The Alleviating Effect of Arginine on Ethanol Stress in Wickerhamomyces anomalus
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Culture Conditions
2.2. Instruments and Equipment
2.3. Ethanol Stress and Exogenous Arginine Supplementation
2.4. Survival Analysis
2.5. ROS Production Detection
2.6. Cellular and Mitochondrial Membrane Integrity Assessment
2.7. Nitric Oxide Synthesis Detection
2.8. RNA Extraction, Transcriptome Sequencing, and Bioinformatics Analysis
2.9. Non-Targeted Metabolomics Analysis
2.10. Statistical Analysis
3. Results
3.1. Arginine Enhanced the Survival of Ethanol-Stressed W. anomalus
3.2. Arginine Reduced ROS Levels in Ethanol-Stressed W. anomalus
3.3. Arginine Preserved the Integrity of Cellular and Mitochondrial Membranes in Ethanol-Stressed W. anomalus
3.4. Arginine Facilitated Nitric Oxide Synthesis in Ethanol-Stressed W. anomalus
3.5. Arginine Regulated Gene Expression Profiles in Ethanol-Stressed W. anomalus
3.5.1. Identification of DEGs
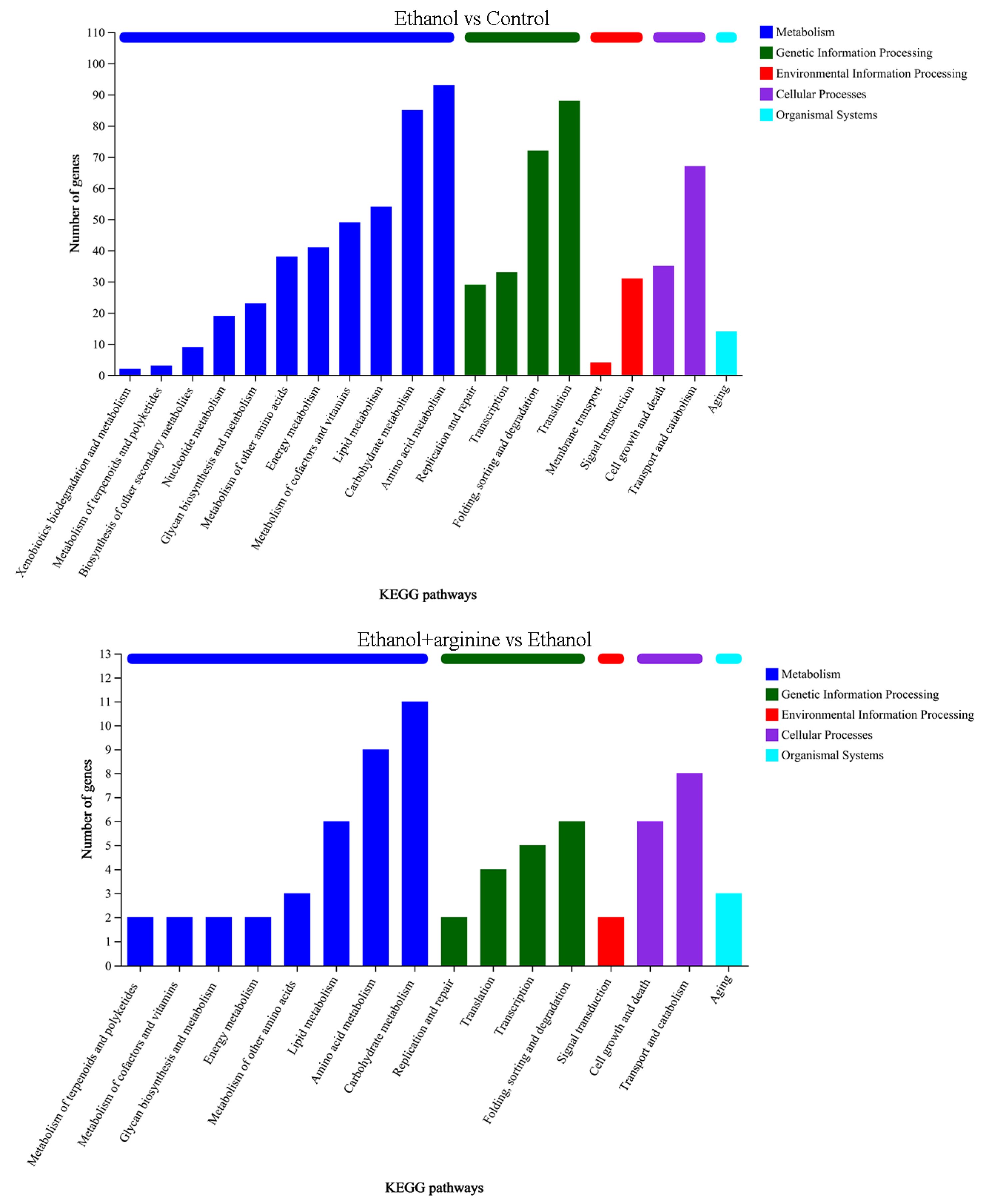
3.5.2. KEGG Annotation Analysis of DEGs
3.5.3. KEGG Enrichment Analysis of DEGs
3.5.4. Validation of Transcriptome Sequencing Data by Quantitative Real-Time PCR
3.6. Arginine Regulated the Metabolite Expression Profiles of Ethanol-Stressed W. anomalus
3.6.1. Identification of DEMs
3.6.2. KEGG Annotation Analysis of DEMs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kavšček, M.; Stražar, M.; Curk, T.; Natter, K.; Petrovič, U. Yeast as a cell factory: Current state and perspectives. Microb. Cell Fact. 2015, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Xu, Y.; Yu, X.W. Overview of yeast environmental stress response pathways and the development of tolerant yeasts. Syst. Microbiol. Biomanuf. 2022, 2, 232–245. [Google Scholar] [CrossRef]
- Stanley, D.; Bandara, A.; Fraser, S.; Chambers, P.J.; Stanley, G.A. The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J. Appl. Microbiol. 2010, 109, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Auesukaree, C. Molecular mechanisms of the yeast adaptive response and tolerance to stresses encountered during ethanol fermentation. J. Biosci. Bioeng. 2017, 124, 133–142. [Google Scholar] [CrossRef]
- Saini, P.; Beniwal, A.; Kokkiligadda, A.; Vij, S. Response and tolerance of yeast to changing environmental stress during ethanol fermentation. Process Biochem. 2018, 72, 1–12. [Google Scholar] [CrossRef]
- de Moura Ferreira, M.A.; da Silveira, F.A.; da Silveira, W.B. Ethanol stress responses in Kluyveromyces marxianus: Current knowledge and perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 1341–1353. [Google Scholar] [CrossRef]
- Cheng, Y.; Du, Z.; Zhu, H.; Guo, X.; He, X. Protective effects of arginine on Saccharomyces cerevisiae against ethanol stress. Sci. Rep. 2016, 6, 31311. [Google Scholar] [CrossRef]
- Li, Y.; Long, H.; Jiang, G.; Gong, X.; Yu, Z.H.; Huang, M.Z.; Guan, T.; Guan, Y.; Liu, X. Analysis of the ethanol stress response mechanism in Wickerhamomyces anomalus based on transcriptomics and metabolomics approaches. BMC Microbiol. 2022, 22, 275. [Google Scholar] [CrossRef]
- Winter, G.; Todd, C.D.; Trovato, M.; Forlani, G.; Funck, D. Physiological implications of arginine metabolism in plants. Front. Plant Sci. 2015, 6, 534. [Google Scholar] [CrossRef]
- Nasibi, F.; Yaghoobi, M.M.; Kalantari, K.M. Effect of exogenous arginine on alleviation of oxidative damage in tomato plant underwater stress. J. Plant Interact. 2011, 6, 291–296. [Google Scholar] [CrossRef]
- Hussein, H.A.; Alshammari, S.O.; Kenawy, S.K.M.; Elkady, F.M.; Badawy, A.A. Grain-priming with L-arginine improves the growth performance of Wheat (Triticum aestivum L.) plants under drought stress. Plants 2022, 11, 1219. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.A.; Abd Elhamid, E.M.; Sadak, M.S. Comparative study for the effect of arginine and sodium nitroprusside on sunflower plants grown under salinity stress conditions. Bull. Natl. Res. Cent. 2019, 43, 118. [Google Scholar] [CrossRef]
- Jiménez-López, C.; Collette, J.R.; Brothers, K.M.; Shepardson, K.M.; Cramer, R.A.; Wheeler, R.T.; Lorenz, M.C. Candida albicans induces arginine biosynthetic genes in response to host-derived reactive oxygen species. Eukaryot. Cell 2013, 12, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhou, J.; Liu, L.; Chen, J. Arginine: A novel compatible solute to protect Candida glabrata against hyperosmotic stress. Process Biochem. 2011, 46, 1230–1235. [Google Scholar] [CrossRef]
- Liu, X.Z.; Li, Y.F.; Zhao, H.B.; Yu, Z.H.; Huang, M.Z. Identification and fermentative properties of an indigenous strain of Wickerhamomyces anomalus isolated from Rosa roxburghii Tratt. Brit. Food J. 2021, 123, 4069–4081. [Google Scholar] [CrossRef]
- Jing, H.; Liu, H.; Zhang, L.; Gao, J.; Song, H.; Tan, X. Ethanol induces autophagy regulated by mitochondrial ROS in Saccharomyces cerevisiae. J. Microbiol. Biotechnol. 2018, 28, 1982–1991. [Google Scholar] [CrossRef]
- Bartlett, T.M.; Sisley, T.A.; Mychack, A.; Walker, S.; Baker, R.W.; Rudner, D.Z.; Bernhardt, T.G. FacZ is a GpsB-interacting protein that prevents aberrant division-site placement in Staphylococcus aureus. Nat. Microbiol. 2024, 9, 801–813. [Google Scholar] [CrossRef]
- Huysseune, A.; Larsen, U.G.; Larionova, D.; Matthiesen, C.L.; Petersen, S.V.; Muller, M.; Witten, P.E. Bone formation in zebrafish: The significance of DAF-FM DA staining for nitric oxide detection. Biomolecules 2023, 13, 1780. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, Y.; Cai, W.; Liu, N.; Zeng, J.; Liu, C.; Peng, H.; Fu, G. Effects on cell membrane integrity of Pichia anomala by the accumulating excessive reactive oxygen species under ethanol stress. Foods 2022, 11, 3744. [Google Scholar] [CrossRef]
- Kaya, C.; Polat, T.; Ashraf, M.; Kaushik, P.; Alyemeni, M.N.; Ahmad, P. Endogenous nitric oxide and its potential sources regulate glutathione-induced cadmium stress tolerance in maize plants. Plant Physiol. Biochem. 2021, 167, 723–737. [Google Scholar] [CrossRef]
- Che, X.; Li, B.; Zeng, J.; Chen, Y.; Liu, J.; Zeng, W.; Zeng, W.; Cai, W.; Wan, Y.; Fu, G. Effect of co-fermentation of non-Saccharomyces yeasts with Saccharomyces cerevisiae on the quality and flavor of blueberry wine. Food Res. Int. 2024, 196, 115051. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Shi, X.; Chen, H.; Zhang, L.; Cen, L.; Li, L. Major active metabolite characteristics of Dendrobium officinale rice wine fermented by Saccharomyces cerevisiae and Wickerhamomyces anomalus cofermentation. Foods 2023, 12, 2370. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Teng, C.; Xu, D.; Fu, Z.; Liu, P.; Wu, Q.; Yang, R.; Li, X. Improving ethyl acetate production in Baijiu manufacture by Wickerhamomyces anomalus and Saccharomyces cerevisiae mixed culture fermentations. Biomed Res. Int. 2019, 13, 1470543. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fan, G.; Li, X.; Fu, Z.; Liang, X.; Sun, B. Application of Wickerhamomyces anomalus in simulated solid-state fermentation for Baijiu production: Changes of microbial community structure and flavor metabolism. Front. Microbiol. 2020, 11, 598758. [Google Scholar] [CrossRef]
- Yan, S.; Chen, X.; Xiang, X. Improvement of the aroma of lily rice wine by using aroma-producing yeast strain Wickerhamomyces anomalus HN006. AMB Express 2019, 9, 89. [Google Scholar] [CrossRef]
- Zhang, J.; He, Y.; Yin, L.; Hu, R.; Yang, J.; Zhou, J. Isolation of aroma-producing Wickerhamomyces anomalus yeast and analysis of its typical flavoring metabolites. Foods 2023, 12, 2934. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zhou, J.; Huang, M. Effects of co-inoculation and sequential inoculation of Wickerhamomyces anomalus and Saccharomyces cerevisiae on the physicochemical properties and aromatic characteristics of longan (Dimocarpus longan Lour.) wine. Qual. Assur. Saf. Crops Foods 2021, 13, 56–66. [Google Scholar] [CrossRef]
- Dong, C.; Fang, W.; Yi, Q.; Zhang, J. A comprehensive review on reactive oxygen species (ROS) in advanced oxidation processes (AOPs). Chemosphere 2022, 308, 136205. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Li, M.; Kim, C. Chloroplast ROS and stress signaling. Plant Commun. 2021, 3, 100264. [Google Scholar] [CrossRef]
- Bleoanca, I.; Silva, A.R.C.; Pimentel, C.; Rodrigues-Pousada, C.; Menezes, R.A. Relationship between ethanol and oxidative stress in laboratory and brewing yeast strains. J. Biosci. Bioeng. 2013, 116, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef] [PubMed]
- Kig, C.; Temizkan, G. Nitric oxide as a signaling molecule in the fission yeast Schizosaccharomyces pombe. Protoplasma 2009, 238, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Mariyam, S.; Bhardwaj, R.; Khan, N.A.; Sahi, S.V.; Seth, C.S. Review on nitric oxide at the forefront of rapid systemic signaling in mitigation of salinity stress in plants: Crosstalk with calcium and hydrogen peroxide. Plant Sci. 2023, 336, 111835. [Google Scholar] [CrossRef]
- Terrón-Camero, L.C.; Peláez-Vico, M.Á.; Del-Val, C.; Sandalio, L.M.; Romero-Puertas, M.C. Role of nitric oxide in plant responses to heavy metal stress: Exogenous application versus endogenous production. J. Exp. Bot. 2019, 70, 4477–4488. [Google Scholar] [CrossRef]
- Sougrakpam, Y.; Babuta, P.; Deswal, R. Nitric oxide (NO) modulates low temperature-stress signaling via S-nitrosation, a NO PTM, inducing ethylene biosynthesis inhibition leading to enhanced post-harvest shelf-life of agricultural produce. Physiol. Mol. Biol. Plants 2023, 29, 2051–2065. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, N.; Ohri, P. Harmonizing hydrogen sulfide and nitric oxide: A duo defending plants against salinity stress. Nitric Oxide 2024, 144, 1–10. [Google Scholar] [CrossRef]
- Feura, E.S.; Yang, L.; Schoenfisch, M.H. Antibacterial activity of nitric oxide-releasing carboxymethylcellulose against periodontal pathogens. J. Biomed. Mater. Res. Part A 2021, 109, 713–721. [Google Scholar] [CrossRef]
- Chiang, K.T.; Shinyashiki, M.; Switzer, C.H.; Valentine, J.S.; Gralla, E.B.; Thiele, D.J.; Thiele, D.J.; Fukuto, J.M. Effects of nitric oxide on the copper-responsive transcription factor Ace1 in Saccharomyces cerevisiae: Cytotoxic and cytoprotective actions of nitric oxide. Arch. Biochem. Biophys. 2000, 377, 296–303. [Google Scholar] [CrossRef]
- Kaur, J.; Goldsmith, J.; Tankka, A.; Bustamante, E.S.; Gimenez, A.A.; Vick, L.; Debnath, J.; Vlahakis, A. Atg32-dependent mitophagy sustains spermidine and nitric oxide required for heat-stress tolerance in Saccharomyces cerevisiae. J. Cell Sci. 2021, 134, jcs253781. [Google Scholar] [CrossRef]
- Astuti, R.I.; Watanabe, D.; Takagi, H. Nitric oxide signaling and its role in oxidative stress response in Schizosaccharomyces pombe. Nitric Oxide 2016, 52, 29–40. [Google Scholar] [CrossRef] [PubMed]
- John, M.J.J.; Song, Y.; Hou, M.; Zhou, L.; Liu, X.; Li, X.; Fu, D.; Li, Q.; Cao, H.; Li, R. Multi-Omics approaches in oil palm research: A comprehensive review of metabolomics, proteomics, and transcriptomics based on low-temperature stress. Int. J. Mol. Sci. 2024, 25, 7695. [Google Scholar] [CrossRef] [PubMed]
- Šoštarić, N.; Arslan, A.; Carvalho, B.; Plech, M.; Voordecker, K.; Verstrepen, K.J. Integrated multi-omics analysis of mechanisms underlying yeast ethanol tolerance. J. Proteome Res. 2021, 20, 3840–3852. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Miao, Y.; Yuan, S.; Li, Y.; Wu, Z.; Weng, P. Integrated transcriptomic and proteomic analysis of the ethanol stress response in Saccharomyces cerevisiae Sc131. J. Proteom. 2019, 203, 103377. [Google Scholar] [CrossRef]
- Gai, Z.; Hu, S.; He, Y.; Yan, S.; Wang, R.; Gong, G.; Zhao, J. L-arginine alleviates heat stress-induced mammary gland injury through modulating CASTOR1-mTORC1 axis mediated mitochondrial homeostasis. Sci. Total Environ. 2024, 926, 172017. [Google Scholar] [CrossRef]
- Siddappa, S.; Marathe, G.K. What we know about plant arginases? Plant Physiol. Biochem. 2020, 156, 600–610. [Google Scholar] [CrossRef]









| Raw Reads | Raw Bases | Clean Reads | Clean Bases | Error Rate (%) | Q20 (%) | Q30 (%) | GC Content (%) | |
|---|---|---|---|---|---|---|---|---|
| Control | 43,065,443 | 6,502,881,943 | 42,245,314 | 6,315,356,769 | 0.02 | 98.25 | 94.71 | 37.133 |
| Ethanol | 46,447,271 | 7,013,537,971 | 44,885,465 | 6,677,724,185 | 0.02 | 98.24 | 94.77 | 40.023 |
| Ethanol + arginine | 45,249,228 | 6,832,633,428 | 44,321,098 | 6,630,839,405 | 0.03 | 98.21 | 94.64 | 38.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, Y.; Liu, S.; Jiang, G.; Huang, M.; Liu, X. The Alleviating Effect of Arginine on Ethanol Stress in Wickerhamomyces anomalus. J. Fungi 2025, 11, 499. https://doi.org/10.3390/jof11070499
Li Y, Wang Y, Liu S, Jiang G, Huang M, Liu X. The Alleviating Effect of Arginine on Ethanol Stress in Wickerhamomyces anomalus. Journal of Fungi. 2025; 11(7):499. https://doi.org/10.3390/jof11070499
Chicago/Turabian StyleLi, Yinfeng, Yujie Wang, Shuangyan Liu, Guilan Jiang, Mingzheng Huang, and Xiaozhu Liu. 2025. "The Alleviating Effect of Arginine on Ethanol Stress in Wickerhamomyces anomalus" Journal of Fungi 11, no. 7: 499. https://doi.org/10.3390/jof11070499
APA StyleLi, Y., Wang, Y., Liu, S., Jiang, G., Huang, M., & Liu, X. (2025). The Alleviating Effect of Arginine on Ethanol Stress in Wickerhamomyces anomalus. Journal of Fungi, 11(7), 499. https://doi.org/10.3390/jof11070499





