New Contributions to the Species Diversity of the Genus Hydnum (Hydnaceae, Cantharellales) in China: Four New Taxa and Newly Recorded Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collecting
2.2. Morphological Studies
2.3. DNA Extraction, PCR, and Sequencing
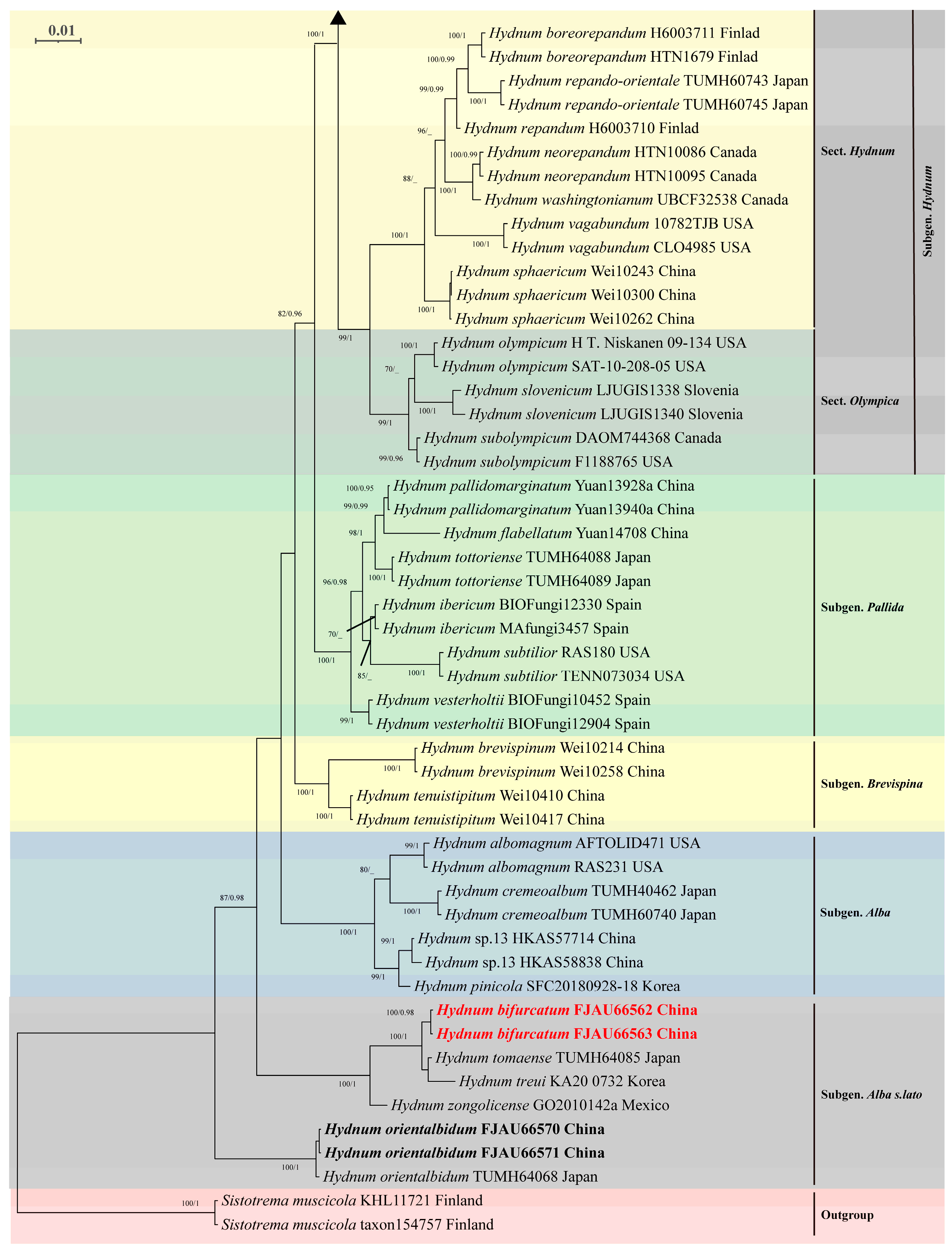
2.4. Phylogenetic Analyses
3. Results
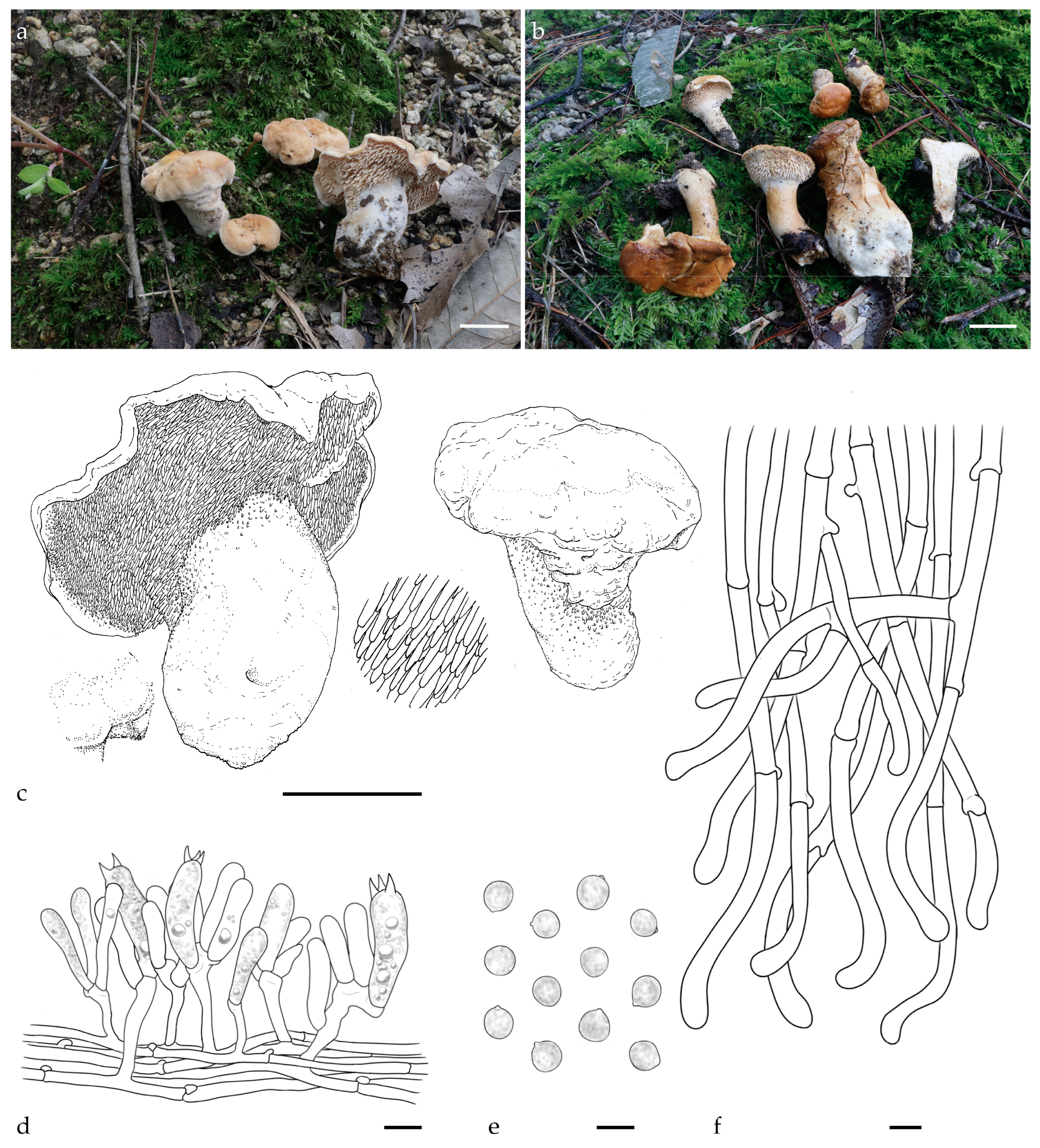
3.1. Taxonomy
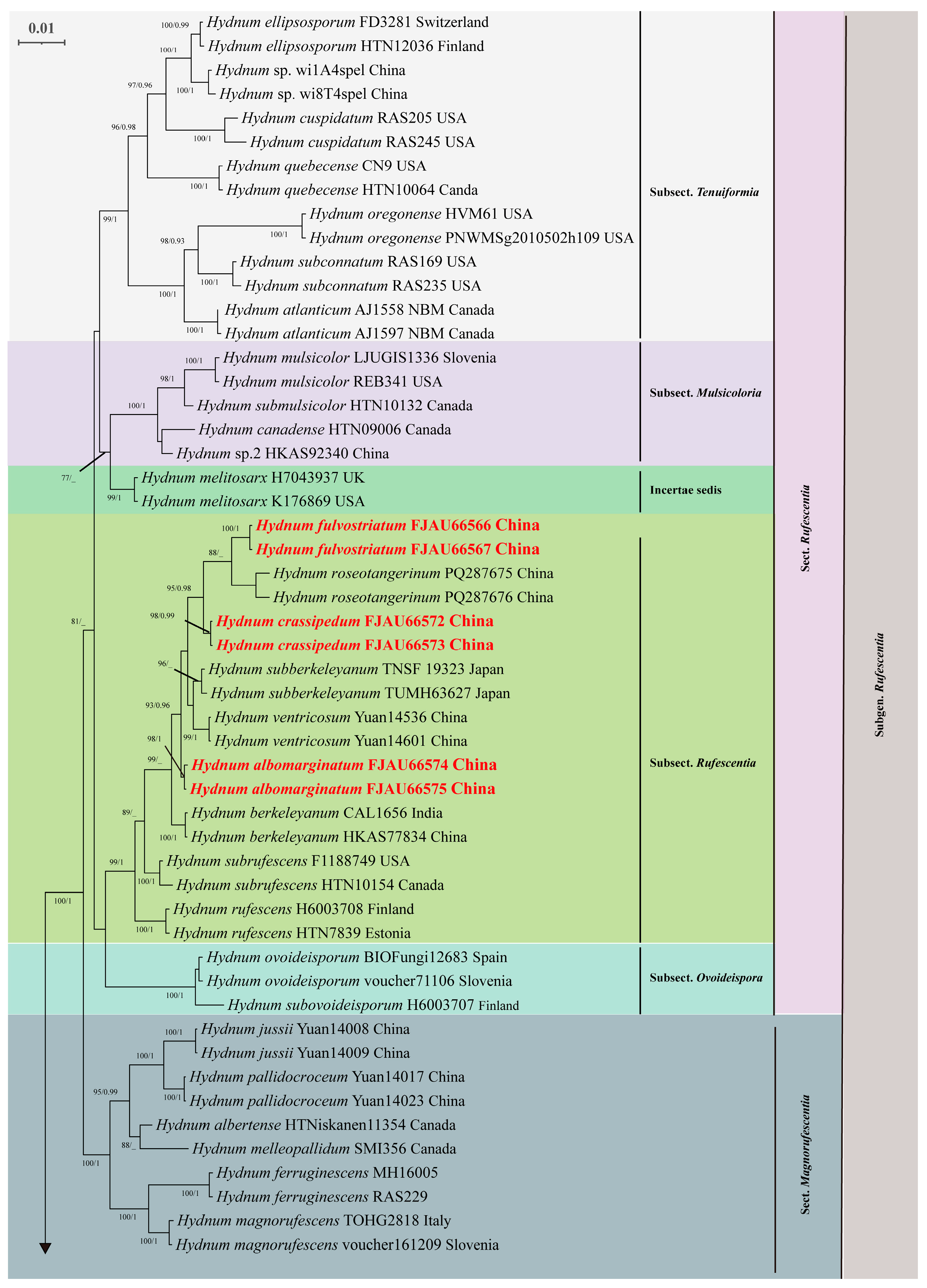
3.2. Molecular Phylogeny
4. Discussion
5. Conclusions
Key to Species of Hydnum in China
| 1. | Basidiomata more or less white to cream yellow | 2 |
| 1. | Basidiomata yellow to orange | 11 |
| 2. | Pileus white | 3 |
| 2. | Pileus cream yellow | 7 |
| 3. | Pileus < 30 mm wide | H. flavidocanum |
| 3. | Pileus > 30 mm wide | 4 |
| 4. | Habitat in broad-leaved forests | 5 |
| 4. | Habitat in Fagaceous forests | 6 |
| 5. | Pileus > 60 mm wide | H. cremeoalbum |
| 5. | Pileus < 60 mm wide | H. orientalbidum |
| 6. | Basidiospores < 5 μm long on average | H. minus |
| 6. | Basidiospores > 5 μm long on average | H. treui |
| 7. | Pileus > 60 mm wide | 8 |
| 7. | Pileus < 60 mm wide | 9 |
| 8. | Habitat in broad-leaved forests | H. roseoalbum |
| 8. | Habitat in Quercus mongolica forests | H. bifurcatum |
| 9. | Spines < 3 mm long | 10 |
| 9. | Spines > 3 mm long | H. albomagnum |
| 10. | Spines pale orange | H. pinicola |
| 10. | Spines cream yellow | H. minum |
| 11. | Basidiomata yellowish-white | 12 |
| 11. | Basidiomata orange | 17 |
| 12. | Basidiospores < 6 μm long on average | 13 |
| 12. | Basidiospores > 6 μm long on average | 14 |
| 13. | Habitat in angiosperm forests | H. brevispinum |
| 13. | Habitat in mixed forests | H. microcarpum |
| 14. | Basidiospores < 7.5 μm long on average | 15 |
| 14. | Basidiospores > 7.5 μm long on average | 16 |
| 15. | Spines 1–3 mm long | H. tenuistipitum |
| 15. | Spines 2–7 mm long | H. fulvostriatum |
| 16. | Habitat in Q. variabilis forests | H. crassipedum |
| 16. | Habitat in Q. serrata forests | H. albomarginatum |
| 17. | Basidiospores 7–8 μm long on average | 18 |
| 17. | Basidiospores > 8 μm long on average | 19 |
| 18. | Habitat in coniferous forests | H. jussii |
| 18. | Habitat in Fagaceous forests | H. erectum |
| 19. | Basidiospores 8–9 μm long on average | 20 |
| 19. | Basidiospores > 9 μm long on average | 27 |
| 20. | Spines white | 21 |
| 20. | Spines orange–white or cream-yellow | 22 |
| 21. | Spines < 3 mm long | H. sphaericum |
| 21. | Spines > 3 mm long | H. sinorepandum |
| 22. | Spines cream-yellow | H. cremeum |
| 22. | Spines orange–white | 23 |
| 23. | Basidiospores 8.1–8.5 μm long on average | 24 |
| 23. | Basidiospores > 8.5 μm long on average | 26 |
| 24. | Spines < 2 mm long | H. vesterholtii |
| 24. | Spines > 2 mm long | 25 |
| 25. | Pileus < 50 mm wide | H. tangerinum |
| 25. | Pileus > 50 mm wide | H. berkeleyanum |
| 26. | Basidiospores Q < 1.2 | H. ventricosum |
| 26. | Basidiospores Q > 1.2 | H. pallidomarginatum |
| 27. | Pileus < 50 mm wide | 28 |
| 27. | Pileus > 50 mm wide | H. roseotangerinum |
| 28. | Pileus orange–white | 29 |
| 28. | Pileus light yellow | 31 |
| 29. | Habitat in mixed forests | H. flabellatum |
| 29. | Habitat in broad forests | 30 |
| 30. | Basidiospores > 9.5 μm long on average | H. longibasidium |
| 30. | Basidiospores < 9.5 μm long on average | H. longipes |
| 31. | Basidiospores Q > 1.3 | H. melitosarxm |
| 31. | Basidiospores Q < 1.3 | 32 |
| 32. | Habitat in Pinus forests | H. pallidocroceum |
| 32. | Habitat in mixed forests | H. flavoquamosum. |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Characteristics of Optical Microscopes and Scanning Electron Microscopes (SEMs)
Appendix A.1. Basidiospores
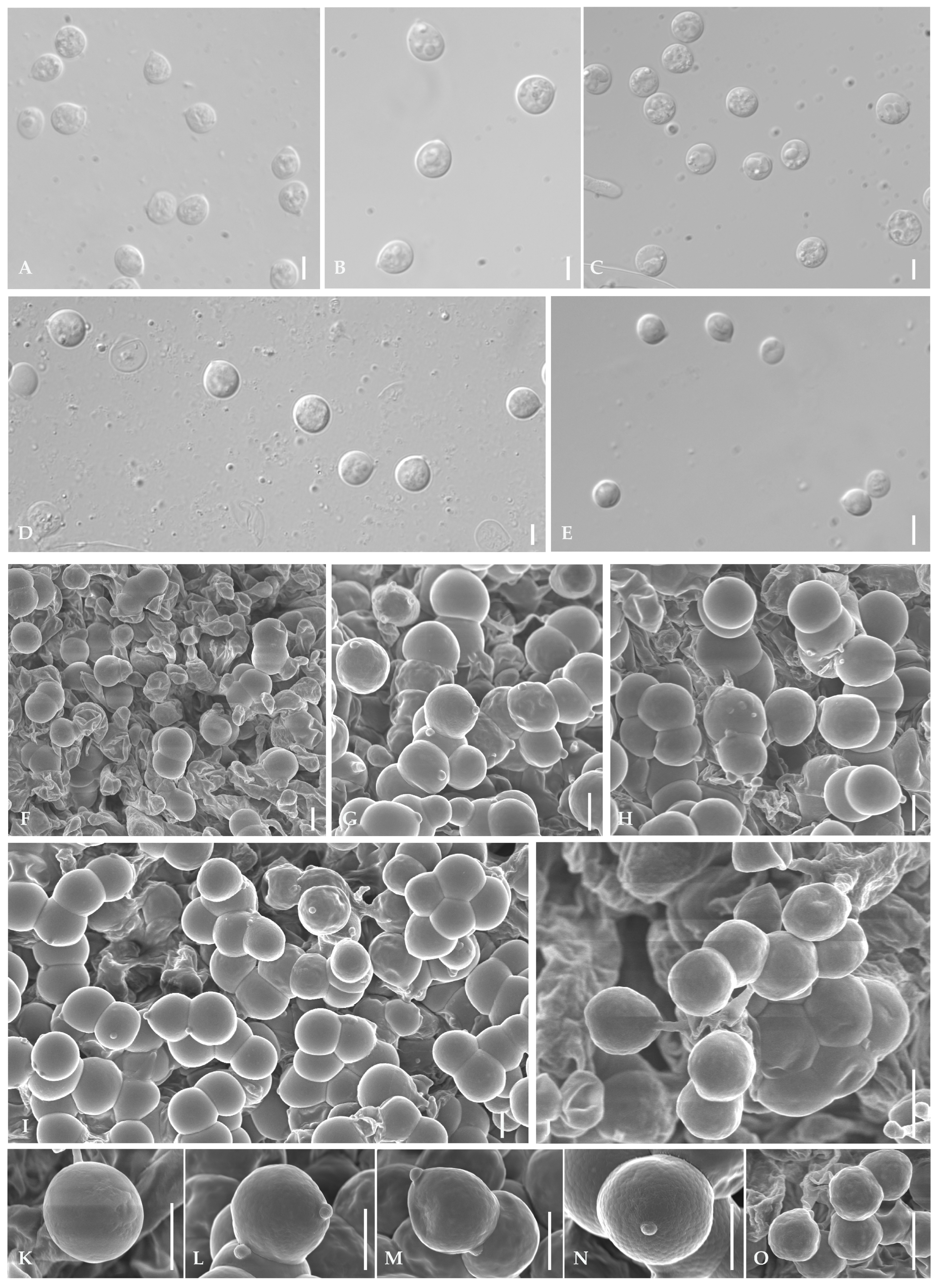
Appendix A.2. Basidia
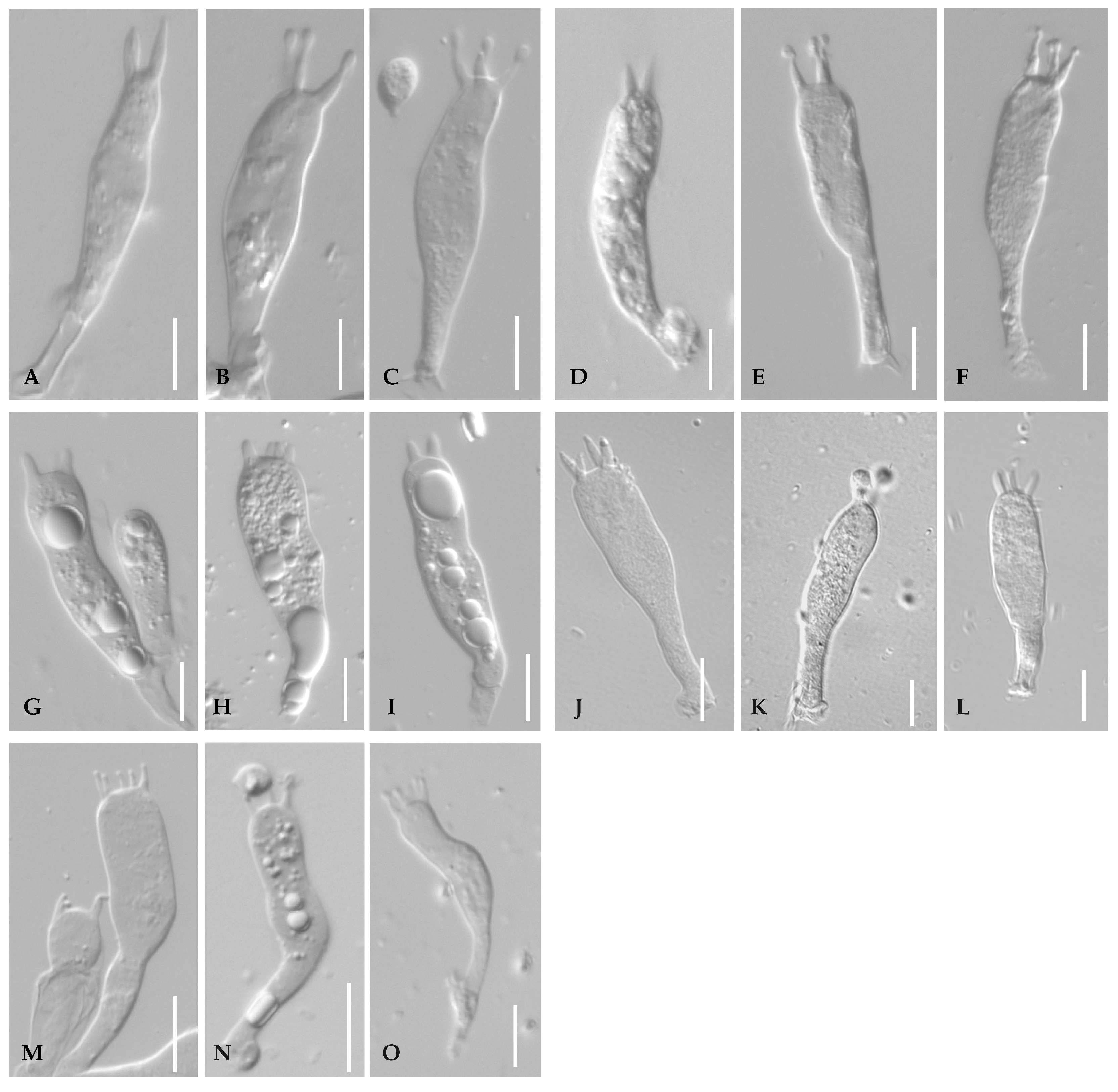
Appendix B
| Species | Pileus | Spines | Spores | Habitat | References | Distribution | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Color | Size (cm) | Color | Length (mm) | Size (µm) | av. L×W (µm) | Q | ||||
| H. albomagnum | Cream-white to pale cream | 25–60 | Cream-white to pale cream | 6–12 | (4.6) 4.8–6.7 (7.1) × (3.5) 3.9–5.4 (5.6) | avg. 5.0–6.5 × 4–5.5 | Q = 1.07–1.36 | Hardwood-oak forests | [2] | China Japan |
| H. albomarginatum | Yellowish-white | 23–66 | Pale cream | 2–6 | (7.0) 8.0–8.5 (9.0) × (7.0) 8.0–8.2 (9.0) | avg. 8.04 × 7.93 | Q = 1.00–1.09 | Quercus serrata forests | This study | Anhui China |
| H. berkeleyanum | Pale orange to light orange | 25–80 | Orange–white | 2–9 | (7.5) 8.4–8.5 (9.5) × (6.9) 7.9–8 (8.8) | avg. 8.4 × 7.95 | Q = 1.01–1.17 | Mixed forests | [3] | India China |
| H. bifurcatum | Pale cream | 65–135 | Yellowish-white | 2–7 | (8.0) 8.5–9.5 (10.0) × (7.5) 8.0–9.5 (10.0) | avg. 9.12 × 8.97 | Q = 1.00–1.06 | Q. mongolica forests | This study | Jilin China |
| H. brevispinum | Yellowish-white | 10–15 | Pure white | 0.2–0.8 | (4.5) 5.0–5.8 (6.0) × (3.5) 3.8–4.8 (5.0) | avg. 5.28 × 4.16 | Q = 1.27–1.31 | Angiosperm forests | [1] | Hunan Province |
| H. crassipedum | Yellowish-white to orange–white | 24–56 | Yellowish | 2–5 | (7.0) 8.0–8.5 (9.0) × (6.5) 7.0–7.5 (6.0) | avg. 8.0× 6.99 | Q = 1.11–1.17 | Q. variabilis forests | This study | Anhui China |
| H. cremeoalbum | White to cream-white | 30–100 | Yellowish-white | 2–5 | 4.0–5.5 × 3.5–4.5 | avg. 4.73 × 3.88 | Q = 1.1–1.42 | Broad-leaved forests Mixed forests | [4] | China Japan |
| H. cremeum | Warm cream to yellowish-white | 25–37 | Cream-yellow | 4–5 | 8.1–9.5 × 7.1–9.0 (9.5) | avg. 8.8 × 8.05 | Q = 1.00–1.11 | Mixed forests | [5] | Yunnan China |
| H. erectum | Creamy yellow to pale salmon | 35–45 | White to pale salmon | 1–2 | (6.5) 7.33–8.0 × (5.5) 6.68–7.5 (8.0) | avg. 7.67 × 7.09 | Q = 1.0–1.1 | Fagaceous forests | [54] | Zhejiang China |
| H. flabellatum | Yellow to grayish-yellow | 30–45 | Orange–white | 0.6–2 | (7.8) 8.5–9.5 (10.0) × (6.0) 6.5–7.8 (8.0) | avg. 9.07 × 7.04 | Q = 1.26–1.29 | Mixed forests | [1] | Liaoning China |
| H. flavidocanum | Yellowish-white or yellowish-gray | 20–30 | Orange–white | 0.5–2 | (7.0) 7.2–8.8 (8.9) × (5.2) 5.5–6.5 (6.8) | avg. 7.75 × 6.01 | Q = 1.29–1.31 | Mixed forests | [1] | Yunnan China |
| H. flavoquamosum | Light yellow to light brownish-orange | 30–50 | Light yellow to light brownish-orange | 0.5 | 8.6–9.5 (10.0) × 7.6–8.6 (9.0) | avg. 9.05 × 8.1 | Q = 1.05–1.23 | Mixed forests | [5] | Yunnan China |
| H. fulvostriatum | Yellowish-white | 44–65 | White | 2–7 | (6.8) 7–7.2 (8) × (6.5) 6.8–7.0 (7.5) | avg. 7.2 × 6.9 | Q = 1.00–1.08 | Q. glauca forests | This study | Anhui China |
| H. jussii | Medium orange ocher | 35–60 | Whitish | 7.2–8.0 × 6.6–7.5 | avg. 7.5 × 7.0 | Q = 1.03–1.18 | Coniferous forests | [6] | Finland China | |
| H. longibasidium | Orange–white to grayish orange | 10–15 | Orange–white to pale orange | 1–4 | (8.0) 8.5–11.0 (11.5) × (7.5) 7.8–9.8 (10.0) | avg. 9.81 × 9.03 | Q = 1.09–1.13 | Angiosperm forests | [1] | Hunan China |
| H. longipes | Orange, light orange | 20–30 | Orange–white to pale orange | 1–3 | (7.5) 8.0–10.0 (10.5) × (6.0) 7.0–8.5 (9.0) | avg. 9.32 × 7.63 | Q = 1.05–1.43 | Q. aquifolioides forests Pinus densata forests | [7] | Yunnan China |
| H.melitosarxm | Light orange to orange | 20–40 | Yellowish-white to pale orange | 2–5 | 8.0–11.0× 7.0–10.0 | avg. 9.37 × 8.71 | Q = 1.0–1.28 | Broad-leaved forests Mixed forests | [6] | Europe Asia |
| H. microcarpum | Yellowish-white to orange–white | 10–20 | White to yellowish-white | 0.5–1.5 | 5.0–6.0 (6.5) × 5.0–5.5 (6.0) | avg. 5.78 × 5.26 | Q = 1.0–1.2 | Mixed forests | [7] | Guangdong China |
| H. minum | Cream-yellow to pale buff | 10–25 | Cream yellow | 0.5–1.7 | 4.5–5.5 × 3.0–4.5 | avg. 4.8 × 3.8 | Q = 1.1–1.5 | Mixed forests | [1] | China Japan |
| H. minus | Cream-white to cream | 15–25 | White to cream-white | 2–4 | (4.0) 4.72–5.0 × (3) 3.68–4.0 | avg. 4.0–5.0 × 3.0–4.0 | Q = 1.13–1.29 | Fagaceous forest | [54] | China Japan |
| H. orientalbidum | White | 30–45 | White | 1–3 | (4.0) 4.5–5 (6.0) × (4.0) 4.5–5.0 (6.0) | avg. 4.6 × 4.4 | Q = 1.0–1.11 | Broad-leaved forests Q. serrata forests | This study | Japan China |
| H. pallidocroceum | Orange–white to pale orange | 25–40 | Light yellow | 1–5 | (7.5) 7.8–9.5 (10.0) × (5.5) 6.0–7.5 (8.0) | avg. 9.09 × 6.72 | Q = 1.32–1.35 | Pinus sp. forests | [1] | Xinjiang China |
| H. pallidomarginatum | Orange–white to pale orange | 20–35 | Orange–white to pale orange | 0.5–2 | (8.0) 8.2–9.8 (10.0) × (6.0) 6.5–7.8 (8.2) | avg. 8.75 × 6.99 | Q = 1.25–1.28 | Angiosperm forests | [1] | Yunnan China |
| H. pinicola | Pale yellow to pale orange | 25–60 | Orange–white to pale orange | 1.5–3 | 4.0–6.0 × 3.5–5.0 | avg. 4.72 × 4.02 | Q = 1.0–1.25 | Mixed forests | [2] | China Japan |
| H. roseoalbum | Cream to whitish | 65 | Pale orange | 8.1–9.0 (9.5) × 8.1–9.0 | Q = 1.00–1.06 | Broad-leaved forests | [5] | Yunnan China | ||
| H. roseotangerinum | Pale brownish-orange | 65 | Yellowish-orange to pinkish-orange | 4–5 | 8.6–9.5 (10.0) × 7.6–9.0 | avg. 9.05 × 8.3 | Q = 1.00–1.18 | Mixed forests | [5] | Yunnan China |
| H. sinorepandum | Light yellow to light orange | 40–120 | White to yellowish-white | 3–7 | (7.5) 8–9 (10) × 6.5–7.5 | avg. 8.16 × 7.12 | Q = 1.0–1.38 | Broad-leaved forests Mixed forests | [7] | Yunnan China |
| H. sphaericum | Orange–white | 20–35 | White | 0.5–3 | (7.5) 8–8.8 (9) × (6) 6.5–7.5 (8) | avg. 8.36 × 6.94 | Q = 1.2–1.23 | Angiosperm forests | [1] | Hunan China |
| H. tangerinum | Orange to brownish-orange | 10–50 | Orange–white | 2–6 | (7) 7.2–8.8 (9) × (5.5) 5.8–7 (7.5) | avg. 8.11 × 6.19 | Q = 1.23–1.31 | Angiosperm forests | [1] | Hunan China |
| H. tenuistipitum | Yellow white to orange–white | 10–30 | Orange–white | 1–3 | (6.5) 6.8–7.2 (7.5) × (5.2) 5.5–6.5 (6.8) | avg. 7.08 × 6.09 | Q = 1.07–1.16 | Angiosperm forests | [1] | Hunan China |
| H. treui | White to creamy white | 28–57 | White to cream-white | 1–4 | (6) 6.44–7 (7.5) × (5) 5.88–6.5 (7) | Q = 1.0–1.2 | Fagaceous forests | [54] | China Papua New Guinea | |
| H. ventricosum | Orange to brown | 28–35 | Orange–white | 1–5 | (7.5) 8.2–9 (9.5) × (7) 7.5–8.5 (9) | avg. 8.64 × 8.17 | Q = 1.05–1.09 | Mixed forests | [1] | Liaoning China |
| H. vesterholtii | Ocher to light ocher | 10–50 | Pale ocher | 1–1.7 | (7) 8–9 (9.5) × 6–7.5 (8) | avg. 8.2–8.7 × 6.4–6.8 | Q = 1.27–1.3 | Abies alba forests Fagus sylvatica forests | [12] | China France |
References
- Niskanen, T.; Liimatainen, K.; Nuytinck, J.; Kirk, P.; Ibarguren, I.O.; Garibay-Orijel, R.; Norvell, L.; Huhtinen, S.; Kytövuori, I.; Ruotsalainen, J. Identifying and naming the currently known diversity of the genus Hydnum, with an emphasis on European and North American taxa. Mycologia 2018, 110, 890–918. [Google Scholar] [CrossRef]
- Swenie, R.A.; Baroni, T.J.; Matheny, P.B. Six new species and reports of Hydnum (Cantharellales) from eastern North America. MycoKeys 2018, 42, 35–72. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.; Stuntz, D. Pileate hydnaceae of the puget sound area. I. White-spored genera: Auriscalpium, Hericium, Dentinum and Phellodon. Mycologia 1971, 63, 1099–1128. [Google Scholar] [CrossRef]
- Lv, Z.; Liu, X.; Jia, B.; Wang, H.; Wu, Y.; Lu, Z. Development of a novel high-entropy alloy with eminent efficiency of degrading azo dye solutions. Sci. Rep. 2016, 6, 34213. [Google Scholar] [CrossRef]
- Hibbett, D.S.; Bauer, R.; Binder, M.; Giachini, A.; Hosaka, K.; Justo, A.; Larsson, E.; Larsson, K.; Lawrey, J.D.; Miettinen, O. 14 Agaricomycetes. In Systematics and Evolution: Part A; Springer: Berlin/Heidelberg, Germany, 2014; pp. 373–429. [Google Scholar] [CrossRef]
- Cao, T.; Hu, Y.; Yu, J.; Wei, T.; Yuan, H. A phylogenetic overview of the Hydnaceae (Cantharellales, Basidiomycota) with new taxa from China. Stud. Mycol. 2021, 99, 100121. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, R.; Maekawa, N.; Sotome, K.; Nakagiri, A.; Endo, N. Systematic revision of Hydnum species in Japan. Mycologia 2022, 114, 413–452. [Google Scholar] [CrossRef]
- Olariaga, I.; Grebenc, T.; Salcedo, I.; Martín, M.P. Two new species of Hydnum with ovoid basidiospores: H. ovoideisporum and H. vesterholtii. Mycologia 2012, 104, 1443–1455. [Google Scholar] [CrossRef]
- Ostrow, H.; Beenken, L. Hydnum ellipsosporum spec. nov. (Basidiomycetes, Cantharellales)–ein Doppelgänger von Hydnum rufescens Fr. Z. Fuer Mykol. 2004, 70, 137–156. [Google Scholar]
- Banker, H.J. A contribution to a revision of the North American Hydnaceae. Mem. Torrey Bot. Club 1906, 12, 99–194. [Google Scholar] [CrossRef][Green Version]
- Huhtinen, S.; Ruotsalainen, J. Variability of Hydnum rufescens in Finland: Three taxa hidden under one name–and appearance. Karstenia 2006, 46, 17–24. [Google Scholar] [CrossRef]
- Feng, B.; Wang, X.; Ratkowsky, D.; Gates, G.; Lee, S.S.; Grebenc, T.; Yang, Z.L. Multilocus phylogenetic analyses reveal unexpected abundant diversity and significant disjunct distribution pattern of the Hedgehog Mushrooms (Hydnum L.). Sci. Rep. 2016, 6, 25586. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Liao, Y.; Zhang, Y.; Lin, W.; Yang, X.; Zeng, N. A Contribution to the Knowledge of Hydnum (Hydnaceae, Cantharellales) in China, Introducing a New Taxon and Amending Descriptions of Five Known Species. Diversity 2024, 16, 166. [Google Scholar] [CrossRef]
- Grebenc, T.; Martín, M.P.; Kraigher, H. Ribosomal ITS diversity among the European species of the genus “Hydnum” (Hydnaceae). In Anales del Jardín Botánico de Madrid; Real Jardín Botánico: Madrid, España, 2009; pp. 121–132. [Google Scholar]
- Vizzini, A.; Picillo, B.; Ercole, E.; Voyron, S.; Contu, M. Detecting the variability of Hydnum ovoideisporum (Agaricomycetes, Cantharellales) on the basis of Italian collections, and H. magnorufescens sp. nov. Mycosphere 2013, 4, 32–44. [Google Scholar] [CrossRef]
- Baroni, T.J.; Swenie, R.A.; Lacey, L.; Ovrebo, C.L.; Cifuentes, J.; Corrales, A.; Smith, M.E.; Lodge, D.J. Hydnum (Cantharellales) of the neotropics: Four new species and new reports from Belize, Costa Rica, Dominican Republic, Mexico, and Panama. Mycol. Prog. 2025, 24, 13. [Google Scholar] [CrossRef]
- Márquez-Sanz, R.; Gorjón, S.P.; Salcedo, I.; Olariaga, I. Hydnum pallidum Raddi, the Correct Name for H. albidum Peck in the Sense of European Authors and the Recently Described H. reginae Kibby, Liimat. & Niskanen. J. Fungi 2023, 9, 1141. [Google Scholar] [CrossRef]
- Ying, J.; Zang, M. Economic Macrofungi from Southwestern China; Science Press: Beijing, China, 1994; pp. 1–398. [Google Scholar]
- Zang, M.; Li, B.; Xi, J.; Zhang, D. Fungi of Hengduan mountains; Science Press: Beijing, China, 1996. [Google Scholar]
- Zhang, M.; Wang, C.; Buyck, B.; Deng, W.; Li, T. Multigene phylogeny and morphology reveal unexpectedly high number of new species of Cantharellus subgenus Parvocantharellus (Hydnaceae, Cantharellales) in China. J. Fungi 2021, 7, 919. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Bai, H.; Deng, W. A Contribution to the Phylogeny and Taxonomy of Hydnum (Cantharellales, Basidiomycota) from China. J. Fungi 2024, 10, 98. [Google Scholar] [CrossRef]
- Su, L.; Yu, T.; Xue, R.; Zhang, W.; Xu, C.; Xia, X.; Li, J.; Lei, H.; Dong, Y.; Zhang, G. New Contributions on Species Diversity of Genus Hydnum and Lentaria sl in China. J. Fungi 2024, 10, 824. [Google Scholar] [CrossRef]
- McNabb, R.F.R. Some new and revised taxa of New Zealand Basidiomycetes (Fungi). New Zealand J. Bot. 1971, 9, 355–370. [Google Scholar] [CrossRef]
- Xie, Y.; MacKinnon, J.; Li, D. Study on biogeographical divisions of China. Biodivers. Conserv. 2004, 13, 1391–1417. [Google Scholar] [CrossRef]
- Xu, B.; Pan, J. Spatial distribution characteristics of national protected areas in China. J. Geogr. Sci. 2019, 29, 2047–2068. [Google Scholar] [CrossRef]
- Khaund, P.; Joshi, S. Micromorphological characterization of wild edible mushroom spores using scanning electron microscopy. Natl. Acad. Sci. Lett. 2014, 37, 521–527. [Google Scholar] [CrossRef]
- Read, N.D.; Lord, K.M. Examination of living fungal spores by scanning electron microscopy. Exp. Mycol. 1991, 15, 132–139. [Google Scholar] [CrossRef]
- Umroong, P. Techniques for Preparing Spores and Hyphae of Schizophyllum commune for Morphological Observation. Microsc. Microanal. Res.–J. Microsc. Soc. Thail. 2020, 33, 22–27. [Google Scholar] [CrossRef]
- Kornerup, A.; Wanscher, J.H. Taschenlexikon der Farben: 1440 Farbnuancen und 600 Farbnamen; Musterschmidt: Göttingen, Germany, 1975. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Xiang, C.; Gao, F.; Jakovlić, I.; Lei, H.P.; Hu, Y.; Zhang, H.; Zou, H.; Wang, G.; Zhang, D. Using PhyloSuite for molecular phylogeny and tree-based analyses. Imeta 2023, 2, e87. [Google Scholar] [CrossRef]
- Justo, A.; Hood, A.W.; Swenie, R.A.; Matheny, P.B. Hydnum atlanticum, a new species from Eastern North America. Fungal Syst. Evol. 2023, 11, 63–70. [Google Scholar] [CrossRef]
- Wang, X.-H.; Das, K.; Horman, J.; Antonin, V.; Baghela, A.; Chakraborty, D.; Hembrom, M.E.; Nakasone, K.; Ortiz-Santana, B.; Vizzini, A. Fungal biodiversity profiles 51–60. Cryptogam. Mycol. 2018, 39, 211–257. [Google Scholar] [CrossRef]
- Baird, R.; Wallace, L.E.; Baker, G.; Scruggs, M. Stipitate hydnoid fungi of the temperate southeastern United States. Fungal Divers. 2013, 62, 41–114. [Google Scholar] [CrossRef]
- Moeller, H.V.; Peay, K.G.; Fukami, T. Ectomycorrhizal fungal traits reflect environmental conditions along a coastal California edaphic gradient. FEMS Microbiol. Ecol. 2014, 87, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, W.; Kim, C.; Park, H.; Kim, C.S.; Lim, Y.W. Unveiling the Diversity of Hydnum in the Republic of Korea with One New Species, Hydnum paucispinum. Mycobiology 2023, 51, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Yanaga, K.; Sotome, K.; Ushijima, S.; Maekawa, N. Hydnum species producing whitish basidiomata in Japan. Mycoscience 2015, 56, 434–442. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Larsson, E.; Kõljalg, U. Fruiting body-guided molecular identification of root-tip mantle mycelia provides strong indications of ectomycorrhizal associations in two species of Sistotrema (Basidiomycota). Mycol. Res. 2006, 110, 1426–1432. [Google Scholar] [CrossRef]
- Ovon Ludwig Freiherrn, Hydnum Schiedermayeri Hflr: Ein neues Hydnum aus Oberösterreich. Österreichische botanische Zeitschrift. 1870, 20, 33–38.
- Lee, S.S.; Watling, R.; Sikin, Y.N. Fructification des basidiorcarpes ectomycorhiziens dans les forêts ombrophiles de faible altitude de la Malaisie péninsulaire. Bois For. Des Trop. 2002, 274, 33–43. [Google Scholar] [CrossRef]
- Arnolds, E. The fate of hydnoid fungi in The Netherlands and Northwestern Europe. Fungal Ecol. 2010, 3, 81–88. [Google Scholar] [CrossRef]
- Morera, A.; LeBlanc, H.; de Aragón, J.M.; Bonet, J.A.; de-Miguel, S. Analysis of climate change impacts on the biogeographical patterns of species-specific productivity of socioeconomically important edible fungi in Mediterranean forest ecosystems. Ecol. Inform. 2024, 81, 102557. [Google Scholar] [CrossRef]
- He, X.; Peng, W.; Wang, D. An Illustration of Important Wild Edible Fungi in Sichuan; Science Press: Beijing, China, 2021; pp. 1–206. [Google Scholar]
- Yang, Z.-L.; Wang, X.-H.; Wu, G. Mushrooms of Yunnan; Science Press: Beijing, China, 2022; pp. 1–378. [Google Scholar]
- Nixon, K.C. Global and neotropical distribution and diversity of oak (genus Quercus) and oak forests. In Ecology and Conservation of Neotropical Montane Oak Forests; Springer: Berlin/Heidelberg, Germany, 2006; pp. 3–13. [Google Scholar]
- Sun, S.; Zhang, Y.; Huang, D.; Wang, H.; Cao, Q.; Fan, P.; Yang, N.; Zheng, P.; Wang, R. The effect of climate change on the richness distribution pattern of oaks (Quercus L.) in China. Sci. Total Environ. 2020, 744, 140786. [Google Scholar] [CrossRef]
- Shen, Z.H.; Fang, J.Y.; Chiu, C.A.; Chen, T.Y. The geographical distribution and differentiation of Chinese beech forests and the association with Quercus. Appl. Veg. Sci. 2015, 18, 23–33. [Google Scholar] [CrossRef]
- Taniguchi, T.; Kitajima, K.; Douhan, G.W.; Yamanaka, N.; Allen, M.F. A pulse of summer precipitation after the dry season triggers changes in ectomycorrhizal formation, diversity, and community composition in a Mediterranean forest in California, USA. Mycorrhiza 2018, 28, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, D.Q.; Zhao, Q.; Zhou, T.X.; Hyde, K.D. Diversity and ecological distribution of macrofungi in the Laojun Mountain region, southwestern China. Biodivers. Conserv. 2010, 19, 3545–3563. [Google Scholar] [CrossRef]
- Setlow, P. Spore germination. Curr. Opin. Microbiol. 2003, 6, 550–556. [Google Scholar] [CrossRef]
- Ayerst, G. The effects of moisture and temperature on growth and spore germination in some fungi. J. Stored Prod. Res. 1969, 5, 127–141. [Google Scholar] [CrossRef]
- Hibbett, D.S.; Pine, E.M.; Langer, E.; Langer, G.; Donoghue, M.J. Evolution of gilled mushrooms and puffballs inferred from ribosomal DNA sequences. Proc. Natl. Acad. Sci. USA 1997, 94, 12002–12006. [Google Scholar] [CrossRef]




| Species | Specimen Voucher | GenBank No. | Country | References | ||
|---|---|---|---|---|---|---|
| nrLSU | ITS | tef1 | ||||
| H. albertense | H T. Niskanen 11-354 | - | KX388664 | - | Canada | [1] |
| H. albomagnum | AFTOL-ID 471 | AY700199 | DQ218305 | DQ234568 | USA | [2] |
| H. albomagnum | RAS231 | - | MH379943 | - | USA | [2] |
| H. albomarginatum | FJAU66574 | PV356813 | PV329855 | PP357262 | China | This study |
| H. albomarginatum | FJAU66575 | PV356814 | PV329856 | PP357263 | China | This study |
| H. atlanticum | AJ1597 | - | OQ235218 | OQ236553 | Canada | [34] |
| H. atlanticum | AJ1558 | - | OQ235214 | OQ236551 | Canada | [34] |
| H. berkeleyanum | CAL 1656 | NG070500 | NR158533 | - | India | [35] |
| H. berkeleyanum | HKAS77834 | KU612667 | KU612525 | - | China | [12] |
| H. bifurcatum | FJAU66562 | - | PV329845 | PP357252 | China | This study |
| H. bifurcatum | FJAU66563 | - | PV329846 | PP357253 | China | This study |
| H. boreorepandum | H T. Niemela 1679 | - | KX388658 | - | Finland | [1] |
| H. boreorepandum | H 6003711 | - | KX388657 | - | Finland | [1] |
| H. brevispinum | IFP 019464 | MW979559 | MW980578 | - | China | [6] |
| H. brevispinum | IFP 019465 | MW979560 | MW980579 | - | China | [6] |
| H. canadense | H T N 09-006 | - | KX388681 | - | Canada | [1] |
| H. crassipedum | FJAU66572 | PV356811 | PV329853 | PP357260 | China | This study |
| H. crassipedum | FJAU66573 | PV356812 | PV329854 | PP357261 | China | This study |
| H. cremeoalbum | TUMH40462 | - | AB906674 | - | Japan | [1] |
| H. cremeoalbum | TUMH60740 | - | AB906678 | - | Japan | [1] |
| H. cuspidatum | RAS246 | - | MH379944 | - | USA | [2] |
| H. cuspidatum | RAS205 | - | MH379936 | - | USA | [2] |
| H. ellipsosporum | FD3281 | KX086217 | KX086215 | - | Switzerland | [9] |
| H. ellipsosporum | H T. Niskanen 12-036 | - | KX388671 | - | Finland | [1] |
| H. ferruginescens | MH16005 | - | MH379905 | - | USA | [2] |
| H. ferruginescens | RAS229 | - | MH379942 | - | USA | [2] |
| H. flabellatum | IFP 019459 | MW979556 | MW980575 | - | China | [6] |
| H. fulvostriatum | FJAU66566 | PV356807 | PV329849 | - | China | This study |
| H. fulvostriatum | FJAU66567 | PV356808 | PV329850 | - | China | This study |
| H. ibericum | BIO:Fungi:12330 | - | HE611086 | - | Spain | [8] |
| H. ibericum | MA-fungi 3457 | - | AJ547879 | - | Spain | [14] |
| H. jussii | IFP 019485 | MW979539 | MW980553 | MW999436 | China | [6] |
| H. jussii | IFP 019486 | MW979540 | MW980554 | MW999437 | China | [6] |
| H. magnorufescens | voucher 161209 | KU612669 | KU612549 | - | Slovenia | [12] |
| H. magnorufescens | TO HG2818 | - | KC293545 | - | Italy | [15] |
| H. melitosarx | H T. Niskanen 11-056 | - | KX388683 | - | USA | [1] |
| H. melitosarx | K 176869 | - | KX388685 | - | UK | [1] |
| H. melleopallidum | SMI356 | - | FJ845406 | - | Canada | [15] |
| H. mulsicolor | LJU GIS 1336 | - | AJ547885 | - | Slovenia | [14] |
| H. mulsicolor | REB-341 | - | JX093560 | - | USA | [36] |
| H. neorepandum | H T. Niskanen 10-095 | - | KX388659 | - | Canada | [1] |
| H. neorepandum | H T. Niskanen 10-086 | - | KX388660 | - | Canada | [1] |
| H. olympicum | H T. Niskanen 09-134 | - | KX388661 | - | USA | [1] |
| H. olympicum | SAT-10-208-05 | - | MT955159 | - | USA | [6] |
| H. oregonense | HVM61 | - | KF879509 | - | USA | [37] |
| H. oregonense | PNW-MS g2010502h1-09 | - | AJ534972 | - | USA | [14] |
| H. orientalbidum | FJAU66570 | PV356809 | PV329857 | PP357258 | China | This study |
| H. orientalbidum | FJAU66571 | PV356810 | PV329858 | PP357259 | China | This study |
| H. orientalbidum | TUMH:64068 | - | LC621862 | - | Japan | [7] |
| H. ovoideisporum | voucher71106 | - | KU612536 | - | Slovenia | [6] |
| H. ovoideisporum | BIO Fungi 12683 | - | NR119818 | - | Spain | [8] |
| H. pallidocroceum | IFP 019466 | MW979554 | MW980568 | MW999449 | China | [6] |
| H. pallidocroceum | IFP 019467 | MW979555 | MW980569 | MW999450 | China | [6] |
| H. pallidomarginatum | IFP 019468 | MW979552 | MW980566 | MW999447 | China | [6] |
| H. pallidomarginatum | IFP 019469 | MW979553 | MW980567 | MW999448 | China | [6] |
| H. pinicola | SFC20180928-18 | OR211401 | OR211383 | OR220059 | Korea | [38] |
| H. quebecense | H T. Niskanen 10-064 | - | KX388662 | - | Canada | [1] |
| H. quebecense | CN9 | - | MH379881 | - | USA | [2] |
| H. repando-orientale | TUMH60745 | - | AB906683 | - | Japan | [39] |
| H. repando-orientale | TUMH60743 | - | AB906684 | - | Japan | [39] |
| H. repandum | H 6003710 | - | NR164553 | - | Finland | [1] |
| H. roseotangerinum | MHKMU LP Tang 3458 | PQ287756 | PQ287675 | PQ295849 | China | [22] |
| H. roseotangerinum | MHKMU LP Tang 3458-1 | PQ287757 | PQ287676 | PQ295850 | China | [22] |
| H. rufescens | H 6003708 | - | KX388688 | - | Finland | [1] |
| H. rufescens | HTN7839 | - | KX388656 | - | Estonia | [1] |
| H. slovenicum | LJU GIS 1338 | - | AJ547870 | - | Slovenia | [14] |
| H. slovenicum | LJU GIS 1340 | - | AJ547884 | - | Slovenia | [14] |
| H. sp. | wi1A4spel | - | KC679833 | - | China | [6] |
| H. sp. | wi8T4spel | - | KC679834 | - | China | [6] |
| H. sp.13 | HKAS57714 | KU612673 | KU612617 | - | China | [12] |
| H. sp.13 | HKAS58838 | KU612675 | KU612616 | - | China | [12] |
| H. sp.2 | HKAS92340 | KU612661 | KU612543 | - | China | [12] |
| H. sphaericum | IFP 019470 | MW979549 | MW980563 | MW999444 | China | [6] |
| H. sphaericum | IFP 019472 | MW979550 | MW980564 | MW999445 | China | [6] |
| H. sphaericum | IFP 019471 | MW979551 | MW980565 | MW999446 | China | [6] |
| H. subberkeleyanum | TNS:F-19323 | - | LC621879 | - | Japan | [7] |
| H. subberkeleyanum | TUMH 63627 | - | LC621880 | LC622505 | Japan | [7] |
| H. subconnatum | RAS235 | - | MH379930 | - | USA | [2] |
| H. subconnatum | RAS169 | - | MH379916 | - | USA | [2] |
| H. submulsicolor | H T. Niskanen 10-132 | - | KX388682 | - | Canada | [1] |
| H. subolympicum | F1188765 | KU612653 | KU612599 | - | USA | [12] |
| H. subolympicum | DAOM744368 | - | MH174257 | - | Canada | [1] |
| H. subovoideisporum | H 6003707 | - | NR158494 | - | Finland | [1] |
| H. subrufescens | H T. Niskanen 10-154 | - | KX388649 | - | Canada | [1] |
| H. subrufescens | F1188749 | KU612663 | KU612535 | - | USA | [12] |
| H. subtilior | RAS180 | - | MH379918 | - | USA | [2] |
| H. subtilior | TENN 073034 | - | NR164029 | - | USA | [2] |
| H. tenuistipitum | IFP 019476 | MW979557 | MW980576 | - | China | [6] |
| H. tenuistipitum | IFP 019477 | MW979558 | MW980577 | - | China | [6] |
| H. tomaense | TUMH64085 | - | LC622508 | - | Japan | [7] |
| H. tottoriense | TUMH64088 | - | LC621887 | LC622511 | Japan | [7] |
| H. tottoriense | TUMH64089 | - | LC621888 | LC622512 | Japan | [7] |
| H. treui | KA20-0732 | ON907772 | ON907793 | OR220061 | Korea | [1] |
| H. vagabundum | 10782TJB | - | MH379949 | - | USA | [2] |
| H. vagabundum | CLO4985 | - | MH379909 | - | USA | [2] |
| H. ventricosum | IFP 019478 | MW979547 | MW980561 | MW999442 | China | [6] |
| H. ventricosum | IFP 019479 | MW979548 | MW980562 | MW999443 | China | [6] |
| H. vesterholtii | BIO:Fungi:10429 | - | HE611084 | - | Spain | [8] |
| H. vesterholtii | BIO:Fungi:10452 | - | HE611085 | - | Spain | [8] |
| H. washingtonianum | UBC F-32538 | - | MF954990 | - | Canada | [6] |
| H. zongolicense | GO-2010-142a | - | KC152121 | - | Mexico | [1] |
| Sistotrema. muscicola | KHL 11721 | - | AJ606040 | - | Sweden | [40] |
| Sistotrema. muscicola | taxon:154757 | - | AJ606041 | - | Sweden | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuo, Y.-L.; Wang, L.; Li, X.-F.; Chu, H.; Liu, M.; Hu, J.; Qi, Z.-X.; Li, X.; Li, Y.; Zhang, B. New Contributions to the Species Diversity of the Genus Hydnum (Hydnaceae, Cantharellales) in China: Four New Taxa and Newly Recorded Species. J. Fungi 2025, 11, 431. https://doi.org/10.3390/jof11060431
Tuo Y-L, Wang L, Li X-F, Chu H, Liu M, Hu J, Qi Z-X, Li X, Li Y, Zhang B. New Contributions to the Species Diversity of the Genus Hydnum (Hydnaceae, Cantharellales) in China: Four New Taxa and Newly Recorded Species. Journal of Fungi. 2025; 11(6):431. https://doi.org/10.3390/jof11060431
Chicago/Turabian StyleTuo, Yong-Lan, Libo Wang, Xue-Fei Li, Hang Chu, Minghao Liu, Jiajun Hu, Zheng-Xiang Qi, Xiao Li, Yu Li, and Bo Zhang. 2025. "New Contributions to the Species Diversity of the Genus Hydnum (Hydnaceae, Cantharellales) in China: Four New Taxa and Newly Recorded Species" Journal of Fungi 11, no. 6: 431. https://doi.org/10.3390/jof11060431
APA StyleTuo, Y.-L., Wang, L., Li, X.-F., Chu, H., Liu, M., Hu, J., Qi, Z.-X., Li, X., Li, Y., & Zhang, B. (2025). New Contributions to the Species Diversity of the Genus Hydnum (Hydnaceae, Cantharellales) in China: Four New Taxa and Newly Recorded Species. Journal of Fungi, 11(6), 431. https://doi.org/10.3390/jof11060431






