RNA Interference in Fungal Plant Pathogens: What Do We Know from Botrytis cinerea with Research Hotspots and Gaps, and What Are the Future Directions?
Abstract
1. Introduction
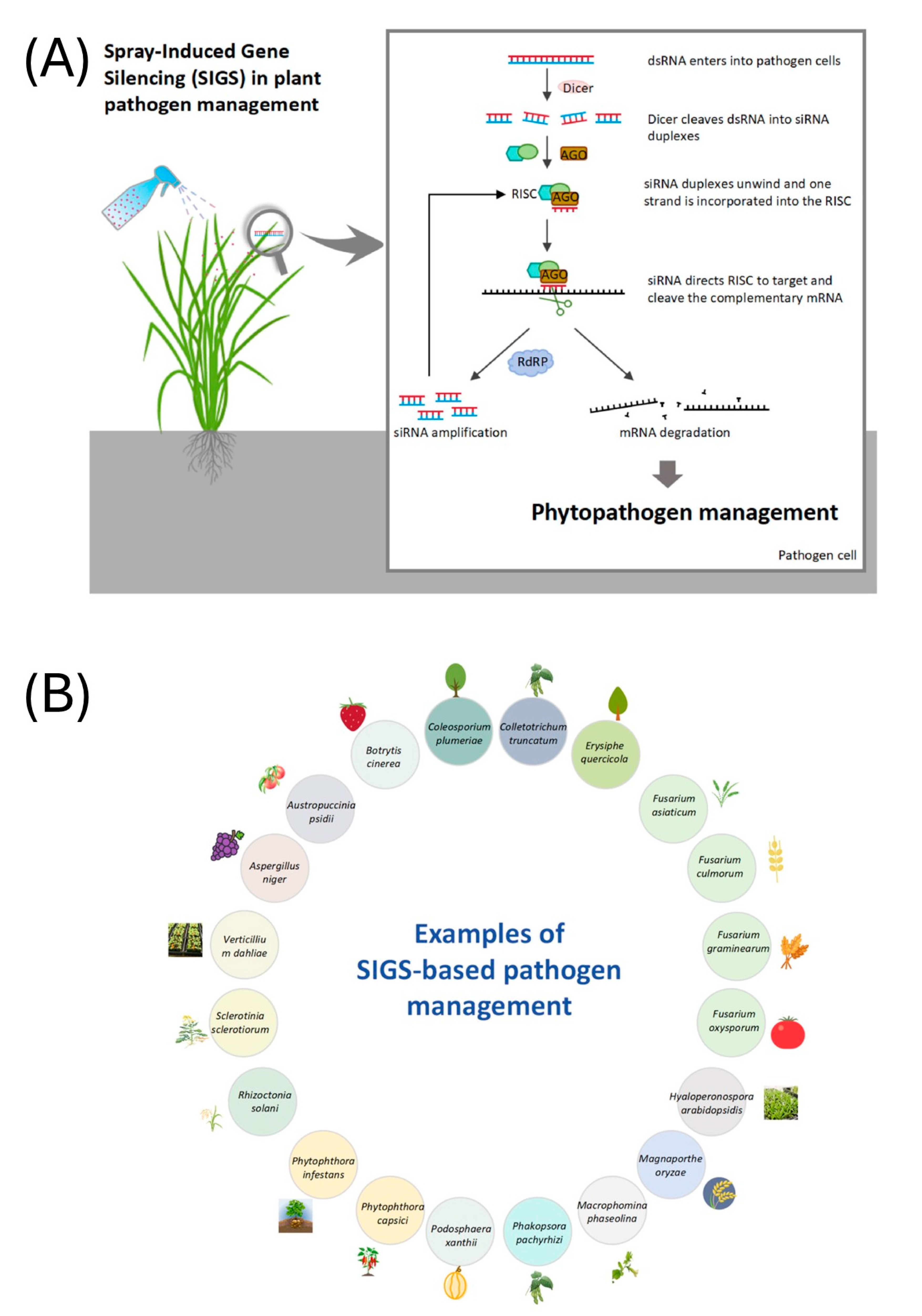
2. Mechanisms of RNAi in B. cinerea
3. Factors Influencing RNAi Efficacy
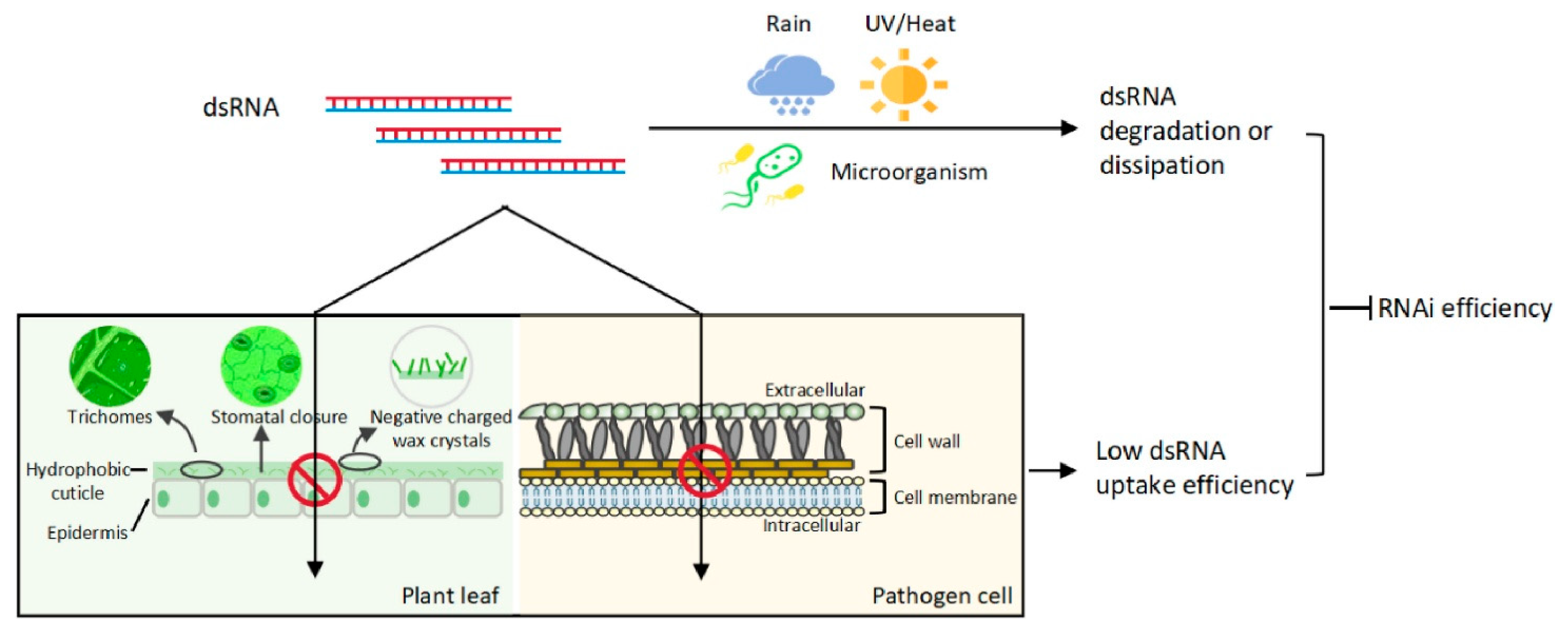
- dsRNA stability: Naked dsRNA is prone to rapid degradation by environmental nucleases, limiting its effectiveness. To address this, formulations like BioClay™, which combines dsRNA with layered double hydroxide (LDH) clay particles, have been developed. This approach has been shown to significantly enhance dsRNA stability and prolong its protective activity. For instance, BioClay™ increased the protection window against B. cinerea from 1 to 3 weeks on tomato leaves and from 5 to 10 days on tomato fruits, compared to naked dsRNA [11,12,13,14,15]. Additionally, other nanocarriers, such as artificial nanovesicles and RNA nanoparticles, have been explored for dsRNA delivery. These carriers can protect dsRNA from degradation and facilitate its uptake by the fungus, thereby enhancing the RNAi effect [16,17]. Figure 4 presents that environmental factors such as rain, UV irradiation/heat, and microorganisms can lead to dsRNA degradation or dissipation. Furthermore, leaf wettability determined by trichomes, stomata, hydrophobic cuticles, and wax crystals acts as a barrier to foliar uptake of sprayed dsRNA. In addition, cell walls and cell membranes of pathogens may hinder cellular uptake of dsRNA. The degraded/dissipated dsRNA or the low dsRNA uptake efficiency will contribute to the inhibition of RNAi efficiency. Thus, dsRNA stability and cellular uptake efficiency are two main factors affecting RNAi efficiency [2].
- Delivery methods: The method of dsRNA application significantly influences its uptake by B. cinerea. SIGS is a prominent technique where dsRNA is sprayed onto plant surfaces, allowing the pathogen to absorb it during infection. Studies have demonstrated the efficacy of SIGS in reducing disease symptoms in various crops. For example, dsRNA targeting specific genes in B. cinerea reduced disease severity in lettuce plants [2,7,15]. Moreover, the use of nanocarriers in SIGS can improve dsRNA delivery efficiency. Nanoparticles can facilitate the penetration of dsRNA through plant cuticles and enhance its stability, leading to more effective gene silencing [12,14,17,18].
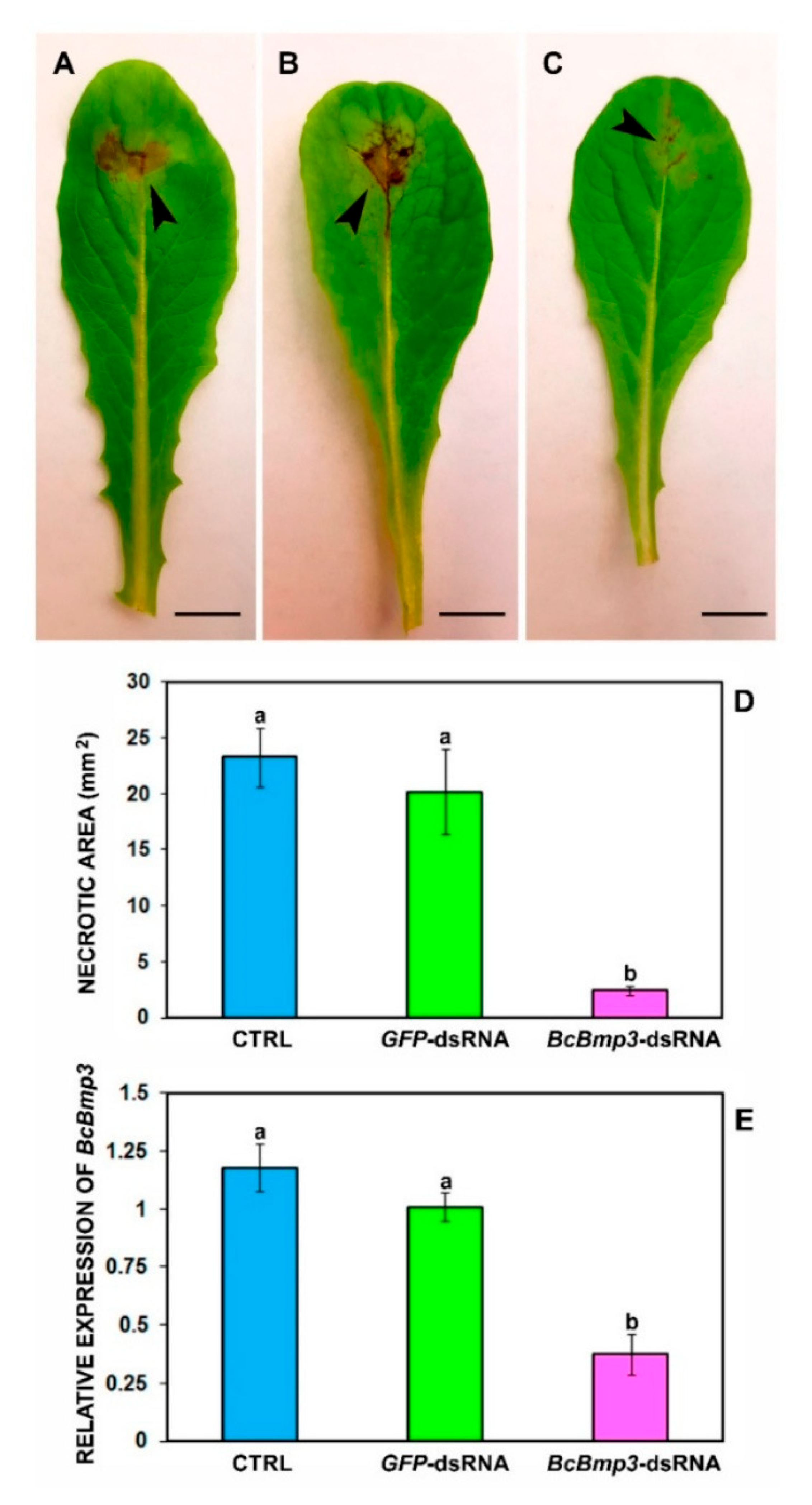
- Target gene selection: Selecting essential and conserved genes in B. cinerea is crucial for effective RNAi-based control. Genes involved in vital processes such as cell wall synthesis, signal transduction, and virulence are prime targets. For instance, targeting the Dicer-like genes (BcDCL1 and BcDCL2), which are involved in the RNAi pathway of the fungus, has been shown to reduce its virulence [7]. Additionally, genes like BcBmp1, BcBmp3, and BcPls1, which play roles in pathogenicity, have been effectively targeted using dsRNA, resulting in decreased disease symptoms in host plants [7]. Interestingly, recent data of Jin et al. [19] demonstrated that BcTRE1-targeting dsRNA (BcTRE1-dsRNA) exhibited the highest inhibitory activity, significantly reducing fungal growth and lesion formation of B. cinerea compared to the other tested dsRNAs. Gene expression analysis confirmed that BcTRE1-dsRNA effectively silenced BcTRE1 expression within 7 days post-treatment, aligning with the transient protection window observed for naked dsRNA applications. To extend this protective period, these authors incorporated LDH nanocarriers for dsRNA delivery, which successfully prolonged the inhibitory effect, reducing lesion formation even at 11 days post-treatment. These findings identify BcTRE1 as a key RNAi target and highlight the potential of dsRNA-LDH formulations for sustainable fungal disease management in crops.
4. Variability in RNAi Responses
- Genetic diversity of B. cinerea: B. cinerea exhibits significant genetic variability, which can influence the efficacy of RNAi-based approaches. Variations in target gene sequences among different strains may affect the binding efficiency of dsRNA molecules, leading to inconsistent gene-silencing outcomes. For instance, Qin et al. [20] reported that certain isolates, known as “vacuma” strains, lack active transposons and have been reported to be less virulent on specific hosts. This genetic diversity necessitates careful selection and validation of target genes for RNAi applications.
- Environmental conditions: Environmental factors such as temperature, humidity, and ultraviolet (UV) exposure can significantly impact the stability and uptake of dsRNA molecules. For example, UV radiation can degrade dsRNA, reducing its availability for uptake by the pathogen. Additionally, environmental conditions can influence the efficiency of dsRNA uptake mechanisms in fungi. Studies have shown that B. cinerea is capable of absorbing external dsRNA, but the efficiency of this process can vary depending on environmental conditions [21].
- Plant species and tissue specificity: The interaction between B. cinerea and its host plant can differ among plant species and even among different tissues within the same plant. These differences can affect the success of RNAi strategies. For instance, the uptake and systemic movement of dsRNA can vary between plant species, influencing the availability of dsRNA to the pathogen. Moreover, the structural and biochemical properties of different plant tissues can affect dsRNA stability and uptake. Therefore, tailoring RNAi approaches to specific plant–pathogen interactions is crucial for effective disease control [7].
5. Field Trials and Efficacy of RNAi to Control B. cinerea
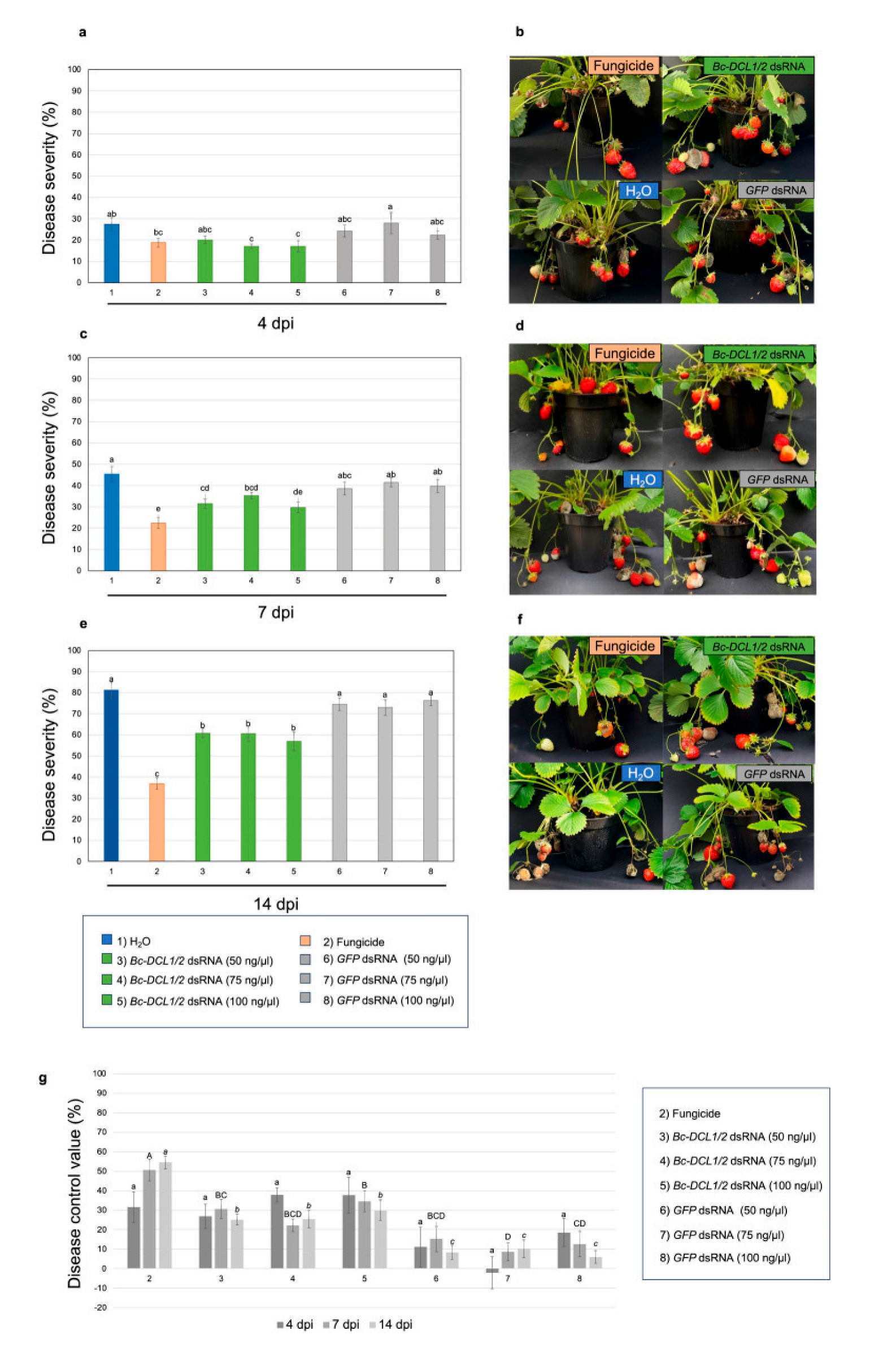
- Strawberries. In greenhouse experiments, topical applications of dsRNAs targeting the BcDCL1 and BcDCL2 genes of B. cinerea were administered to strawberry plants. Capriotti et al. [7] report that the treatments led to a significant reduction in fungal susceptibility compared to untreated controls (Figure 2). However, the efficacy was somewhat lower than that achieved with chemical fungicides. These results underscore the potential of RNAi-based approaches for gray mold management in strawberries, while also highlighting the need for improved formulations to enhance dsRNA stability and longevity.
- Lettuce. Field trials on lettuce involved the application of dsRNAs targeting the BcBmp1, BcBmp3, and BcPls1 genes of B. cinerea using SIGS. When combined with nanocarriers like synthetic LDH (sLDH) clay nanosheets, these treatments effectively reduced gray mold symptoms (Figure 3). The use of sLDH nanocarriers not only protected the dsRNA from environmental degradation but also facilitated controlled release, enhancing the duration of protection [26].
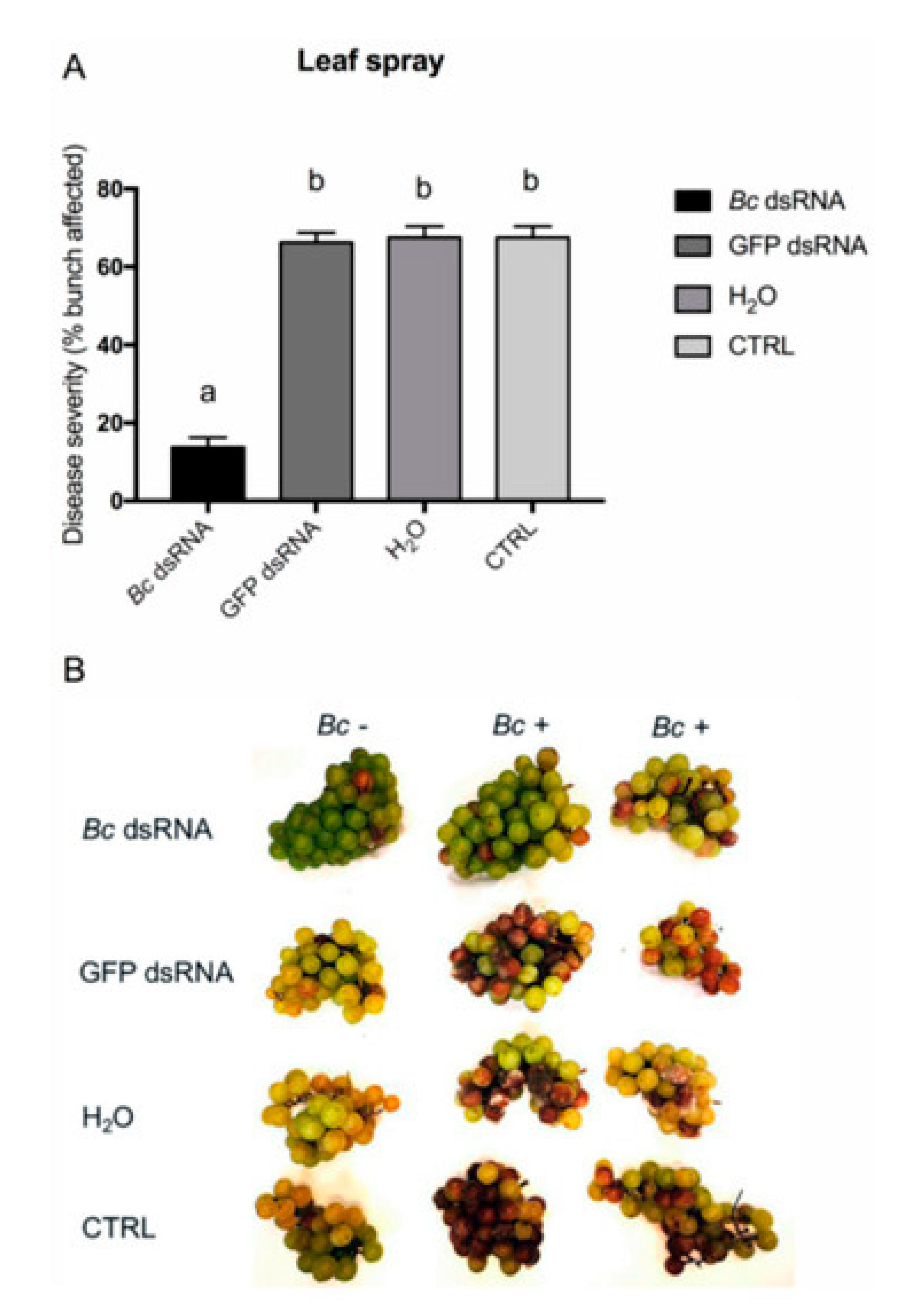
- Grapevines. Field experiments on grapevines demonstrated that dsRNA applications could control both pre- and post-harvest gray mold infections. Various delivery methods were tested, including high-pressure spraying of leaves, petiole adsorption, and post-harvest spraying of grape bunches. All methods effectively reduced fungal virulence, with post-harvest spraying showing particularly promising results. For instance, Figure 5 shows a significant decrease in disease severity on bunches recorded for the in vivo high-pressure leaf spray application method with Bc dsRNA. These findings highlight the applicability of RNAi-based strategies in fruit crops susceptible to B. cinerea [27].
6. Optimization Strategies
- Nanoparticle-based delivery systems: Incorporating dsRNA into nanoparticles can protect it from environmental degradation and facilitate controlled release, thereby extending the duration of protection. For instance, Niño-Sánchez et al. [32] reported on LDH nanoparticles, such as those used in BioClay™, and these have been shown to enhance dsRNA stability and prolong its protective effects against B. cinerea infections in crops like tomatoes. Similarly, chitosan-based nanoparticles have demonstrated potential in shielding dsRNA and improving its delivery efficiency.
- Combination therapies: Employing RNAi in conjunction with other control methods can enhance overall effectiveness and mitigate resistance development. Integrating RNAi with biological control agents (BCAs), such as microbial biofungicides, or applying reduced doses of chemical fungicides has shown promise in managing B. cinerea infections. This integrated approach can lead to synergistic effects, improving disease control while reducing reliance on chemical inputs.
- Targeting multiple genes: Designing dsRNA molecules that simultaneously target multiple essential genes in B. cinerea can increase the likelihood of successful gene silencing and reduce the risk of resistance development. For example, targeting both Dicer-like genes (BcDCL1 and BcDCL2), which are crucial for the fungus’s RNAi machinery, has been effective in compromising its virulence. Additionally, RNA nanostructures capable of delivering multiple small interfering RNAs (siRNAs) have been developed to enhance the breadth and durability of RNAi responses [13,33,34].
- Advancements in dsRNA production: Developing cost-effective and scalable methods for dsRNA synthesis is vital for the widespread adoption of RNAi-based strategies. Utilizing microbial biofactories, such as genetically engineered bacteria, to produce dsRNA offers a sustainable and economical approach. These microbial systems can be harnessed to generate large quantities of dsRNA targeting specific fungal genes, facilitating practical field applications [35].
7. Conclusions: Hotspots, Gaps, and Future Directions
- SIGS: This technique involves the topical application of dsRNA to plants, leading to gene silencing in pathogens upon contact. SIGS has shown promise against B. cinerea, offering a non-transgenic method for disease control.
- Nanocarrier systems: To enhance the stability and delivery of dsRNA, researchers are exploring nanotechnology-based carriers, such as artificial nanovesicles and LDHs. These systems protect dsRNA from environmental degradation and facilitate its uptake by fungal pathogens.
- Cross-kingdom RNAi mechanisms: Cross-kingdom RNAi is a bidirectional communication mechanism wherein small RNAs (sRNAs) are exchanged between plants and pathogens like Botrytis cinerea, leading to gene silencing across species boundaries [21,33]. Understanding how plants and pathogens exchange small RNAs is crucial. Studies have shown that pathogens like B. cinerea can deliver small RNAs into plant cells to suppress immunity, while plants can reciprocate by sending silencing RNAs into the pathogen [18]. In detail, B. cinerea secretes sRNAs that can enter plant cells and suppress host immunity. These fungal sRNAs are packaged into extracellular vesicles (EVs) and delivered into plant cells via clathrin-mediated endocytosis. Once inside, they hijack the plant’s Argonaute 1 (AGO1) protein to silence defense-related genes, facilitating infection [29,34,36]. Conversely, plants can produce sRNAs that target and silence genes in B. cinerea. These plant-derived sRNAs are also delivered via EVs into the fungal cells, where they can suppress fungal virulence genes, enhancing plant resistance. Recently, Cheng et al. [37] revealed that members of fungal plant pathogen B. cinerea BcAGO family contribute to plant infection. Specifically, BcAGO1 binds to both fungal and plant small RNAs during infection and acts in bidirectional cross-kingdom RNAi, from fungus to plant and vice versa. BcAGO2 also binds fungal and plant small RNAs but acts independent from BcAGO1 by regulating distinct genes. Nevertheless, BcAGO2 is important for infection, as it is required for effective pathogen small RNA delivery into host cells and fungal induced cross-kingdom RNAi. Providing these mechanistic insights of pathogen AGOs promises to improve RNAi-based crop protection strategies.
- Mechanistic studies in different fungi: While the uptake of dsRNA by fungi like B. cinerea has been observed, the specific cellular and molecular mechanisms facilitating this process remain incompletely understood, especially in other fungi than B. cinerea. Studies have shown that B. cinerea can internalize exogenous dsRNA, leading to gene silencing; however, the pathways and proteins involved in dsRNA uptake are not fully elucidated. Further research into the cellular uptake pathways of dsRNA in B. cinerea and also in other fungal pathogens will aid in optimizing delivery methods. Understanding these mechanisms is crucial for enhancing the efficiency of RNAi-based control methods against fungal plant pathogens. Indeed, addressing these research gaps through targeted studies and policy development will be pivotal in advancing RNAi-based strategies for sustainable crop protection against important plant pathogens like B. cinerea.
- Environmental stability: Ensuring the stability of dsRNA under field conditions is a significant challenge. Unformulated dsRNA is susceptible to rapid degradation due to environmental factors such as UV radiation, microbial activity, and soil composition. To address this, researchers are exploring protective delivery systems, including nanocarriers like chitosan and LDH nanoparticles, which can shield dsRNA from degradation and facilitate controlled release, thereby extending its protective effects [31,38].
- Field trials: Conducting large-scale field trials will be essential to assess the real-world efficacy and environmental impact of RNAi-based fungicides. While laboratory studies have demonstrated the potential of RNAi strategies, their performance under field conditions remains to be thoroughly evaluated. Field trials will provide valuable data on factors such as dsRNA stability, delivery efficiency, and overall effectiveness in diverse environmental settings. In parallel, resistance development can be followed, similar to the proactive management strategy as proposed for RNAi-based control of pest insects [39].
- Integration into integrated pest management (IPM): Incorporating RNAi strategies into IPM programs can provide a holistic approach to disease control. RNAi-based applications, such as SIGS, offer species-specific targeting of pathogens with minimal impact on non-target organisms (NTOs). By integrating RNAi with other control methods, such as biological control agents and cultural practices, it is possible to enhance overall pest management efficacy and sustainability.
- Regulatory frameworks: The deployment of RNA-based fungicides necessitates comprehensive regulatory guidelines to assess their safety and efficacy. Establishing clear regulatory guidelines is vital to facilitate the adoption of RNA-based plant protection products. Currently, regulatory frameworks for RNAi-based biopesticides are still evolving. In the European Union, these products are subject to a two-step approval process involving the European Food Safety Authority (EFSA) and individual Member States. In the United States, the Environmental Protection Agency (EPA) evaluates RNAi-based biopesticides as biochemical pesticides, requiring data to demonstrate their safety for humans and the environment. Developing clear and harmonized regulatory policies is essential to facilitate the adoption of RNAi technologies in agriculture [40,41,42].
Funding
Acknowledgments
Conflicts of Interest
References
- Smagghe, G.; Palli, S.R.; Swevers, L. RNA Interference in Agriculture: Basic Science to Applications. From Bioinformatics and Laboratory Assays over Regulatory Issues to Field Uses; Springer Nature: Berlin/Heidelberg, Germany, 2025; 711p, ISBN 978-3-031-81548-5. [Google Scholar]
- He, L.; Zhou, Y.; Mo, Q.; Huang, Y.; Tang, X. Spray-induced gene silencing in phytopathogen: Mechanisms, applications, and progress. Adv. Agrochem 2024, 3, 289–297. [Google Scholar] [CrossRef]
- Habib, W.; Saab, C.; Malek, R.; Kattoura, L.; Rotolo, C.; Gerges, E.; Baroudy, F.; Pollastro, S.; Faretra, F.; De Miccolis Angelini, R.M. Resistance profiles of Botrytis cinerea populations to several fungicide classes on greenhouse tomato and strawberry in Lebanon. Plant Pathol. 2020, 69, 1453–1468. [Google Scholar] [CrossRef]
- CABI. Botrytis cinerea (Grey Mould-Rot); CABI Compendium 9611; CABI: Wallingford, UK, 2021. [Google Scholar] [CrossRef]
- Shao, W.; Zhao, Y.; Ma, Z. Advances in Understanding Fungicide Resistance in Botrytis cinerea in China. Phytopathology 2021, 111, 455–463. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.S.; Roy, M.; Abbasi, P.A.; Carisse, O.; Yurgel, S.N.; Ali, S. Why Do We Need Alternative Methods for Fungal Disease Management in Plants? Plants 2023, 12, 3822. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, L.; Molesini, B.; Pandolfini, T.; Jin, H.; Baraldi, E.; Cecchin, M.; Mezzetti, B.; Sabbadini, S. RNA interference-based strategies to control Botrytis cinerea infection in cultivated strawberry. Plant Cell Rep. 2024, 43, 201. [Google Scholar] [CrossRef]
- Spada, M.; Pugliesi, C.; Fambrini, M.; Pecchia, S. Challenges and Opportunities Arising from Host–Botrytis cinerea Interactions to Outline Novel and Sustainable Control Strategies: The Key Role of RNA Interference. Int. J. Mol. Sci. 2024, 25, 6798. [Google Scholar] [CrossRef]
- Kostov, K.; Smagghe, G. RNA interference: View on its current status since the 2006 Nobel Prize and impact on the agriculture looking to the future. In RNAi in Arthropods: Powerful Tool for Basic Research and Product for Entrepreneurs; Smagghe, G., Palli, S.R., Swevers, L., Eds.; Springer: Dordrecht, The Netherlands, 2025; pp. 25–50. [Google Scholar]
- Spada, M.; Pugliesi, C.; Fambrini, M.; Pecchia, S. Silencing of the Slt2-Type MAP Kinase Bmp3 in Botrytis cinerea by Application of Exogenous dsRNA Affects Fungal Growth and Virulence on Lactuca sativa. Int. J. Mol. Sci. 2021, 22, 5362. [Google Scholar] [CrossRef]
- Islam, M.T.; Sherif, S.M. RNAi-Based Biofungicides as a Promising Next-Generation Strategy for Controlling Devastating Gray Mold Diseases. Int. J. Mol. Sci. 2020, 21, 2072. [Google Scholar] [CrossRef] [PubMed]
- Quilez-Molina, A.I.; Sanchez, J.N.; Merino, D. The role of polymers in enabling RNAi-based technology for sustainable pest management. Nat. Commun. 2024, 15, 9158. [Google Scholar] [CrossRef]
- Wu, F.; Yan, L.; Zhao, X.; Lv, C.; Jin, W. Development of an RNA Nanostructure for Effective Botrytis cinerea Control through Spray-Induced Gene Silencing without an Extra Nanocarrier. J. Fungi 2024, 10, 483. [Google Scholar] [CrossRef]
- Germing, K.; Diaz Navarrete, C.A.; Schiermeyer, A.; Hommen, U.; Zuhl, L.; Eilebrecht, S.; Eilebrecht, E. Crop protection by RNA interference: A review of recent approaches, current state of developments and use as of 2013. Environ. Sci. Eur. 2025, 37, 15. [Google Scholar] [CrossRef]
- Spada, M.; Pugliesi, C.; Fambrini, M.; Palpacelli, D.; Caneo, A.; Pecchia, S. Spray-Induced Gene Silencing (SIGS): Nanocarrier-Mediated dsRNA Delivery Improves RNAi Efficiency in the Management of Lettuce Gray Mold Caused by Botrytis cinerea. Agronomy 2025, 15, 194. [Google Scholar] [CrossRef]
- Qiao, L.; Nino-Sanchez, J.; Hamby, R.; Capriotti, L.; Chen, A.; Mezzetti, B.; Jin, H. Artificial nanovesicles for dsRNA delivery in spray-induced gene silencing for crop protection. Plant Biotechnol. J. 2023, 21, 854–865. [Google Scholar] [CrossRef]
- Chen, C.; Imran, M.; Feng, X.; Shen, X.; Sun, Z. Spray-induced gene silencing for crop protection: Recent advances and emerging trends. Front. Plant Sci. 2025, 16, 1527944. [Google Scholar] [CrossRef]
- Imran, M.; Feng, X.; Sun, Z.; Al Omari, H.; Zhang, G.; Zhu, J.; Aldayel, M.F.; Li, C. Nanotechnology-driven gene silencing: Advancements in SIGS–dsRNA technology for sustainable disease management. Chem. Biol. Technol. Agric. 2025, 12, 31. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, X.; Wu, F.; Zhang, P. Selection of BcTRE1 as an effective RNAi target for dsRNA-Based control of Botrytis cinerea. Physiol. Mol. Plant Pathol. 2025, 139, 102773. [Google Scholar] [CrossRef]
- Qin, S.; Veloso, J.; Baak, M.; Boogmans, B.; Bosman, T.; Puccetti, G.; Shi-Kunne, X.; Smit, S.; Grant-Downton, R.; Leisen, T.; et al. Molecular characterization reveals no functional evidence for naturally occurring cross-kingdom RNA interference in the early stages of Botrytis cinerea–tomato interaction. Mol. Plant Pathol. 2023, 24, 3–15. [Google Scholar] [CrossRef]
- Wang, M.; Thomas, N.; Jin, H. Cross-kingdom RNA trafficking and environmental RNAi for powerful innovative pre- and post-harvest plant protection. Curr. Opin. Plant Biol. 2017, 38, 133–141. [Google Scholar] [CrossRef]
- Patel, R.M.; van Kan, J.A.L.; Bailey, A.M.; Foster, G.D. RNA-Mediated Gene Silencing of Superoxide Dismutase (bcsod1) in Botrytis cinerea. Phytopathology 2008, 98, 1335. [Google Scholar] [CrossRef]
- Ding, Z.-T.; Zhang, Z.; Luo, D.; Zhou, J.Y.; Zhong, J.; Yang, J.; Xiao, L.; Shu, D.; Tan, H. Gene Overexpression and RNA Silencing Tools for the Genetic Manipulation of the S-(+)-Abscisic Acid Producing Ascomycete Botrytis cinerea. Int. J. Mol. Sci. 2015, 16, 10301–10323. [Google Scholar] [CrossRef]
- Wang, M.; Weiberg, A.; Lin, F.M.; Thomma, B.P.; Huang, H.D.; Jin, H. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2016, 2, 16151. [Google Scholar] [CrossRef] [PubMed]
- Sabbadini, S.; Capriotti, L.; Jin, H.; Ricci, A.; Giovanetti, G.; Mezzetti, B. RNAi-based approaches to induce resistance against grey mould disease in strawberry. Acta Hortic. 2021, 1309, 209–216. [Google Scholar] [CrossRef]
- Duanis-Assaf, D.; Shlar, I.; Galsurker, O.; Davydov, O.; Maurer, D.; Feygenberg, O.; Poverenov, E.; Fluhr, R.; Alkan, N. Nano-clay, layered-double hydroxide (LDH), improves the efficacy of double-stranded RNA in controlling postharvest decay. Postharvest Biol. Technol. 2022, 193, 112051. [Google Scholar] [CrossRef]
- Nerva, L.; Sandrini, M.; Gambino, G.; Chitarra, W. Double-Stranded RNAs (dsRNAs) as a Sustainable Tool against Gray Mold (Botrytis cinerea) in Grapevine: Effectiveness of Different Application Methods in an Open-Air Environment. Biomolecules 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Sahu, D.; Aminedi, R.; Chandran, D. Concepts and considerations for enhancing RNAi efficiency in phytopathogenic fungi for RNAi-based crop protection using nanocarrier-mediated dsRNA delivery systems. Front. Fungal Biol. 2022, 3, 977502. [Google Scholar] [CrossRef]
- Yang, W.; Wang, B.; Lei, G.; Chen, G.; Liu, D. Advances in nanocarriers to improve the stability of dsRNA in the environment. Front. Bioeng. Biotechnol. 2022, 10, 974646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luo, H.; Zhang, X.; Yang, R.; Yan, S.; Yang, A.; Yang, J. Extracellular Vesicles Mimetic Design of Membrane Chimeric Nanovesicles for dsRNA Delivery in Spray-Induced Gene Silencing for Crop Protection. ACS Nano 2024, 18, 32468–32480. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.W.G.; Sawyer, A.; Gardiner, D.M.; Mitter, N.; Carroll, B.J.; Eamens, A.L. RNA-Based Control of Fungal Pathogens in Plants. Int. J. Mol. Sci. 2023, 24, 12391. [Google Scholar] [CrossRef]
- Niño-Sánchez, J.; Chen, L.-H.; De Souza, J.T.; Mosquera, S.; Stergiopoulos, I. Targeted Delivery of Gene Silencing in Fungi Using Genetically Engineered Bacteria. J. Fungi 2021, 7, 125. [Google Scholar] [CrossRef]
- Qin, S.; Veloso, J.; Puccetti, G.; van Kan, J.A.L. Molecular characterization of cross-kingdom RNA interference in Botrytis cinerea by tomato small RNAs. Front. Plant Sci. 2023, 14, 1107888. [Google Scholar] [CrossRef]
- He, B.; Wang, H.; Liu, G.; Chen, A.; Calvo, A.; Cai, Q.; Jin, H. Fungal small RNAs ride in extracellular vesicles to enter plant cells through clathrin-mediated endocytosis. Nat. Commun. 2023, 14, 4383. [Google Scholar] [CrossRef] [PubMed]
- Niño-Sánchez, J.; Sambasivam, P.T.; Sawyer, A.; Hamby, R.; Chen, A.; Czislowski, E.; Li, P.; Manzie, N.; Gardiner, D.M.; Ford, R.; et al. BioClay™ prolongs RNA interference-mediated crop protection against Botrytis cinerea. J. Integr. Plant Biol. 2022, 64, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Ruf, A.; Robatzek, S. “Messenger RNA just entered the chat’’: The next layer of cross-kingdom RNA transfer. Cell Host Microbe 2024, 32, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-P.; Huanga, L.; Oberkofler, L.; Johnson, N.R.; Glodeanu, A.-S.; Stillmana, K.; Weiberg, A. Fungal Argonaute proteins act in bidirectional cross-kingdomRNA interference during plant infection. Proc. Natl. Acad. Sci. USA 2025, 122, e2422756122. [Google Scholar] [CrossRef]
- Cheng, A.-P.; Kwon, S.; Adeshara, T.; Göhre, V.; Feldbrügge, M.; Weiberg, A. Extracellular RNAs released by plant-associated fungi: From fundamental mechanisms to biotechnological applications. Appl. Microbiol. Biotechnol. 2023, 107, 5935–5945. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Alyokhin, A.; Zhang, R.; Smagghe, G.; Palli, S.R.; Tabashnik, B.; Jurat-Fuentes, J.L. Proactive resistance management for sustaining the efficacy of RNA interference biotechnology for pest control. J. Econ. Entomol. 2024, 117, 1306–1308. [Google Scholar] [CrossRef]
- Luo, X.; Nanda, S.; Zhang, Y.; Zhou, X.; Yang, C.; Pan, H. Risk assessment of RNAi-based biopesticides. New Crops 2024, 1, 100019. [Google Scholar] [CrossRef]
- Gathman, A.; Veromann, E.; Smagghe, G.; Arpaia, S. Regulatory landscape surrounding approval and authorisation of dsRNA-based plant protection products in Europe. In RNAi in Arthropods: Powerful Tool for Basic Research and Product for Entrepreneurs; Smagghe, G., Palli, S.R., Swevers, L., Eds.; Springer: Dordrecht, The Netherlands, 2025; pp. 647–662. [Google Scholar]
- Tardin-Coelho, R.; Fletcher, S.; Manzie, N.; Gunasekara, S.N.; Fidelman, P.; Mitter, N.; Ashworth, P. A systematic review on public perceptions of RNAi-based biopesticides: Developing social licence to operate. Sustain. Agric. 2025, 3, 15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smagghe, G. RNA Interference in Fungal Plant Pathogens: What Do We Know from Botrytis cinerea with Research Hotspots and Gaps, and What Are the Future Directions? J. Fungi 2025, 11, 498. https://doi.org/10.3390/jof11070498
Smagghe G. RNA Interference in Fungal Plant Pathogens: What Do We Know from Botrytis cinerea with Research Hotspots and Gaps, and What Are the Future Directions? Journal of Fungi. 2025; 11(7):498. https://doi.org/10.3390/jof11070498
Chicago/Turabian StyleSmagghe, Guy. 2025. "RNA Interference in Fungal Plant Pathogens: What Do We Know from Botrytis cinerea with Research Hotspots and Gaps, and What Are the Future Directions?" Journal of Fungi 11, no. 7: 498. https://doi.org/10.3390/jof11070498
APA StyleSmagghe, G. (2025). RNA Interference in Fungal Plant Pathogens: What Do We Know from Botrytis cinerea with Research Hotspots and Gaps, and What Are the Future Directions? Journal of Fungi, 11(7), 498. https://doi.org/10.3390/jof11070498





