Growth-Promoting Effects of Dark Septate Endophytes Fungus Acrocalymma on Tomato (Solanum lycopersicum)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Fungal Isolation
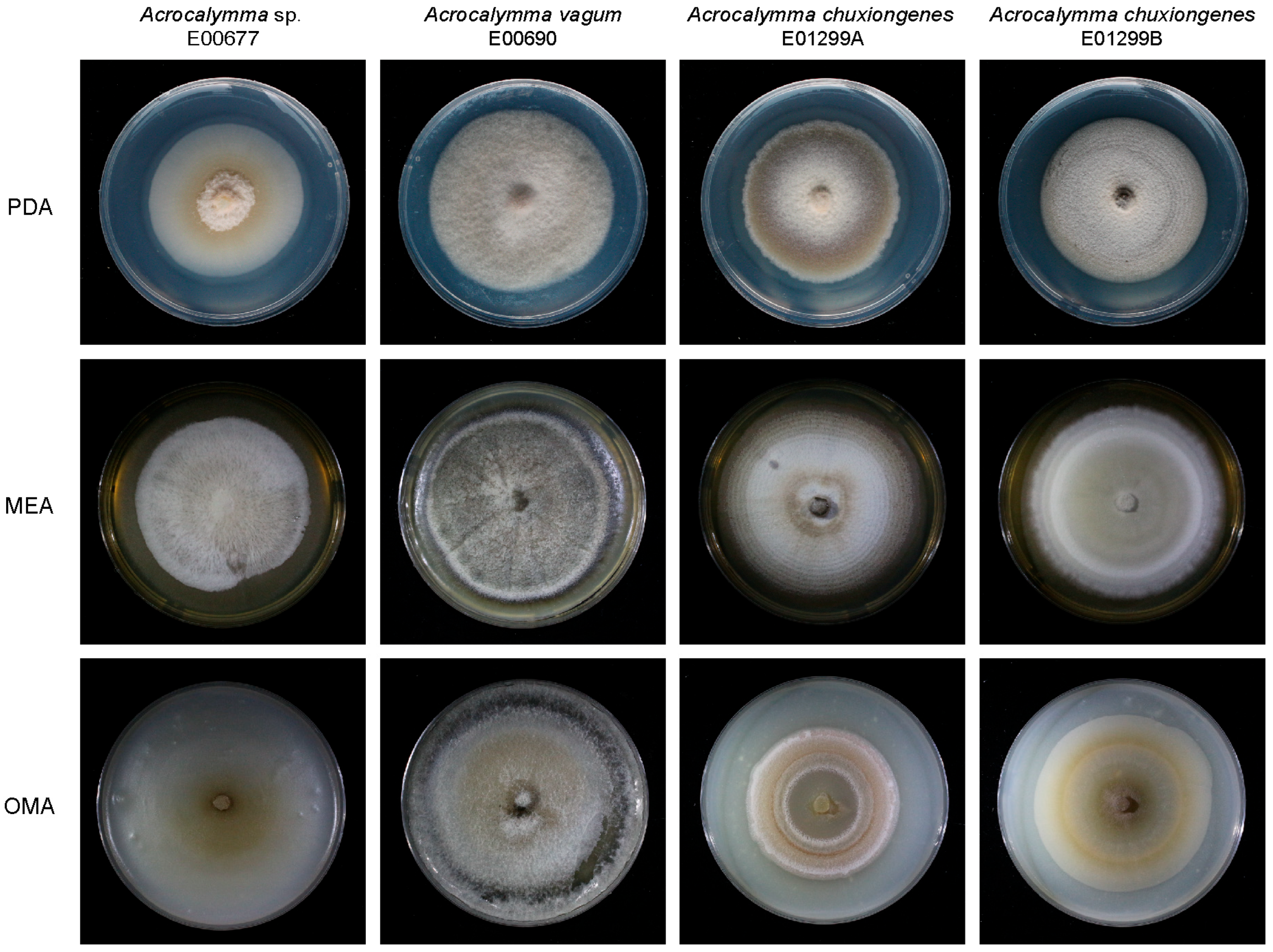
2.2. Morphological Characterization
2.3. Molecular Identification
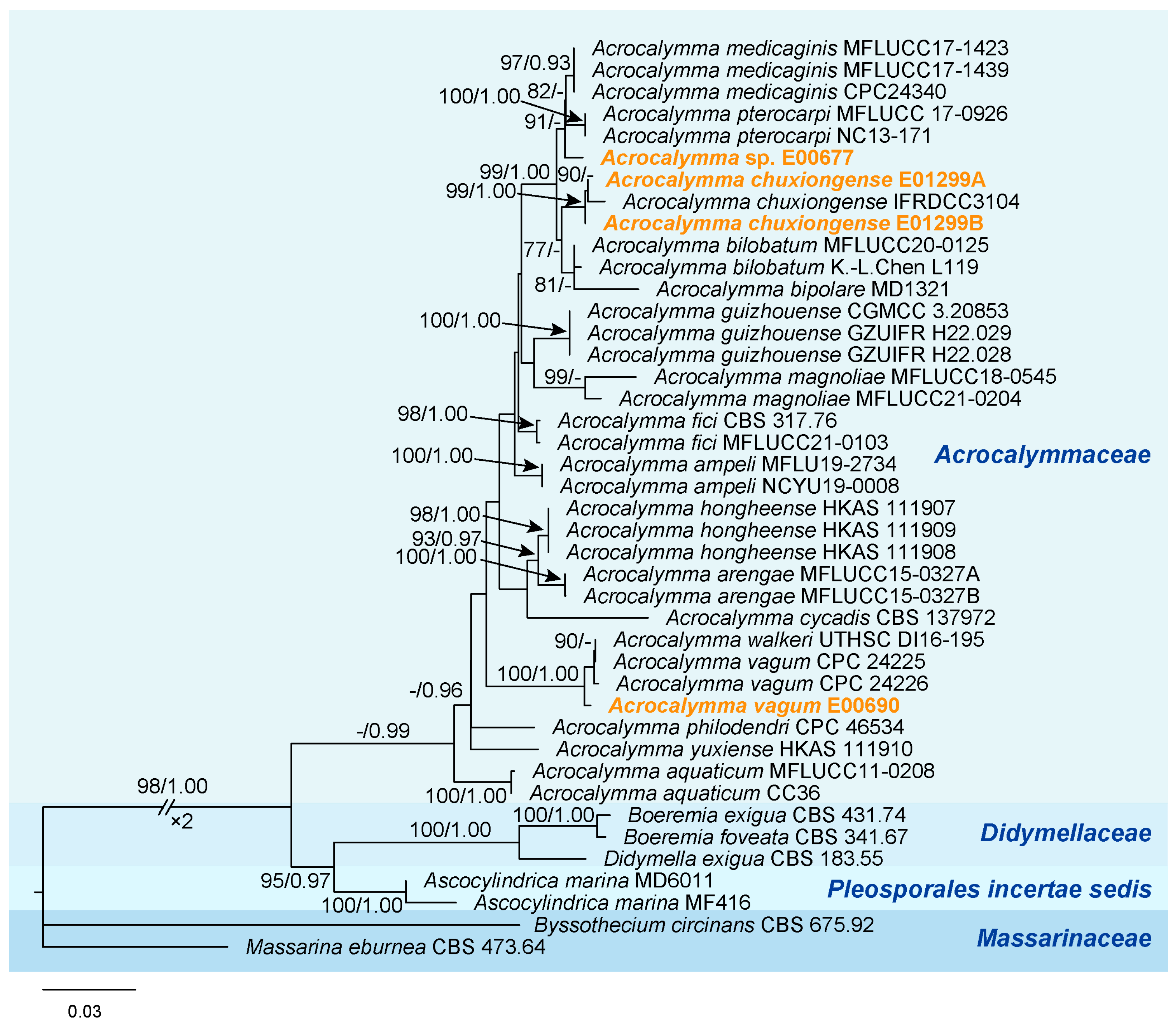
2.4. Phylogenetic Analyses
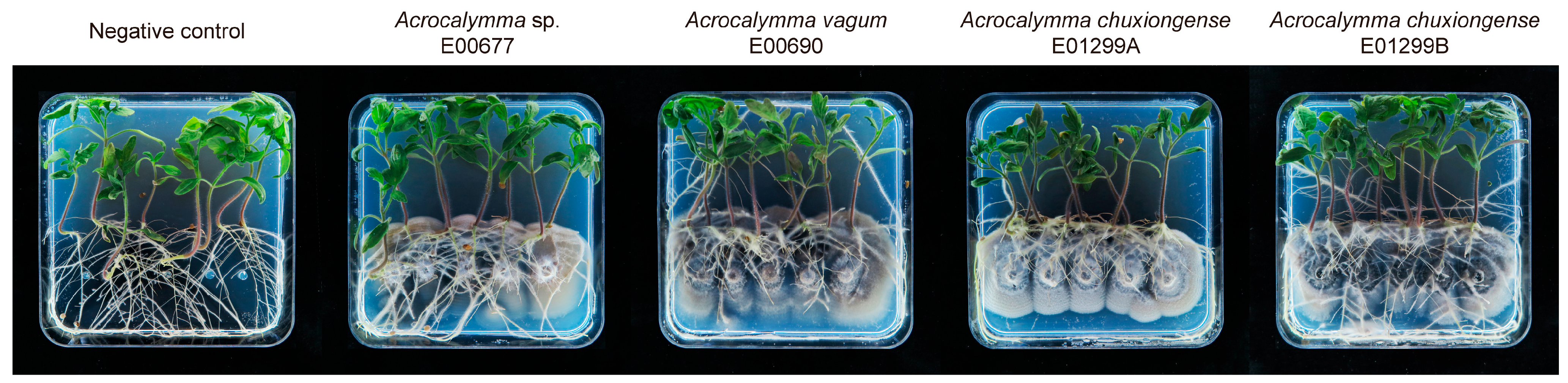
2.5. Evaluation of Plant Growth Promotion by Endophytic Strains Under Greenhouse Conditions
2.6. Physiological Indexes Measurement
2.6.1. Morphological Measurements
2.6.2. Physiological and Biochemical Analyses
2.7. Geographic Coordinates Analysis
2.8. Statistics and Analysis
3. Results
3.1. Morphological Characteristics of Isolated DSE
3.2. Phylogenetic Analysis
3.3. Effect of Acrocalymma Inoculation on Physiological Indexes
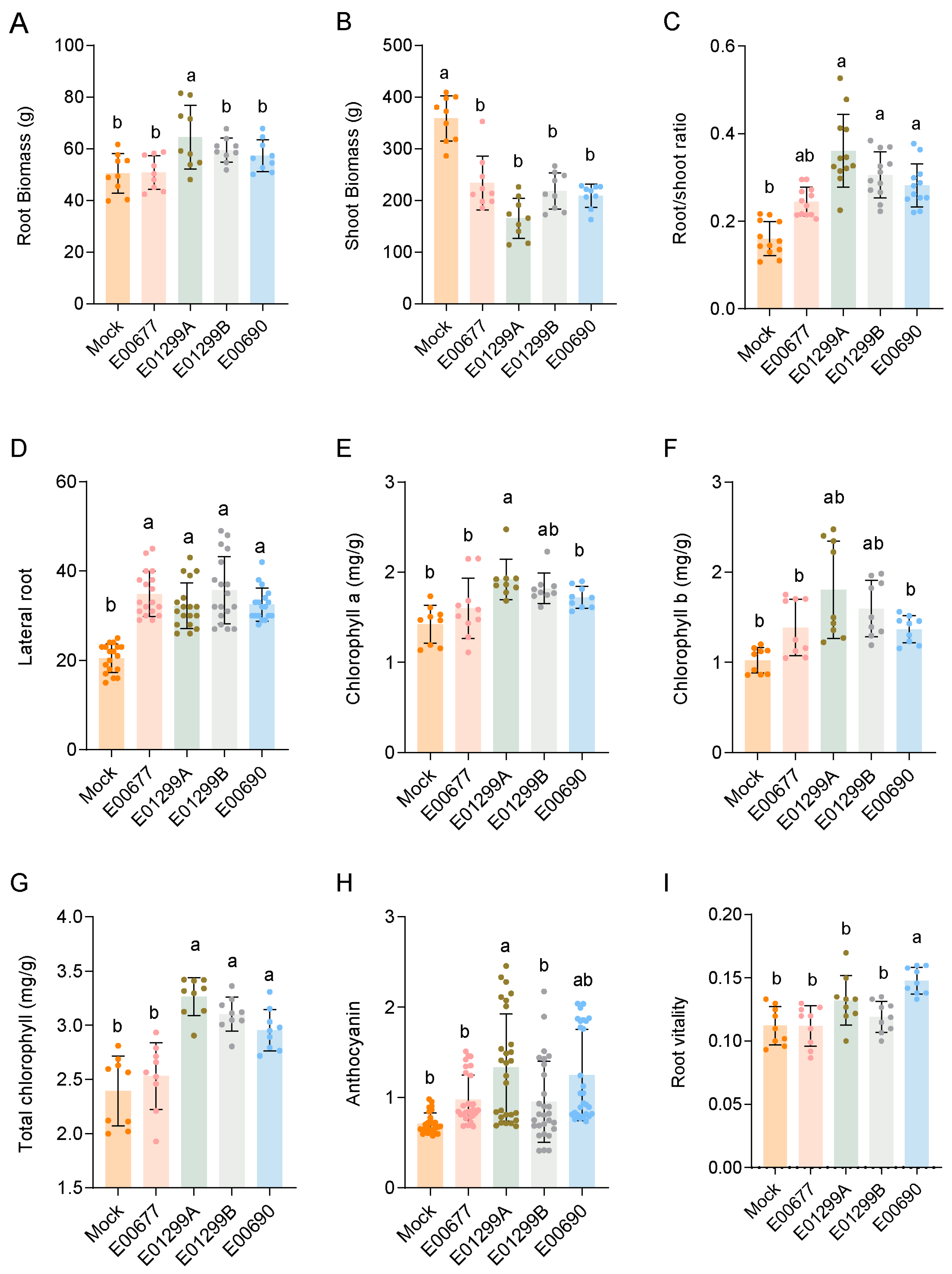
3.3.1. Root Development and Biomass Allocation
3.3.2. Photosynthetic Pigments and Stress-Related Compounds
3.3.3. Anthocyanin Accumulation
3.3.4. Root Vitality
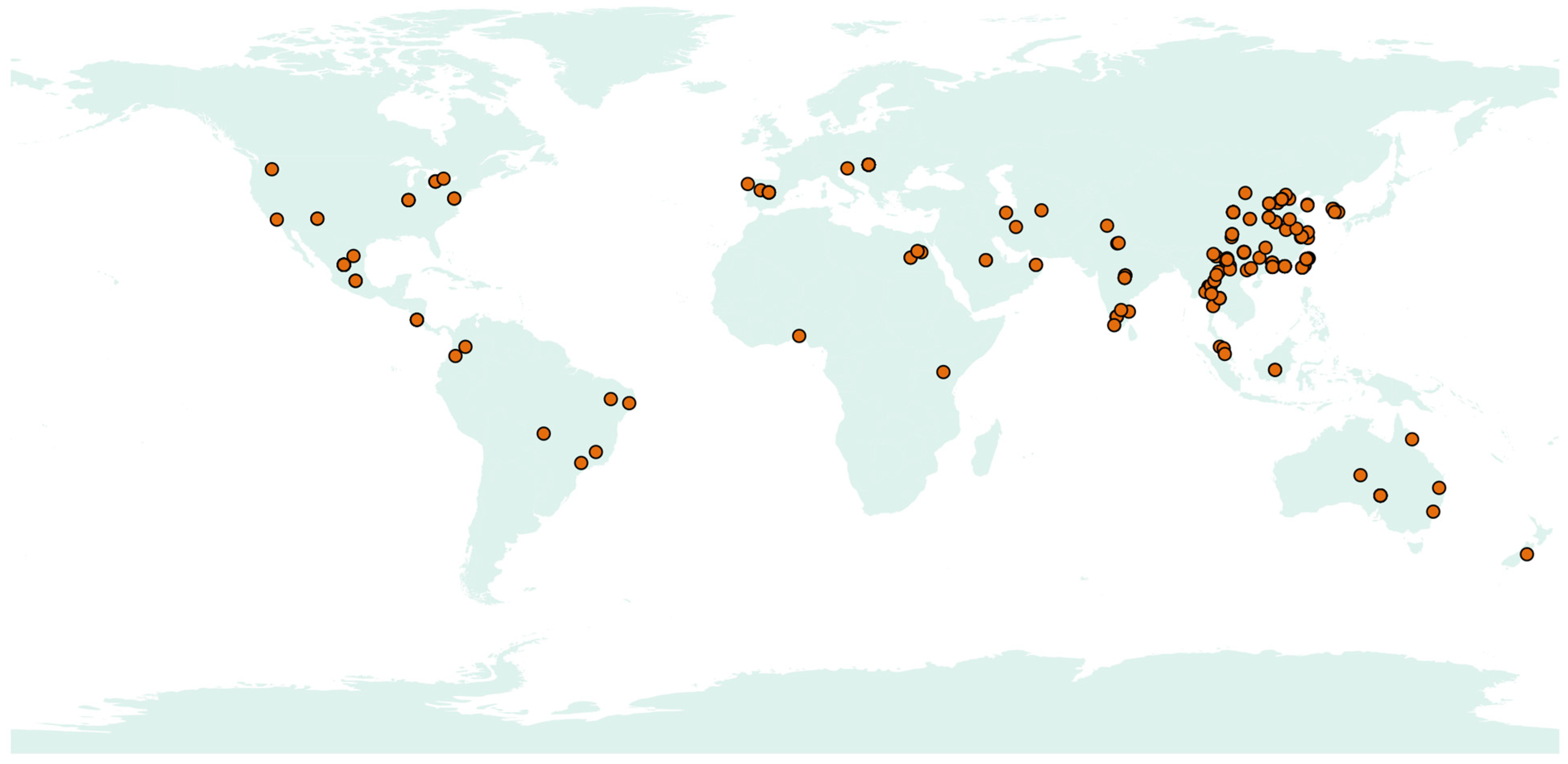
3.4. Global Distribution and Ecological Adaptability on Acrocalymma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, L.; Gonda, I.; Sun, H.; Ma, Q.; Bao, K.; Tieman, D.M.; Burzynski-Chang, E.A.; Fish, T.L.; Stromberg, K.A.; Sacks, G.L.; et al. The tomato pan-genome uncovers new genes and a rare allele regulating fruit flavor. Nat. Genet. 2019, 51, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Lopez, L.G.; Lopez-Espinoza, M.Y.; Juarez-Verdayes, M.A.; Lopez-Meyer, M. Genome-wide characterization of the xyloglucan endotransglucosylase/hydrolase gene family in Solanum lycopersicum L. and gene expression analysis in response to arbuscular mycorrhizal symbiosis. Peerj 2023, 11, e15257. [Google Scholar] [CrossRef] [PubMed]
- Argentel-Martinez, L.; Penuelas-Rubio, O.; Amador, C.A.; Steiner, F.; Aguilera, J.G.; Shin, J.H.; Zuffo, A.M.; Ratke, R.F.; Teodoro, P.E.; Azizoglu, U. Mitigating salinity stress on tomato growth, water regime, gas exchange, and yield with the application of QuitoMax. Sci. Rep. 2024, 14, 31755. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Cesanelli, I.; Diánez, F.; Sánchez-Montesinos, B.; Moreno-Gavíra, A. Advances in the role of dark septate endophytes in the plant resistance to abiotic and biotic stresses. J. Fungi 2021, 7, 939. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Yang, F.; Zhu, L.; Wang, L.; Li, Z.; Qi, Z.; Fotopoulos, V.; Yu, J.; Zhou, J. Loss of cold tolerance is conferred by absence of the WRKY34 promoter fragment during tomato evolution. Nat Commun. 2024, 15, 6667. [Google Scholar] [CrossRef]
- Becerril-Espinosa, A.; Hernandez-Herrera, R.M.; Meza-Canales, I.D.; Perez-Ramirez, R.; Rodriguez-Zaragoza, F.A.; Mendez-Moran, L.; Sanchez-Hernandez, C.V.; Palmeros-Suarez, P.A.; Palacios, O.A.; Choix, F.J.; et al. Habitat-adapted heterologous symbiont Salinispora arenicola promotes growth and alleviates salt stress in tomato crop plants. Front. Plant Sci. 2022, 13, 920881. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, S.; Zhao, X.; Xu, M.; Xu, J.; Xing, G.; Zhang, Y.; Ahammed, G.J. Comparative physiological and transcriptomics analysis revealed crucial mechanisms of silicon-mediated tolerance to iron deficiency in tomato. Front. Plant Sci. 2022, 13, 1094451. [Google Scholar] [CrossRef]
- Rangseekaew, P.; Barros-Rodriguez, A.; Pathom-Aree, W.; Manzanera, M. Plant Beneficial Deep-Sea Actinobacterium, Dermacoccus abyssi mt1.1T promote growth of tomato (Solanum lycopersicum) under salinity stress. Biology 2022, 11, 191. [Google Scholar] [CrossRef]
- Ntanasi, T.; Karavidas, I.; Zioviris, G.; Ziogas, I.; Karaolani, M.; Fortis, D.; Conesa, M.A.; Schubert, A.; Savvas, D.; Ntatsi, G. Assessment of growth, yield, and nutrient uptake of mediterranean tomato landraces in response to salinity stress. Plants 2023, 12, 3551. [Google Scholar] [CrossRef]
- Chen, E.; Chao, S.; Shi, B.; Liu, L.; Chen, M.; Zheng, Y.; Feng, X.; Wu, H. Bacillus velezensis ZN-S10 reforms the rhizosphere microbial community and enhances tomato resistance to TPN. Plants 2023, 12, 3636. [Google Scholar] [CrossRef]
- Ke, J.; Zhu, W.; Yuan, Y.; Du, X.; Xu, A.; Zhang, D.; Cao, S.; Chen, W.; Lin, Y.; Xie, J.; et al. Duality of immune recognition by tomato and virulence activity of the Ralstonia solanacearum exo-polygalacturonase PehC. Plant Cell 2023, 35, 2552–2569. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, J.; Liu, F.; Liang, J.; Zhao, P.; Tsui, C.; Cai, L. Cross-kingdom synthetic microbiota supports tomato suppression of Fusarium wilt disease. Nat. Commun. 2022, 13, 7890. [Google Scholar] [CrossRef]
- Syed, N.R.; Shahzad, R.; Tayade, R.; Shahid, M.; Hussain, A.; Ali, M.W.; Yun, B.W. Evaluation potential of PGPR to protect tomato against Fusarium wilt and promote plant growth. Peerj 2021, 9, e11194. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Aparicio, F.; Lison, P.; Rodrigo, I.; Belles, J.M.; Lopez-Gresa, M.P. Signaling in the tomato immunity against Fusarium oxysporum. Molecules 2021, 26, 1818. [Google Scholar] [CrossRef]
- Takken, F.; Rep, M. The arms race between tomato and Fusarium oxysporum. Mol. Plant Pathol. 2010, 11, 309–314. [Google Scholar] [CrossRef]
- Peeters, N.; Guidot, A.; Vailleau, F.; Valls, M. Ralstonia solanacearum, a widespread bacterial plant pathogen in the post-genomic era. Mol. Plant Pathol. 2013, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Chen, H.; Huang, J.; Zhang, Y.; Qi, M.; Liu, Y.; Li, T. Photosynthetic response mechanism of soil salinity-induced cross-tolerance to subsequent drought stress in tomato plants. Plants 2020, 9, 363. [Google Scholar] [CrossRef]
- Upson, R.; Read, D.J.; Newsham, K.K. Nitrogen form influences the response of Deschampsia antarctica to dark septate root endophytes. Mycorrhiza 2009, 20, 1–11. [Google Scholar] [CrossRef]
- Ali, M.; Aboelhasan, F.; Abdelhameed, A.A.; Soudy, F.A.; Eldin, D.D.; Zeinab, I.M.E.; Khalil, R.; El-Absy, K.M.; Kawy, A. Physiological and transcriptomic evaluation of salt tolerance in Egyptian tomato landraces at the seedling stage. BMC Plant Biol. 2025, 25, 507. [Google Scholar] [CrossRef]
- Zhu, L.; Li, T.; Wang, C.; Zhang, X.; Xu, L.; Xu, R.; Zhao, Z. The effects of dark septate endophyte (DSE) inoculation on tomato seedlings under Zn and Cd stress. Environ. Sci. Pollut. Res. 2018, 25, 35232–35241. [Google Scholar] [CrossRef]
- Vergara, C.; Araujo, K.; Urquiaga, S.; Schultz, N.; Balieiro, F.C.; Medeiros, P.S.; Santos, L.A.; Xavier, G.R.; Zilli, J.E. Dark septate endophytic fungi help tomato to acquire nutrients from ground plant material. Front. Microbiol. 2017, 8, 2437. [Google Scholar] [CrossRef]
- Gaber, D.A.; Berthelot, C.; Blaudez, D.; Kovacs, G.M.; Franken, P. Impact of dark septate endophytes on salt stress alleviation of tomato plants. Front. Microbiol. 2023, 14, 1124879. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.S.; Narisawa, K. A new fungal endophyte, Scolecobasidium humicola, promotes tomato growth under organic nitrogen conditions. PLoS ONE 2013, 8, e78746. [Google Scholar] [CrossRef] [PubMed]
- Jumpponen, A.; Trappe, J.M. Dark septate endophytes: A review of facultative biotrophic root-colonizing fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef]
- Yuan, Z.; Su, Z.; Mao, L.; Peng, Y.; Yang, G.; Lin, F.; Zhang, C. Distinctive endophytic fungal assemblage in stems of wild rice (Oryza granulata) in China with special reference to two species of Muscodor (Xylariaceae). J. Microbiol. 2011, 49, 15–23. [Google Scholar] [CrossRef]
- Jin, H.; Liu, H.; Xie, Y.; Zhang, Y.; Xu, Q.; Mao, L.; Li, X.; Chen, J.; Lin, F.; Zhang, C. Effect of the dark septate endophytic fungus Acrocalymma vagum on heavy metal content in tobacco leaves. Symbiosis 2018, 74, 89–95. [Google Scholar] [CrossRef]
- Ruotsalainen, A.L.; Kauppinen, M.; Wali, P.R.; Saikkonen, K.; Helander, M.; Tuomi, J. Dark septate endophytes: Mutualism from by-products? Trends Plant Sci. 2022, 27, 247–254. [Google Scholar] [CrossRef]
- Wang, J.L.; Li, T.; Liu, G.Y.; Smith, J.M.; Zhao, Z.W. Unraveling the role of dark septate endophyte (DSE) colonizing maize (Zea mays) under cadmium stress: Physiological, cytological and genic aspects. Sci. Rep. 2016, 6, 22028. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Liu, M.; Shi, X.; Zhao, Z. Dark septate endophyte (DSE) fungi isolated from metal polluted soils: Their taxonomic position, tolerance, and accumulation of heavy metals in vitro. J. Microbiol. 2008, 46, 624–632. [Google Scholar] [CrossRef]
- Xie, L.; Bi, Y.; Ma, S.; Shang, J.; Hu, Q.; Christie, P. Combined inoculation with dark septate endophytes and arbuscular mycorrhizal fungi: Synergistic or competitive growth effects on maize? BMC Plant Biol. 2021, 21, 498. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Chiocchio, V.M. Tolerance of dark septate endophytic fungi (DSE) to agrochemicals in vitro. Rev. Argent. Microbiol. 2020, 52, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Lu, X.; Wang, M.; Chen, R.; Li, Q.; Zhu, J.; Su, Z.; Lin, F. Endophyte Acrocalymma vagum establishes the holobiont with rice to attract beneficial microorganisms and promote disease resistance. J. Adv. Res. 2025; in press. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, G.; Liang, X.; Wang, L.; Li, Z.; He, Y.; Li, B.; Zhan, F. A dark septate endophyte improves cadmium tolerance of maize by modifying root morphology and promoting cadmium binding to the cell wall and phosphate. J Fungi. 2023, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Z.; Dai, M.D.; Zhu, J.N.; Liu, X.H.; Li, L.; Zhu, X.M.; Wang, J.Y.; Yuan, Z.L.; Lin, F.C. Dark septate endophyte Falciphora oryzae-assisted alleviation of cadmium in rice. J. Hazard. Mater. 2021, 419, 126435. [Google Scholar] [CrossRef] [PubMed]
- Vergara, C.; Araujo, K.; Urquiaga, S.; Santa-Catarina, C.; Schultz, N.; Da, S.A.E.; de Carvalho, B.F.; Xavier, G.R.; Zilli, J.E. Dark septate endophytic fungi increase green manure-15N recovery efficiency, N contents, and micronutrients in rice grains. Front. Plant Sci. 2018, 9, 613. [Google Scholar] [CrossRef]
- Su, Z.Z.; Mao, L.J.; Li, N.; Feng, X.X.; Yuan, Z.L.; Wang, L.W.; Lin, F.C.; Zhang, C.L. Evidence for biotrophic lifestyle and biocontrol potential of dark septate endophyte Harpophora oryzae to rice blast disease. PLoS ONE 2013, 8, e61332. [Google Scholar] [CrossRef]
- He, Y.; Yang, Z.; Li, M.; Jiang, M.; Zhan, F.; Zu, Y.; Li, T.; Zhao, Z. Effects of a dark septate endophyte (DSE) on growth, cadmium content, and physiology in maize under cadmium stress. Environ. Sci. Pollut. Res. 2017, 24, 18494–18504. [Google Scholar] [CrossRef]
- Xiao, Y.; Dai, M.X.; Zhang, G.Q.; Yang, Z.X.; He, Y.M.; Zhan, F.D. Effects of the dark septate endophyte (DSE) Exophiala pisciphila on the growth of root cell wall polysaccharides and the cadmium content of Zea mays L. under cadmium stress. J. Fungi 2021, 7, 1035. [Google Scholar] [CrossRef]
- Lalthazuali, E.; Marwein, B.; Sailo, H.; Lalbiaktluanga, P.S.; Vanlalmuana, M.F.; Ralte, L. Enhancing rice growth in adverse conditions: The role of dark septate endophytes in salt and water scarcity tolerance. Mycologia 2025, 117, 546–558. [Google Scholar] [CrossRef]
- He, C.; Wang, W.; Hou, J. Plant growth and soil microbial impacts of enhancing licorice with inoculating dark septate endophytes under drought stress. Front. Microbiol. 2019, 10, 2277. [Google Scholar] [CrossRef]
- Armengol, J.; Vicent, A.; Martinez-Culebras, P.; Bruton, B.D.; Garcia-Jimnez, J. Identification, occurrence and pathogenicity of Rhizopycnis vagum on muskmelon in Spain. Plant. Pathol. 2003, 52, 68–73. [Google Scholar] [CrossRef]
- Girlanda, M.; Ghignone, S.; Luppi, A.M. Diversity of sterile root-associated fungi of two Mediterranean plants. New Phytol. 2002, 155, 481–498. [Google Scholar] [CrossRef] [PubMed]
- Netherway, T.; Bengtsson, J.; Buegger, F.; Fritscher, J.; Oja, J.; Pritsch, K.; Hildebrand, F.; Krab, E.J.; Bahram, M. Pervasive associations between dark septate endophytic fungi with tree root and soil microbiomes across Europe. Nat. Commun. 2024, 15, 159. [Google Scholar] [CrossRef]
- Barresi, O.; Lavado, R.S.; Chiocchio, V.M. Can dark septate endophytic fungi (DSE) mobilize selectively inorganic soil phosphorus thereby promoting sorghum growth? A preliminary study. Rev. Argent. Microbiol. 2022, 54, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Hou, R.; Shang, X.; Li, S. Effects of Phosphorus-dissolving dark septate endophytes on the growth of blueberry. J. Microbiol. 2023, 61, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Mikheev, V.S.; Struchkova, I.V.; Ageyeva, M.N.; Brilkina, A.A.; Berezina, E.V. The Role of Phialocephala fortinii in improving plants’ phosphorus nutrition: New puzzle pieces. J. Fungi 2022, 8, 1225. [Google Scholar] [CrossRef]
- Wang, K.; Wen, Z.; Asiegbu, F.O. The dark septate endophyte Phialocephala sphaeroides suppresses conifer pathogen transcripts and promotes root growth of Norway spruce. Tree Physiol. 2022, 42, 2627–2639. [Google Scholar] [CrossRef]
- Kurczynska, E.U.; Gaj, M.D.; Ujczak, A.; Mazur, E. Histological analysis of direct somatic embryogenesis in Arabidopsis thaliana (L.) Heynh. Planta 2007, 226, 619–628. [Google Scholar] [CrossRef]
- Gulzar, U.; Hussain, A.; Hamayun, M.; Iqbal, A.; Seleiman, M.F.; Alotaibi, M.; Lee, B. Gibberellins producing endophytic Aspergillus nidulans DSE-2 biosorbs Cd and down-regulates OsNRAMP5 and OsCd1 genes to improve rice growth in contaminated soil. Plant Physiol. Biochem. 2025, 221, 109650. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Zhang, G.; Liang, X.; Hu, L.; Li, Y.; He, Y.; Zhan, F. Dark septate endophyte Exophiala pisciphila promotes maize growth and alleviates cadmium toxicity. Front. Microbiol. 2023, 14, 1165131. [Google Scholar] [CrossRef]
- Habib, H.M.; El-Fakharany, E.M.; Souka, U.D.; Elsebaee, F.M.; El-Ziney, M.G.; Ibrahim, W.H. Polyphenol-rich date palm fruit seed (Phoenix dactylifera L.) extract inhibits labile iron, enzyme, and cancer cell activities, and dna and protein damage. Nutrients 2022, 14, 3536. [Google Scholar] [CrossRef]
- Bi, Y.; Xue, Z. Dark septate endophyte inoculation enhances antioxidant activity in Astragalus membranaceus var. mongholicus under heat stress. Physiol. Plant. 2023, 175, e14054. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Xu, M.; Ye, Q.; Gao, H.; He, X. Improved tolerance of Artemisia ordosica to drought stress via dark septate endophyte (DSE) symbiosis. J. Fungi 2022, 8, 730. [Google Scholar] [CrossRef]
- Legeay, J.; Basiru, S.; Ziami, A.; Errafii, K.; Hijri, M. Response of Alternaria and Fusarium species to low precipitation in a drought-tolerant plant in Morocco. Microb. Ecol. 2024, 87, 127. [Google Scholar] [CrossRef]
- Gramaje, D.; Berlanas, C.; Martinez-Diz, M.; Diaz-Losada, E.; Antonielli, L.; Beier, S.; Gorfer, M.; Schmoll, M.; Compant, S. Comparative genomic analysis of dactylonectria torresensis strains from grapevine, soil and weed highlights potential mechanisms in pathogenicity and endophytic lifestyle. J. Fungi 2020, 6, 255. [Google Scholar] [CrossRef]
- Feng, X.; Chen, J.; Liu, F.; Hu, W.; Lin, F.; Zhang, C. Diversity of non-mycorrhizal endophytic fungi from five epiphytic orchids from Xishuangbanna, China. Mycosystema 2019, 38, 1876–1885. [Google Scholar] [CrossRef]
- Feng, X.; Chen, J.; Wang, G.; Ccao, T.; Zheng, Y.; Zhang, C. Diaporthe sinensis, a new fungus from Amaranthus sp. in China. Phytotaxa 2019, 425, 259–268. [Google Scholar] [CrossRef]
- Liu, F.; Deng, Y.; Wang, F.; Jeewon, R.; Zeng, Q.; Xu, X.; Liu, Y.; Yang, C. Morphological and molecular analyses reveal two new species of Microcera (Nectriaceae, Hypocreales) associated with scale insects on walnut in China. Mycokeys 2023, 98, 19–35. [Google Scholar] [CrossRef] [PubMed]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Rehner, S.A.; Samuels, G.J. Molecular systematics of the Hypocreales: A teleomorph gene phylogeny and the status of their anamorphs. Can. J. Bot. 1995, 73, 816–823. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sarver, B.A.; Brandt, M.; Chang, D.C.; Noble-Wang, J.; Park, B.J.; Sutton, D.A.; Benjamin, L.; Lindsley, M.; Padhye, A.; et al. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic Fusaria, including isolates from the multistate contact lens-associated U.S. keratitis outbreaks of 2005 and 2006. J. Clin. Microbiol. 2007, 45, 2235–2248. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A Method for designing primer sets for speciation studies in filamentous Ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z.; Risede, J.M.; Simoneau, P.; Hyde, K.D. Calonectria species and their Cylindrocladium anamorphs: Species with clavate vesicles. Stud. Mycol. 2006, 55, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.Y.; Gao, F.; Jakovlić, I.; Lei, H.P.; Hu, Y.; Zhang, H.; Zou, H.; Wang, G.T.; Zhang, D. Using PhyloSuite for molecular phylogeny and tree-based analyses. iMeta 2023, 2, e87. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Fengjun, G. Experimental Guidance for Plant Physiology; Higher Education Press: Beijing, China, 2006; pp. 74–76. [Google Scholar]
- Yin, C.; Sun, A.; Zhou, Y.; Liu, K.; Wang, P.; Ye, W.; Fang, Y. The dynamics of H2A.Z on SMALL AUXIN UP RNAs regulate abscisic acid-auxin signaling crosstalk in Arabidopsis. J. Exp. Bot. 2023, 74, 4158–4168. [Google Scholar] [CrossRef] [PubMed]
- Weiting, L. Research on Endophytic Fungal Resources in Poaceae Plants and the Biological Functions of Endophytic Fungi in the Order Sclerotiniaceae; Zhejiang University: Hangzhou, China, 2022. [Google Scholar]
- Taylor, J.W.; Jacobson, D.J.; Kroken, S.; Kasuga, T.; Geiser, D.M.; Hibbett, D.S.; Fisher, M.C. Phylogenetic species recognition and species concepts in fungi. Fungal Genet. Biol. 2000, 31, 21–32. [Google Scholar] [CrossRef]
- Masoudi, K.F.; Ganjeali, A.; Asili, J.; Cheniany, M. Beneficial effects of endophytic fungi inoculation on tanshinones and phenolic compounds of Salvia abrotanoides. Iran. J. Basic Med. Sci. 2023, 26, 408–413. [Google Scholar] [CrossRef]
- Mortimer, P.E.; Jeewon, R.; Xu, J.C.; Lumyong, S.; Wanasinghe, D.N. Morpho-Phylo taxonomy of novel Dothideomycetous fungi associated with dead woody twigs in Yunnan province, China. Front. Microbiol. 2021, 12, 654683. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, G.; Lv, X.; Wang, Y.; Long, Y.; Wang, X.; Liu, H. First complete mitogenome of Massarineae and its contribution to phylogenetic implications in Pleosporales. Sci. Rep. 2023, 13, 22431. [Google Scholar] [CrossRef] [PubMed]
- Azuddin, N.F.; Mohd, M.H.; Rosely, N.; Mansor, A.; Zakaria, L. Molecular phylogeny of endophytic fungi from rattan (Calamus castaneus Griff.) spines and their antagonistic activities against plant pathogenic fungi. J. Fungi 2021, 7, 301. [Google Scholar] [CrossRef]
- Liu, Y.W.; Zeng, X.Y. Acrocalymma chuxiongense sp. nov., a new species of Acrocalymmaceae (Pleosporales) on leaves of Quercus. Biodivers. Data J. 2022, 10, e89635. [Google Scholar] [CrossRef]
- Boonmee, S.; Wanasinghe, D.N.; Calabon, M.S.; Huanraluek, N.; Chandrasiri, S.; Jones, G.; Rossi, W.; Leonardi, M.; Singh, S.K.; Rana, S.; et al. Fungal diversity notes 1387-1511: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2021, 111, 1–335. [Google Scholar] [CrossRef]
- Sidhoum, W.; Dib, S.; Alim, Y.; Anseur, S.; Benlatreche, S.; Belaidouni, Z.M.; Chamouma, F. Growth-promoting effects of Aspergillus elegans and the dark septate endophyte (DSE) Periconia macrospinosa on cucumber. Arch. Microbiol. 2024, 206, 226. [Google Scholar] [CrossRef]
- Berthelot, C.; Blaudez, D.; Leyval, C. Differential growth promotion of poplar and birch inoculated with three dark septate endophytes in two trace element-contaminated soils. Int. J. Phytoremediation 2017, 19, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, E.A.; Guardini, Z.; Dall’Osto, L.; Bassi, R. A paler shade of green: Engineering cellular chlorophyll content to enhance photosynthesis in crowded environments. New Phytol. 2023, 239, 1567–1583. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, L.; Liang, R.; Huang, S.; Li, X.; Huang, B.; Luo, H.; Zhang, M.; Wang, X.; Zhu, H. The role of light in regulating plant growth, development and sugar metabolism: A review. Front. Plant Sci. 2024, 15, 1507628. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin pigments: Beyond aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Fang, Y.; Gao, Y.; Yang, S.; Su, J.; Ni, J.; Teng, Y.; Bai, S. Phosphorylated transcription factor PuHB40 mediates ROS-dependent anthocyanin biosynthesis in pear exposed to high light. Plant Cell 2024, 36, 3562–3583. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Isayenkov, S.V. The role of anthocyanins in plant tolerance to drought and salt stresses. Plants 2023, 12, 2558. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Lee, C.M.; Kim, H.S.; Kim, C.; Jeon, J.H.; Lee, H.J. Ethylene signals modulate the survival of Arabidopsis leaf explants. BMC Plant Biol. 2023, 23, 281. [Google Scholar] [CrossRef] [PubMed]
- Asim, M.; Ullah, Z.; Xu, F.; An, L.; Aluko, O.O.; Wang, Q.; Liu, H. Nitrate signaling, functions, and regulation of root system architecture: Insights from Arabidopsis thaliana. Genes 2020, 11, 633. [Google Scholar] [CrossRef]
- He, C.; Wang, W.; Hou, J.; Li, X. Dark septate endophytes isolated from wild licorice roots grown in the desert regions of northwest China enhance the growth of host plants under water deficit stress. Front. Microbiol. 2021, 12, 522449. [Google Scholar] [CrossRef]
- Newsham, K.K. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011, 190, 783–793. [Google Scholar] [CrossRef]
| Gene Regions | Primers | Sequences of Primers 5′-3′ | References |
|---|---|---|---|
| ITS | ITS5 | GGAAGTAAAAGTCGTAACAAGG | (White et al., 1990) [59] |
| ITS4 | TCCTCCGCTTATTGATATGC | ||
| LSU | LR0R | ACCCGCTGAACTTAAGC | (Rehner & Samuels, 1995) [60] |
| LR5 | ATCCTGAGGGAAACTTC | ||
| SSU | SSU-SR1R | TACCTGGTTGATTCTGC | (White et al., 1990) [59] |
| SSU-SR7 | GTTCAACTACGAGCTTTTTAA | ||
| rpb2 | RPB2-5F2 | GGGGWGAYCAGAAGAAGGC | (O’Donnell et al., 2007) [61] |
| RPB2-7cR | CCCATRGCTTGYTTRCCCAT | ||
| tef1 | EF1-728F | CATCGAGAAGTTCGAGAAGG | (Carbone & Kohn, 1999) [62] |
| EF2 | GGARGTACCAGTSATCATG | (O’Donnell et al., 2007) [61] | |
| tub2 | T1 | AACATGCGTGAGATTGTAAGT | (O’Donnell et al., 2007) [61] |
| CYLTUB1R | AGTTGTCGG GACGGAAGAG | (Crous et al., 2006) [63] |
| Taxon | Strain/Voucher Number | Genbank Accession Number | |||||
|---|---|---|---|---|---|---|---|
| ITS | LSU | SSU | rpb2 | tef1 | tub2 | ||
| Acrocalymma ampeli | MFLU19-2734 | MW063150 | MW063211 | MW079341 | _ | _ | _ |
| Acrocalymma ampeli | NCYU19-0008 | MW063151 | MW063212 | MW079342 | _ | _ | _ |
| Acrocalymma aquaticum | MFLUCC11-0208 | NR_121544 | NG_042698 | JX276953 | _ | _ | _ |
| Acrocalymma aquaticum | CC36 | MT875395 | MT875393 | _ | _ | MT897894 | _ |
| Acrocalymma arengae | MFLUCC15-0327A | ON650154 | ON650673 | ON650177 | ON734014 | _ | ON745966 |
| Acrocalymma arengae | MFLUCC15-0327B | ON650155 | ON650674 | ON650178 | ON734015 | _ | |
| Acrocalymma bilobatum | K.-L.Chen L119 | KX034339 | _ | _ | _ | _ | _ |
| Acrocalymma bilobatum | MFLUCC20-0125 | MT875396 | MT875394 | _ | _ | MT897895 | _ |
| Acrocalymma bipolare | MD1321 | _ | NG_075326 | _ | _ | _ | _ |
| Acrocalymma chuxiongense | IFRDCC3104 | ON595715 | ON596248 | _ | _ | _ | _ |
| Acrocalymma chuxiongense | E01299A | PV716432 | PV731392 | PV739235 | PV763383 | PV763379 | PV763388 |
| Acrocalymma chuxiongense | E01299B | PV716431 | PV731393 | PV739234 | PV763384 | PV763380 | PV763387 |
| Acrocalymma cycadis | CBS 137972 | NR_137884 | NG_057046 | _ | _ | _ | _ |
| Acrocalymma fici | CBS 317.76 | NR_137953 | NG_057056 | _ | _ | KP170663 | KP170687 |
| Acrocalymma fici | MFLUCC21-0103 | MT864351 | MT860429 | _ | _ | _ | _ |
| Acrocalymma guizhouense | CGMCC 3.20853 | OM838410 | OM838474 | OM838471 | _ | _ | _ |
| Acrocalymma guizhouense | GZUIFR H22.028 | OM838411 | OM838475 | OM838472 | _ | _ | _ |
| Acrocalymma guizhouense | GZUIFR H22.029 | OM838412 | OM838476 | OM838473 | _ | _ | _ |
| Acrocalymma hongheense | HKAS 111907 | MW424763 | MW424777 | MW424792 | _ | _ | _ |
| Acrocalymma hongheense | HKAS 111908 | MW424762 | MW424776 | MW424791 | _ | _ | _ |
| Acrocalymma hongheense | HKAS 111909 | MW424761 | MW424775 | MW424790 | _ | _ | _ |
| Acrocalymma magnoliae | MFLUCC18-0545 | OL413439 | OK655819 | OL331094 | _ | _ | _ |
| Acrocalymma magnoliae | MFLUCC21-0204 | OL413440 | OK655820 | OL331095 | _ | _ | _ |
| Acrocalymma medicaginis | CPC 24340 | KP170620 | KP170713 | _ | _ | _ | _ |
| Acrocalymma medicaginis | MFLUCC17-1423 | MT214338 | MT214432 | MT214387 | _ | _ | _ |
| Acrocalymma medicaginis | MFLUCC17-1439 | MT214339 | MT214433 | MT214388 | _ | _ | _ |
| Acrocalymma philodendri | CPC 46534 | PQ498969 | PQ499018 | PQ497740 | PQ497755 | _ | |
| Acrocalymma pterocarpi | MFLUCC17-0926 | MK347732 | NG_066306 | MK347840 | MK434897 | MK360040 | _ |
| Acrocalymma pterocarpi | NC13-171 | LC517880 | LC517881 | _ | _ | _ | _ |
| Acrocalymma sp. | E00677 | PV716433 | PV731390 | PV739232 | PV763382 | PV763377 | PV763386 |
| Acrocalymma vagum | E00690 | PV716434 | PV731391 | PV739236 | PV763381 | PV763378 | PV763385 |
| Acrocalymma vagum | CPC24225 | KP170635 | _ | _ | _ | _ | _ |
| Acrocalymma vagum | CPC24226 | KP170636 | _ | _ | _ | _ | _ |
| Acrocalymma walkeri | UTHSC Dl16-195 | LT796832 | LN907338 | _ | LT796992 | LT797072 | LT796912 |
| Acrocalymma yuxiense | HKAS 111910 | _ | MW424778 | MW424793 | _ | _ | _ |
| Ascocylindrica marina | MD6011 | _ | KT252905 | KT252907 | _ | _ | _ |
| Ascocylindrica marina | MF416 | _ | MK007123 | MK007124 | _ | _ | _ |
| Boeremia exigua | CBS 431.74 | FJ427001 | EU754183 | EU754084 | GU371780 | GU349080 | FJ427112 |
| Boeremia foveata | CBS 341.67 | GU237834 | GU237947 | GU238203 | MN983393 | _ | GU237509 |
| Didymella exigua | CBS 183.55 | NR_135936 | NG_069119 | NG_061065 | EU874850 | GCA_010094145.1 | GCA_010094145.1 |
| Byssothecium circinans | CBS 675.92 | OM337536 | GU205217 | _ | GCA_010015675.1 | GU349061 | GCA_010015675.1 |
| Massarina eburnea | CBS 473.64 | OM337528 | GU301840 | _ | GU371732 | GU349040 | GCA_010093635.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Jin, Y.; Zhong, Z.; Zheng, Y.; Wu, H. Growth-Promoting Effects of Dark Septate Endophytes Fungus Acrocalymma on Tomato (Solanum lycopersicum). J. Fungi 2025, 11, 510. https://doi.org/10.3390/jof11070510
Feng X, Jin Y, Zhong Z, Zheng Y, Wu H. Growth-Promoting Effects of Dark Septate Endophytes Fungus Acrocalymma on Tomato (Solanum lycopersicum). Journal of Fungi. 2025; 11(7):510. https://doi.org/10.3390/jof11070510
Chicago/Turabian StyleFeng, Xiaoxiao, Ying Jin, Zhupeiqi Zhong, Yongli Zheng, and Huiming Wu. 2025. "Growth-Promoting Effects of Dark Septate Endophytes Fungus Acrocalymma on Tomato (Solanum lycopersicum)" Journal of Fungi 11, no. 7: 510. https://doi.org/10.3390/jof11070510
APA StyleFeng, X., Jin, Y., Zhong, Z., Zheng, Y., & Wu, H. (2025). Growth-Promoting Effects of Dark Septate Endophytes Fungus Acrocalymma on Tomato (Solanum lycopersicum). Journal of Fungi, 11(7), 510. https://doi.org/10.3390/jof11070510







