangporus baumii (Pilát) LW Zhou and YC Dai. Plants 2023, 12, 3565. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Cao, N.; Huang, L.; Tang, L.; Liu, X.; Zhang, W.; Huang, S.; Xie, X.; Yan, Y. Structural Characterization, and Antioxidant, Hypoglycemic and Immunomodulatory Activity of Exopolysaccharide from Sanghuangporus sanghuang JM-1. Molecules 2024, 29, 4546. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Su, M.; Zhou, X.; Li, D.; Niu, X.; Wang, Y. Research Progress of Bioactive Components in Sanghuangporus spp. Molecules 2024, 29,1195. Molecules 2024, 29, 1195. [Google Scholar] [CrossRef]
- Zhang, X.; An, S.; Zhou, L.; Bao, Y. Comparison of Morphology, Components, and Activity of Four Species of Sanghuangporus Mushrooms (Agaricomycetes). Int. J. Med. Mushrooms 2025, 27, 45–65. [Google Scholar] [CrossRef]
- Wu, S.-H.; Dai, Y.-C.; Hattori, T.; Yu, T.-W.; Wang, D.-M.; Parmasto, E.; Chang, H.-Y.; Shih, S.-Y. Species clarification for the medicinally valuable’sanghuang’mushroom. Bot. Stud. 2012, 53, 135–149. [Google Scholar]
- Cheng, J.; Song, J.; Wei, H.; Wang, Y.; Huang, X.; Liu, Y.; Lu, N.; He, L.; Lv, G.; Ding, H.; et al. Structural characterization and hypoglycemic activity of an intracellular polysaccharide from Sanghuangporus sanghuang mycelia. Int. J. Biol. Macromol. 2020, 164, 3305–3314. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Balik, M.; Muszyńska, B. Selected Species of the Genus Phellinus-Chemical Composition, Biological Activity, and Medicinal Applications. Chem. Biodivers. 2021, 18, e2100609. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, R.; Tong, X.; Zou, L. New Insights into Methyl Jasmonate Regulation of Triterpenoid Biosynthesis in Medicinal Fungal Species Sanghuang porusbaumii (Pilát) LW Zhou & YC Dai. J. Fungi. 2022, 8, 889. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.; Wang, S.; Sun, T.; Zou, L. Salicylic acid promotes terpenoid synthesis in the fungi Sanghuangporus baumii. Microb. Biotechnol. 2023, 16, 1360–1372. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Wang, X.; Liu, R.; Zou, L. Mushrooms Do Produce Flavonoids: Metabolite Profiling and Transcriptome Analysis of Flavonoid Synthesis in the Medicinal Mushroom Sanghuangporus baumii. J. Fungi 2022, 8, 582. [Google Scholar] [CrossRef]
- He, P.Y.; Hou, Y.H.; Yang, Y.; Li, N. The anticancer effect of extract of medicinal mushroom Sanghuangprous vaninii against human cervical cancer cell via endoplasmic reticulum stress-mitochondrial apoptotic pathway. J. Ethnopharmacol. 2021, 279, 114345. [Google Scholar] [CrossRef]
- Wan, X.; Jin, X.; Xie, M.; Liu, J.; Gontcharov, A.A.; Wang, H.; Lv, R.; Liu, D.; Wang, Q.; Li, Y. Characterization of a polysaccharide from Sanghuangporus vaninii and its antitumor regulation via activation of the p53 signaling pathway in breast cancer MCF-7 cells. Int. J. Biol. Macromol. 2020, 163, 865–877. [Google Scholar] [CrossRef]
- Zuo, K.; Tang, K.; Liang, Y.; Xu, Y.; Sheng, K.; Kong, X.; Wang, J.; Zhu, F.; Zha, X.; Wang, Y. Purification and antioxidant and anti-Inflammatory activity of extracellular polysaccharopeptide from sanghuang mushroom, Sanghuangporus lonicericola. J. Sci. Food Agric. 2021, 101, 1009–1020. [Google Scholar] [CrossRef]
- Cai, C.; Ma, J.; Han, C.; Jin, Y.; Zhao, G.; He, X. Extraction and antioxidant activity of total triterpenoids in the mycelium of a medicinal fungus, Sanghuangporus sanghuang. Sci. Rep. 2019, 9, 7418. [Google Scholar] [CrossRef]
- Rajput, S.A.; Wang, X.Q.; Yan, H.C. Morin hydrate: A comprehensive review on novel natural dietary bioactive compound with versatile biological and pharmacological potential. Biomed. Pharmacother. 2021, 138, 111511. [Google Scholar] [CrossRef]
- Jung, G.H.; Kang, J.H. Efficacy of Phellinus linteus (sanghuang) extract for improving immune functions: Study protocol for a randomized, double-blinded, placebo-controlled pilot trial. Medicine 2020, 99, e18829. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, J.X.; Zhou, M.; Si, J.; Cui, B.K. Current advances and potential trends of the polysaccharides derived from medicinal mushrooms sanghuang. Front. Microbiol. 2022, 13, 965934. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Chang, C.C.; Wei, C.L.; Jiang, G.Z.; Cui, B.K. Sanghuangporus toxicodendri sp. nov. (Hymenochaetales, Basidiomycota) from China. MycoKeys 2019, 57, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Gika, H.; Virgiliou, C.; Theodoridis, G.; Plumb, R.S.; Wilson, I.D. Untargeted LC/MS-based metabolic phenotyping (metabonomics/metabolomics): The state of the art. J. Chromatogr. B 2019, 1117, 136–147. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, M.; Zhao, Y.; Zha, L.; Yang, H.; Wu, Y. GC–MS-Based Nontargeted and Targeted Metabolic Profiling Identifies Changes in the Lentinula edodes Mycelial Metabolome under High-Temperature Stress. Int. J. Mol. Sci. 2019, 20, 2330. [Google Scholar] [CrossRef]
- Bobiş, O.; Bonta, V.; Cornea-Cipcigan, M.; Nayik, G.A.; Dezmirean, D.S. Bioactive Molecules for Discriminating Robinia and Helianthus Honey: High-Performance Liquid Chromatography-Electron Spray Ionization-Mass Spectrometry Polyphenolic Profile and Physicochemical Determinations. Molecules 2021, 26, 4433. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Zhao, Y.; Wu, F.; Yue, Y.; Zhang, Y.; Li, D. Ultrasound-assisted optimization extraction and biological activities analysis of flavonoids from Sanghuangporus sanghuang. Ultrason. Sonochem. 2025, 117, 107326. [Google Scholar] [CrossRef]
- Zhang, N.; Nao, J.; Dong, X. Efficacy and Safety of Natural Apigenin Treatment for Alzheimer′s Disease: Focus on In vivo Research Advancements. Curr. Neuropharmacol. 2025, 23, 728–754. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhang, X.; Zhou, N.; Hu, T.; Shen, Y.; Chen, T.J.; Liu, Y.; Cui, H.; Zhu, S. Apigenin inhibits lipid metabolism of hepatocellular carcinoma cells by targeting the histone demethylase KDM1A. Phytomedicine 2024, 135, 156024. [Google Scholar] [CrossRef] [PubMed]
- Pereyra-Vergara, F.; Olivares-Corichi, I.M.; Luna-Arias, J.P.; Méndez-Luna, D.; García-Sánchez, J.R. Epicatechin Decreases UCP2 Gene Expression in MDA-MB-231 Breast Cancer Cells by the Presence of a Regulatory Element in the Promoter. Int. J. Mol. Sci. 2025, 26, 4102. [Google Scholar] [CrossRef] [PubMed]
- Hirai, S.; Kim, Y.I.; Goto, T.; Kang, M.S.; Yoshimura, M.; Obata, A.; Yu, R.; Kawada, T. Inhibitory effect of naringenin chalcone on inflammatory changes in the interaction between adipocytes and macrophages. Life Sci. 2007, 81, 1272–1279. [Google Scholar] [CrossRef]
- Guo, L.T.; Wang, S.Q.; Su, J.; Xu, L.X.; Ji, Z.Y.; Zhang, R.Y.; Zhao, Q.W.; Ma, Z.Q.; Deng, X.Y.; Ma, S.P. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J. Neuroinflammation 2019, 16, 95. [Google Scholar] [CrossRef]
- Deng, L.; Yu, Q.; Kuang, G.; Wang, L.; Fan, J.; Ye, L. Luteolin modulates liver macrophage subtype polarization and play protective role in sepsis induced acute hepatic injury. Inflamm. Res. 2025, 74, 59. [Google Scholar] [CrossRef]
- Wang, X.; Gong, M.; Zhu, Z.; Zhang, B.; Han, L.; Li, W.; Wu, Z.; Ma, Q.; Wang, Z.; Qian, W. Rutin protects the pancreas from inflammatory injury and oncogene-driven tumorigenesis by inhibiting acinar to ductal metaplasia. Eur. J. Pharmacol. 2025, 998, 177536. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.; Wang, Z.; Li, Z.; Geng, Y. Effects of Pine Pollen Polysaccharides and Sulfated Polysaccharides on Ulcerative Colitis and Gut Flora in Mice. Polymers 2023, 15, 1414. [Google Scholar] [CrossRef]
- Liu, G.; Das, S.K. D-Xylose Ameliorates Non-Alcoholic Fatty Liver Disease by Targeting Macrophage-expressed LYZ Gene. Cell Biochem. Biophys. 2025, 83, 1617–1629. [Google Scholar] [CrossRef]
- Shen, Y.; Miao, Z.; Zheng, Y.; Dong, Y.; Han, M.; Huang, C.; Bai, R.; Xia, C.; Shi, S.; Li, J. D-Glucuronolactone Supplementation Enhances Production Performance, Eggshell Quality, and Liver Health in Laying Hens. Animals 2025, 15, 1317. [Google Scholar] [CrossRef]
- Khadbaatar, S.; Bao, H.; Gao, X.; Huo, H. Study on Differences of Metabolites among Different Ganoderma Species with Comprehensive Metabolomics. J. Fungi. 2024, 10, 524. [Google Scholar] [CrossRef]
- Zhang, D.X.; Liu, B.Y.; Xue, F.F.; Tang, Y.L.; Yan, M.J.; Wang, S.X.; Guo, L.; Tong, T.; Wan, L.N.; Liu, Y.N.; et al. Paclobutrazol induces triterpenoid biosynthesis via downregulation of the negative transcriptional regulator SlMYB in Sanghuangporus lonicericola. Commun. Biol. 2025, 8, 551. [Google Scholar] [CrossRef]
- Tian, C.; Sun, L.T.; Jin, T.; Yuan, L.L.; Xu, W.F.; Yang, H.X.; Feng, T.; Liu, J.K. Phellintremulins A-C, antinociceptive sesquiterpenoids from the medicinal fungus Phellinus tremulae. Phytochemistry 2024, 223, 114112. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Deng, J.S.; Huang, S.S.; Wu, S.H.; Chen, C.C.; Lin, W.R.; Lin, H.Y.; Huang, G.J. Anti-Inflammatory Activity of Sanghuangporus sanghuang Mycelium. Int. J. Mol. Sci. 2017, 18, 347. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Shen, S.; Zhou, L.W. Modeling current geographic distribution and future range shifts of Sanghuangporus under multiple climate change scenarios in China. Front. Microbiol. 2022, 13, 1064451. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Liu, S.L.; Jiang, J.H.; Zhou, L.W. Addressing widespread misidentifications of traditional medicinal mushrooms in Sanghuangporus (Basidiomycota) through ITS barcoding and designation of reference sequences. IMA Fungus 2021, 12, 10. [Google Scholar] [CrossRef]
- Qi, Y.; Guo, X.Y.; Xu, X.Y.; Hou, J.X.; Liu, S.L.; Guo, H.B.; Xu, A.G.; Yang, R.H.; Yu, X.D. Widely targeted metabolomics analysis of Sanghuangporus vaninii mycelia and fruiting bodies at different harvest stages. Front. Microbiol. 2024, 15, 1391558. [Google Scholar] [CrossRef]
- Alhalmi, A.; Amin, S.; Ralli, T.; Ali, K.S.; Kohli, K. Therapeutic role of naringin in cancer: Molecular pathways, synergy with other agents, and nanocarrier innovations. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 3595–3615. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Alenezi, S.K.; Alsahli, T.; Afzal, M.; Mantargi, M.J.S.; Kazmi, I.; Sayyed, N. Effect of sakuranetin against cyclophosphamide-induced immunodeficiency mice: Role of IFN-γ/TNF-α/IgG/IgM/interleukins. Naunyn. Schmiedebergs Arch. Pharmacol. 2025, 398, 1–12. [Google Scholar] [CrossRef]
- Jiang, Z.B.; Wang, W.J.; Xu, C.; Xie, Y.J.; Wang, X.R.; Zhang, Y.Z.; Huang, J.M.; Huang, M.; Xie, C.; Liu, P.; et al. Luteolin and its derivative apigenin suppress the inducible PD-L1 expression to improve anti-tumor immunity in KRAS-mutant lung cancer. Cancer Lett. 2021, 515, 36–48. [Google Scholar] [CrossRef]
- Song, G.; Wang, K.; Zhang, H.; Sun, H.; Wu, B.; Ju, X. Structural characterization and immunomodulatory activity of a novel polysaccharide from Pteridium aquilinum. Int. J. Biol. Macromol. 2017, 102, 599–604. [Google Scholar] [CrossRef]
- Pierman, B.; Toussaint, F.; Bertin, A.; Lévy, D.; Smargiasso, N.; De Pauw, E.; Boutry, M. Activity of the purified plant ABC transporter NtPDR1 is stimulated by diterpenes and sesquiterpenes involved in constitutive and induced defenses. J. Biol. Chem. 2017, 292, 19491–19502. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Xu, M.; Cheng, X.; Li, N.; Xu, H.; Feng, Y.; Guan, T.; Xiao, L. Patrinia scabiosaefolia L. Modulates the Intestinal Microecology to Treat DSS-Induced Ulcerative Colitis: UHPLC-OE-MS/MS, Network Pharmacology, and Experimental Validation. Foods 2025, 14, 1145. [Google Scholar] [CrossRef]

| Group | Classification | Compound | Up/Downregulated |
|---|---|---|---|
| SB_vs._SS1 | Benzene and substituted derivatives | Phenelzine | up |
| SB_vs._SS2 | Organic acids | Geranylgeranyl diphosphate | down |
| SB_vs._SS3 | Phenolic acids | Cinnamic acid | up |
| SB_vs._SS4 | Flavonoids | Naringin | down |
| SB_vs._SS5 | Flavonoids | Chrysin | down |
| SB_vs._SS6 | Alkaloids | Ergothioneine | up |
| SB_vs._SS7 | Organic acids | 3-Carboxy-4-methyl-5-propyl-2-furanpropionic acid | up |
| SB_vs._SS8 | Benzene and substituted derivatives | 2,3-Dihydroxy-4-methoxybenzoic | up |
| SB_vs._SS9 | Amino acids and derivatives | Glutamylproline | up |
| SB_vs._SS10 | Phenolic acids | 4-Hydroxy-3-methoxybenzenemethanol | up |
| SB_vs._SS11 | Benzene and substituted derivatives | Benzimidazole | up |
| SB_vs._SS12 | Amino acids and derivatives | His-Glu | up |
| SB_vs._SS13 | Alcohol and amines | Lipoamide | down |
| SB_vs._SS14 | Organic acids | 4-Hydroxycyclohexylcarboxylic acid | up |
| SB_vs._SS15 | Carbohydrates and substituted derivatives | Gentianose | down |
| SB_vs._SS16 | Benzene and substituted derivatives | 4-Hydroxytoluene sulfonamide | up |
| SB_vs._SS17 | Flavonoids | Licoisoflavone A | down |
| SB_vs._SS18 | Organic acids | Adenylocuccinic Acid | up |
| SB_vs._SS19 | Phenolic acids | Eudesmic acid | down |
| SB_vs._SS20 | Benzene and substituted derivatives | Cyperine | up |
| SV_vs._SS1 | Others | 15-Hydroxynorandrostene-3,17-dione glucuronide | up |
| SV_vs._SS2 | Flavonoids | Sakuranetin | up |
| SV_vs._SS3 | Alkaloids | 2-Phenylacetamide | up |
| SV_vs._SS4 | Alkaloids | Dopamine | up |
| SV_vs._SS5 | Organic acids | Geranylgeranyl diphosphate | down |
| SV_vs._SS6 | Phenolic acids | Coniferin | up |
| SV_vs._SS7 | Carbohydrates and derivatives | Di-N-acetylchitobiose | up |
| SV_vs._SS8 | Carbohydrates and derivatives | D-Xylose | up |
| SV_vs._SS9 | Lignans and coumarins | Coumarin | up |
| SV_vs._SS10 | Flavonoids | Naringenin | up |
| SV_vs._SS11 | Flavonoids | (S)-7-(((2-O-6-Deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-2,3-dihydro-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one | up |
| SV_vs._SS12 | Lignans and coumarins | Matairesinol | up |
| SV_vs._SS13 | Flavonoids | Naringin | down |
| SV_vs._SS14 | Others | Resveratrol | up |
| SV_vs._SS15 | Organic acids | Porphobilinogen | up |
| SV_vs._SS16 | Phenolic acids | Salicin | up |
| SV_vs._SS17 | Alkaloids | Pyridoxamine | up |
| SV_vs._SS18 | Phenolic acids | Agnuside | up |
| SV_vs._SS19 | Others | 12a-Hydroxyrotenone | up |
| SV_vs._SS20 | Flavonoids | alpha-Mangostin | down |
| SB_vs._SV1 | Benzene and substituted derivatives | Phenelzine | up |
| SB_vs._SV2 | Flavonoids | Sakuranetin | down |
| SB_vs._SV3 | Alkaloids | Dopamine | down |
| SB_vs._SV4 | Phenolic acids | 1′-Acetoxychavicol acetate | down |
| SB_vs._SV5 | Others | 2-Aminoacetophenone | down |
| SB_vs._SV6 | Lignans and coumarins | Coumarin | down |
| SB_vs._SV7 | Lipids | 3-Hydroxyhexadecanoic acid | down |
| SB_vs._SV8 | Benzene and substituted derivatives | Benzimidazole | up |
| SB_vs._SV9 | Phenolic acids | Agnuside | down |
| SB_vs._SV10 | Flavonoids | Naringenin | down |
| SB_vs._SV11 | Phenolic acids | Cinnamic acid | up |
| SB_vs._SV12 | Others | 15-Hydroxynorandrostene-3,17-dione glucuronide | down |
| SB_vs._SV13 | Flavonoids | Genistein | down |
| SB_vs._SV14 | Flavonoids | Naringenin chalcone | down |
| SB_vs._SV15 | Phenolic acids | Coniferin | down |
| SB_vs._SV16 | Phenolic acids | 4-Hydroxy-3-methoxycinnamaldehyde | up |
| SB_vs._SV17 | Lipids | 17,18-EpETE | up |
| SB_vs._SV18 | Organic acids | Porphobilinogen | down |
| SB_vs._SV19 | Alkaloids | Pyridoxamine | down |
| SB_vs._SV20 | Flavonoids | 5,7-Dihydroxy-2-(4-hydroxy-3-methoxy-phenyl)-3,6,8-trimethoxy-chromen-4-one | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Chen, S.; Song, J.; Xie, T.; Xue, Y.; Yang, Q. Comparative Untargeted Metabolomic Analysis of Fruiting Bodies from Three Sanghuangporus Species. J. Fungi 2025, 11, 558. https://doi.org/10.3390/jof11080558
Jiang Z, Chen S, Song J, Xie T, Xue Y, Yang Q. Comparative Untargeted Metabolomic Analysis of Fruiting Bodies from Three Sanghuangporus Species. Journal of Fungi. 2025; 11(8):558. https://doi.org/10.3390/jof11080558
Chicago/Turabian StyleJiang, Zixuan, Shimao Chen, Jia Song, Tao Xie, Yu Xue, and Qingshan Yang. 2025. "Comparative Untargeted Metabolomic Analysis of Fruiting Bodies from Three Sanghuangporus Species" Journal of Fungi 11, no. 8: 558. https://doi.org/10.3390/jof11080558
APA StyleJiang, Z., Chen, S., Song, J., Xie, T., Xue, Y., & Yang, Q. (2025). Comparative Untargeted Metabolomic Analysis of Fruiting Bodies from Three Sanghuangporus Species. Journal of Fungi, 11(8), 558. https://doi.org/10.3390/jof11080558






