UvPomt, an O-Methyltransferase Interacting with UvMAT1-1-3, for Regulating Growth, Stress Tolerance, and Virulence in Ustilaginoidea virens †
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Phylogenetic Analysis of Homologous Proteins
2.3. Construction of UvPomt Knockout and Complementation Strains
2.4. Phenotypic Analysis of U. virens Strains
2.5. Pathogenicity and Plant Infection Assays
2.6. RNA Manipulations and qRT-PCR
2.7. Yeast Two-Hybrid Assay
2.8. Western Blotting Assay
2.9. Statistical Analysis
3. Results
3.1. Identification of UvPomt in U. virens by Y2H
3.2. UvPomt Is Essential for U. virens Virulence
3.3. UvPomt Is Involved in Hyphae and Conidiation Capacity
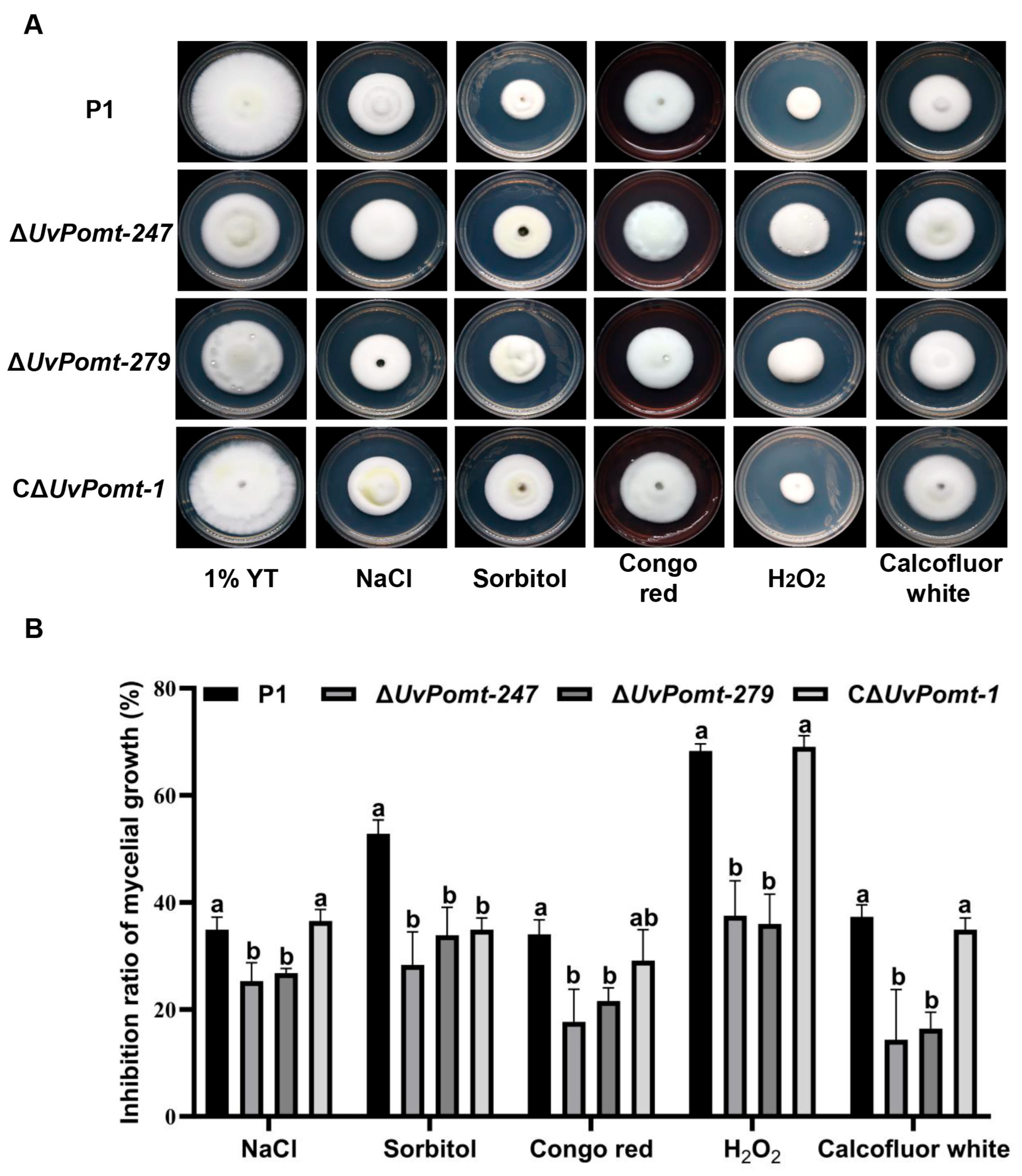
3.4. UvPomt’s Role in Response to Environmental Stresses
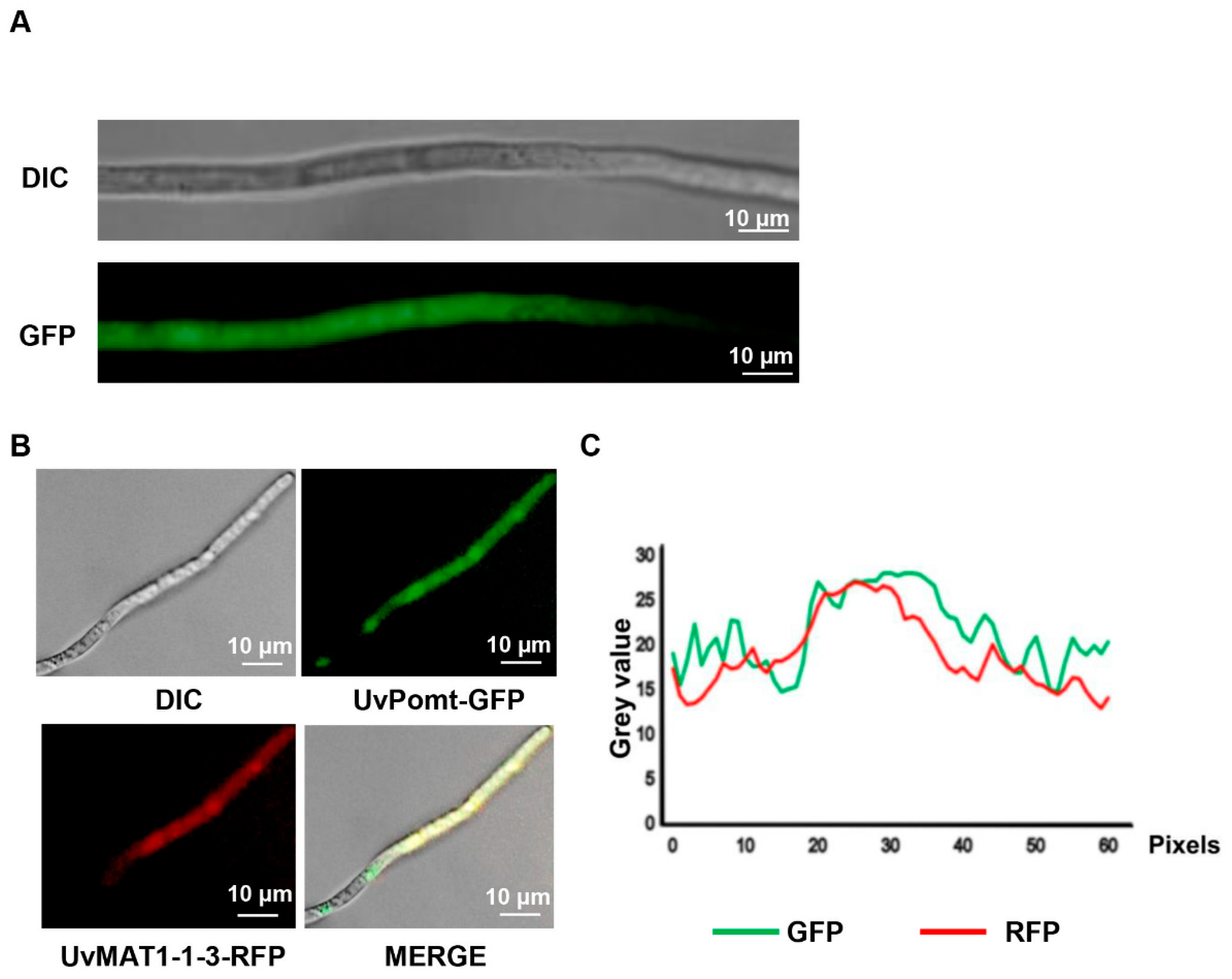
3.5. UvPomt Affects the Protein Expression Level of UvMAT1-1-3 in U. virens
3.6. UvPomt Affects On the Gene Expression level of Pathogenicity and Sexual Reproduction-Related Genes in U. virens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, W.X.; Fan, J.; Fang, A.F.; Li, Y.J.; Tariqjaveed, M.; Li, D.Y.; Hu, D.W.; Wang, W.M. Ustilaginoidea virens: Insights into an emerging rice pathogen. Annu. Rev. Phytopathol. 2020, 58, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Wang, M.; Liu, Z.H.; Zhang, K.; Cui, F.H. Towards understanding the biosynthetic pathway for Ustilaginoidea mycotoxins in Ustilaginoidea virens. Environ. Microbiol. 2020, 22, 2629–2643. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.Y.; Wang, P.Y.; Yu, Q.H.; Wang, B.; Li, D.Y.; Yang, C.; Liu, L.; Duan, G.H.; Sun, W.X. The histone deacetylase UvHOS2 regulates vegetative growth, conidiation, ustilaginoidin synthesis, and pathogenicity in Ustilaginoidea virens. Phytopathol. Res. 2024, 6, 11. [Google Scholar] [CrossRef]
- Liu, X.Y.; Matsumoto, H.; Lv, T.X.; Zhan, C.F.; Fang, H.D.; Pan, Q.Q.; Xu, H.R.; Fan, X.Y.; Chu, T.Y.; Chen, S.L.; et al. Phyllosphere microbiome induces host metabolic defence against rice false-smut disease. Nat. Microbiol. 2023, 8, 1419–1433. [Google Scholar] [CrossRef]
- Song, J.H.; Wei, W.; Lv, B.; Lin, Y.; Yin, W.X.; Peng, Y.L.; Schnabel, G.; Huang, J.B.; Jiang, D.H.; Luo, C.X. Rice false smut fungus hijacks the rice nutrient supply by blocking and mimicking the fertilization of rice ovary. Environ. Microbiol. 2016, 18, 3840–3849. [Google Scholar] [CrossRef]
- Deng, Q.D. The Function of Sclerotia in the Life History of Rice False Smut Pathogen. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2015. [Google Scholar]
- Whittle, C.A.; Nygren, K.; Johannesson, H. Consequences of reproductive mode on genome evolution in fungi. Fungal Genet. Biol. 2011, 48, 661–667. [Google Scholar] [CrossRef]
- Pyrzak, W.; Miller, K.Y.; Miller, B.L. Mating type protein Mat1-2 from asexual Aspergillus fumigatus drives sexual reproduction in fertile Aspergillus nidulans. Eukaryot. Cell 2008, 7, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; Li, Y.; Chen, D.P.; Qi, Z.M.; Wang, Q.H.; Wang, J.H.; Jiang, C.; Xu, J.R. A-to-I RNA editing is developmentally regulated and generally adaptive for sexual reproduction in Neurospora crassa. Proc. Natl. Acad. Sci. USA 2017, 114, E7756–E7765. [Google Scholar] [CrossRef]
- Svedberg, J.; Nygren, K.; Menkis, A.; James, T.Y.; Wik, L.; Stajich, J.E.; Johannesson, H. Conflict between reproductive gene trees and species phylogeny among heterothallic and pseudohomothallic members of the filamentous ascomycete genus Neurospora. Fungal Genet. Biol. 2010, 47, 869–878. [Google Scholar] [CrossRef]
- Nolting, N.; Pöggele, S.A. MADS box protein interacts with a mating-type protein and is required for fruiting body development in the homothallic ascomycete Sordaria macrospora. Eukaryot. Cell 2006, 5, 1043–1056. [Google Scholar] [CrossRef]
- Elías-Villalobos, A.; Fernández-Álvarez, A.; Moreno-Sánchez, I.; Helmlinger, D.; Ibeas, J.I. The Hos2 histone deacetylase controls Ustilago maydis virulence through direct regulation of mating-type genes. PLoS Pathog. 2015, 11, e1005134. [Google Scholar] [CrossRef]
- Yu, J.J.; Sun, W.X.; Yu, M.N.; Yin, X.L.; Meng, X.K.; Zhao, J.; Huang, L.; Liu, Y.F. Characterization of mating-type loci in rice false smut fungus Villosiclava virens. FEMS Microbiol. Lett. 2015, 362, fnv014. [Google Scholar] [CrossRef]
- Yong, M.L.; Yu, J.J.; Pan, X.Y.; Yu, M.N.; Cao, H.J.; Song, T.Q.; Qi, Z.Q.; Du, Y.; Zhang, R.S.; Yin, X.L.; et al. Two mating-type genes MAT1-1-1 and MAT1-1-2 with significant functions in conidiation, stress response, sexual development, and pathogenicity of rice false smut fungus Villosiclava virens. Curr. Genet. 2020, 66, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Yong, M.L.; Yu, J.J.; Pan, X.Y.; Yu, M.N.; Cao, H.J.; Qi, Z.Q.; Du, Y.; Zhang, R.S.; Song, T.Q.; Yin, X.L.; et al. MAT1-1-3, a mating type gene in Villosiclava virens, is required for fruiting bodies and sclerotia formation, asexual development and pathogenicity. Front. Microbiol. 2020, 11, 1337. [Google Scholar] [CrossRef]
- Yang, W.J.; Du, Y.T.; Zhou, Y.B.; Chen, J.; Xu, Z.S.; Ma, Y.Z.; Chen, M.; Min, D.H. Overexpression of TaCOMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis. Int. J. Mol. Sci. 2019, 20, 652. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Cloning and Functional Analysis of Caffeine-Induced LrCOMT Gene by Botrytis cinerea in Lily of the Minjiang River. Master’s Thesis, Chongqing Three Gorges University, Chongqing, China, 2024. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.Q.; Fu, J.F.; Zhang, Q.; Ma, C.Y.; Li, Y.Z.; Zhang, L.; Wang, G.G. Genome-wide identification and functional analysis of caffeic acid from maize. Fujian J. Agric. Sci. 2023, 11, 1259–1266. [Google Scholar] [CrossRef]
- Ou, P.P.; He, Q.L.; Zhao, Q. Structural diversification of natural substrates modified by the AurJ from Fusarium graminearum. Biochem. Biophys. Res. Commun. 2023, 678, 158–164. [Google Scholar] [CrossRef]
- Xue, M.Y.; Hou, X.W.; Gu, G.; Dong, J.; Yang, Y.L.; Pan, X.Q.; Zhang, X.; Xu, D.; Lai, D.W.; Zhou, L.G. Activation of ustilaginoidin biosynthesis Gene uvpks1 in Villosiclava virens albino strain LN02 influences development, stress responses, and inhibition of rice seed germination. J. Fungi 2024, 10, 31. [Google Scholar] [CrossRef]
- Wang, B.; Duan, G.; Liu, L.; Long, Z.; Bai, X.; Ou, M.M.; Wang, P.Y.; Jiang, D.; Li, D.Y.; Sun, W.X. UvHOS3-mediated histone deacetylation is essential for virulence and negatively regulates ustilaginoidin biosynthesis in Ustilaginoidea virens. Mol. Plant Pathol. 2024, 25, e13429. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, B.; Duan, G.H.; Wang, J.; Pan, Z.Q.; Ou, M.M.; Bai, X.L.; Wang, P.Y.; Zhao, D.; Nan, N.; et al. Histone deacetylase UvHST2 is a global regulator of secondary metabolism in Ustilaginoidea virens. J. Agric. Food Chem. 2023, 71, 13124–13136. [Google Scholar] [CrossRef]
- Qu, J.S.; Wang, Y.F.; Xia, M.Z.; Liu, Y.R.; Gu, L.F.; Zhou, P.; Du, Y.L.; Xu, C.H.; Wang, R.; Yin, W.X.; et al. The bZIP transcription factor UvbZIP6 mediates fungal growth, stress response, and false smut formation in Ustilaginoidea virens. Phytopathol. Res. 2022, 4, 32. [Google Scholar] [CrossRef]
- Xu, Y.D.; Wu, S.; Yu, Z.M.; Moeketsi, E.K.; Yang, Z.X.; Zhang, Z.G.; Zhang, H.F. Transcription factor UvMsn2 is important for vegetative growth, conidiogenesis, stress response, mitochondrial morphology, and pathogenicity in the rice false smut fungus Ustilaginoidea virens. Phytopathol. Res. 2021, 3, 16. [Google Scholar] [CrossRef]
- Chen, X.Y.; Li, P.P.; Liu, H.; Chen, X.L.; Huang, J.B.; Luo, C.X.; Li, G.T.; Hsiang, T.; Collinge, D.B.; Zheng, L. A novel transcription factor UvCGBP1 regulates development and virulence of rice false smut fungus Ustilaginoidea virens. Virulence 2021, 12, 1563–1579. [Google Scholar] [CrossRef]
- Zhao, J. Research on the Mating-Type Gene Expression Characteristics of Ustilaginoidea virens and Preliminary Study on MAT1-1-3 Interacting Proteins. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2015. [Google Scholar]
- Gu, Z.M.; Li, L.Z.; Yin, H.X. Genome-wide identification and expression analysis of caffeic acid COMT gene family in Capsicum. J. Plant Physiol. 2024, 12, 1810–1822. [Google Scholar] [CrossRef]
- Chen, X.Y.; Hai, D.; Tang, J.T.; Liu, H.; Huang, J.B.; Luo, C.X.; Hsiang, T.; Zheng, L. UvCom1 is an important regulator required for development and infection in the rice false smut fungus Ustilaginoidea virens. Phytopathology 2020, 110, 483–493. [Google Scholar] [CrossRef]
- Yang, Q.; He, Y.J.; Kabahuma, M.; Chaya, T.; Kelly, A.; Borrego, E.; Bian, Y.; Kasmi, F.E.; Yang, L.; Teixeira, P.; et al. A gene encoding maize caffeoyl-CoA confers quantitative resistance to multiple pathogens. Nat. Genet. 2017, 49, 1364–1372. [Google Scholar] [CrossRef]
- Li, H.M.; Mo, P.C.; Zhang, J.; Xie, Z.E.; Liu, X.Y.; Chen, H.; Yang, L.Y.; Liu, M.X.; Zhang, H.F.; Wang, P.; et al. Methionine biosynthesis enzyme MoMet2 is required for rice blast fungus pathogenicity by promoting virulence gene expression via reducing 5mC modification. PLoS Genet. 2023, 19, e1010943. [Google Scholar] [CrossRef]
- Xu, H.J.; Ye, M.; Xia, A.L.; Jiang, H.; Huang, P.P.; Liu, H.Q.; Hou, R.; Wang, Q.H.; Li, D.G.; Xu, J.R.; et al. The Fng3 ING protein regulates H3 acetylation and H4 deacetylation by interacting with two distinct histone-modifying complexes. New Phytol. 2023, 239, 807–809. [Google Scholar] [CrossRef]
- Mayrhofer, S.; Weber, J.M.; Pöggeler, S. Pheromones and pheromone receptors are required for proper sexual development in the homothallic ascomycete Sordaria macrospora. Genetics 2006, 172, 1521–1533. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yu, J.; Yu, M.; Cao, H.; Song, T.; Wang, S.; Qi, Z.; Du, Y.; Pan, X.; Liu, Y. UvPomt, an O-Methyltransferase Interacting with UvMAT1-1-3, for Regulating Growth, Stress Tolerance, and Virulence in Ustilaginoidea virens. J. Fungi 2025, 11, 426. https://doi.org/10.3390/jof11060426
Li Z, Yu J, Yu M, Cao H, Song T, Wang S, Qi Z, Du Y, Pan X, Liu Y. UvPomt, an O-Methyltransferase Interacting with UvMAT1-1-3, for Regulating Growth, Stress Tolerance, and Virulence in Ustilaginoidea virens. Journal of Fungi. 2025; 11(6):426. https://doi.org/10.3390/jof11060426
Chicago/Turabian StyleLi, Zhi, Junjie Yu, Mina Yu, Huijuan Cao, Tianqiao Song, Shuchen Wang, Zhongqiang Qi, Yan Du, Xiayan Pan, and Yongfeng Liu. 2025. "UvPomt, an O-Methyltransferase Interacting with UvMAT1-1-3, for Regulating Growth, Stress Tolerance, and Virulence in Ustilaginoidea virens" Journal of Fungi 11, no. 6: 426. https://doi.org/10.3390/jof11060426
APA StyleLi, Z., Yu, J., Yu, M., Cao, H., Song, T., Wang, S., Qi, Z., Du, Y., Pan, X., & Liu, Y. (2025). UvPomt, an O-Methyltransferase Interacting with UvMAT1-1-3, for Regulating Growth, Stress Tolerance, and Virulence in Ustilaginoidea virens. Journal of Fungi, 11(6), 426. https://doi.org/10.3390/jof11060426







