Pine Forest Plantations in the Neotropics: Challenges and Potential Use of Ectomycorrhizal Fungi and Bacteria as Inoculants
Abstract
1. Introduction
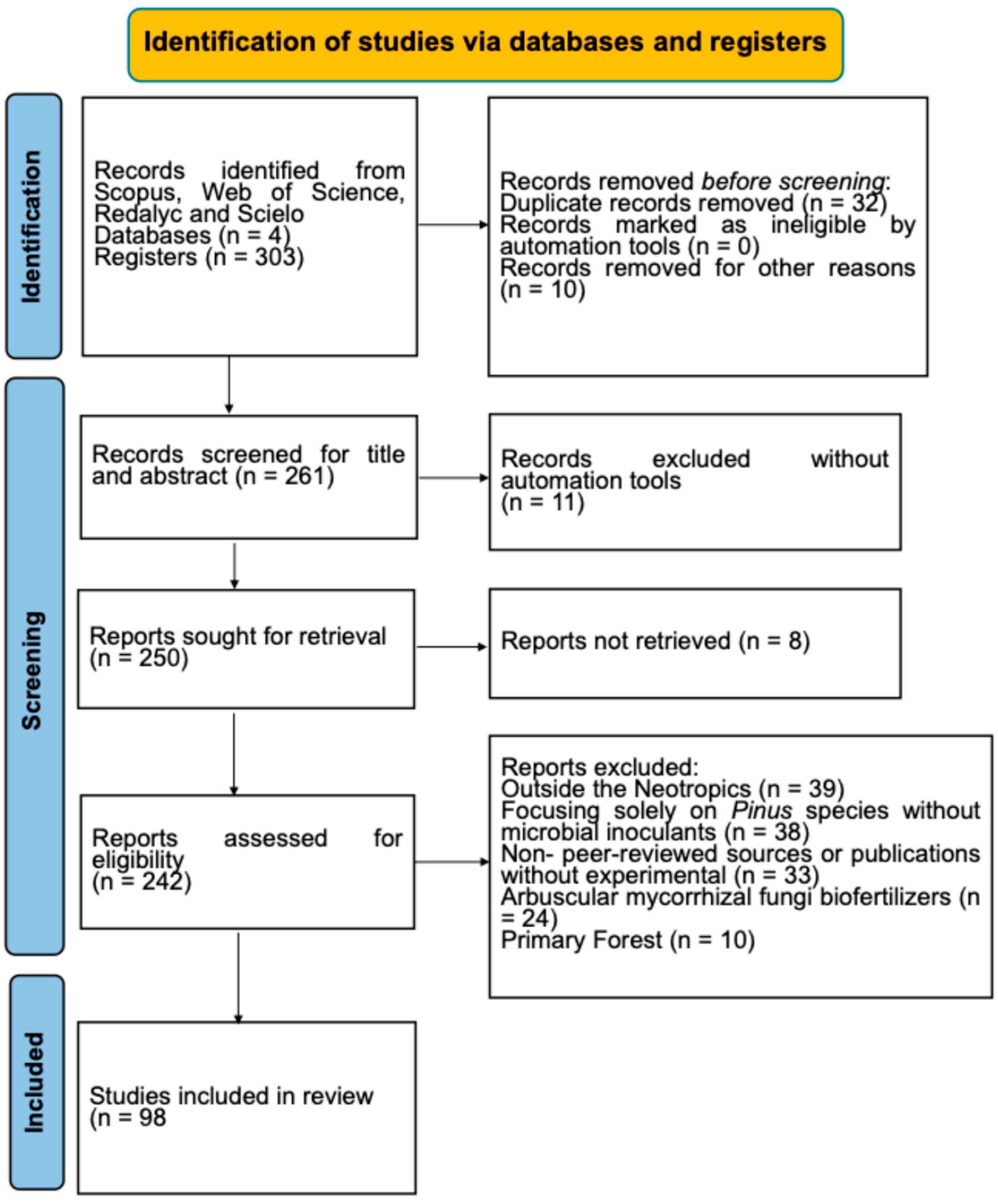
2. Methods
3. Results
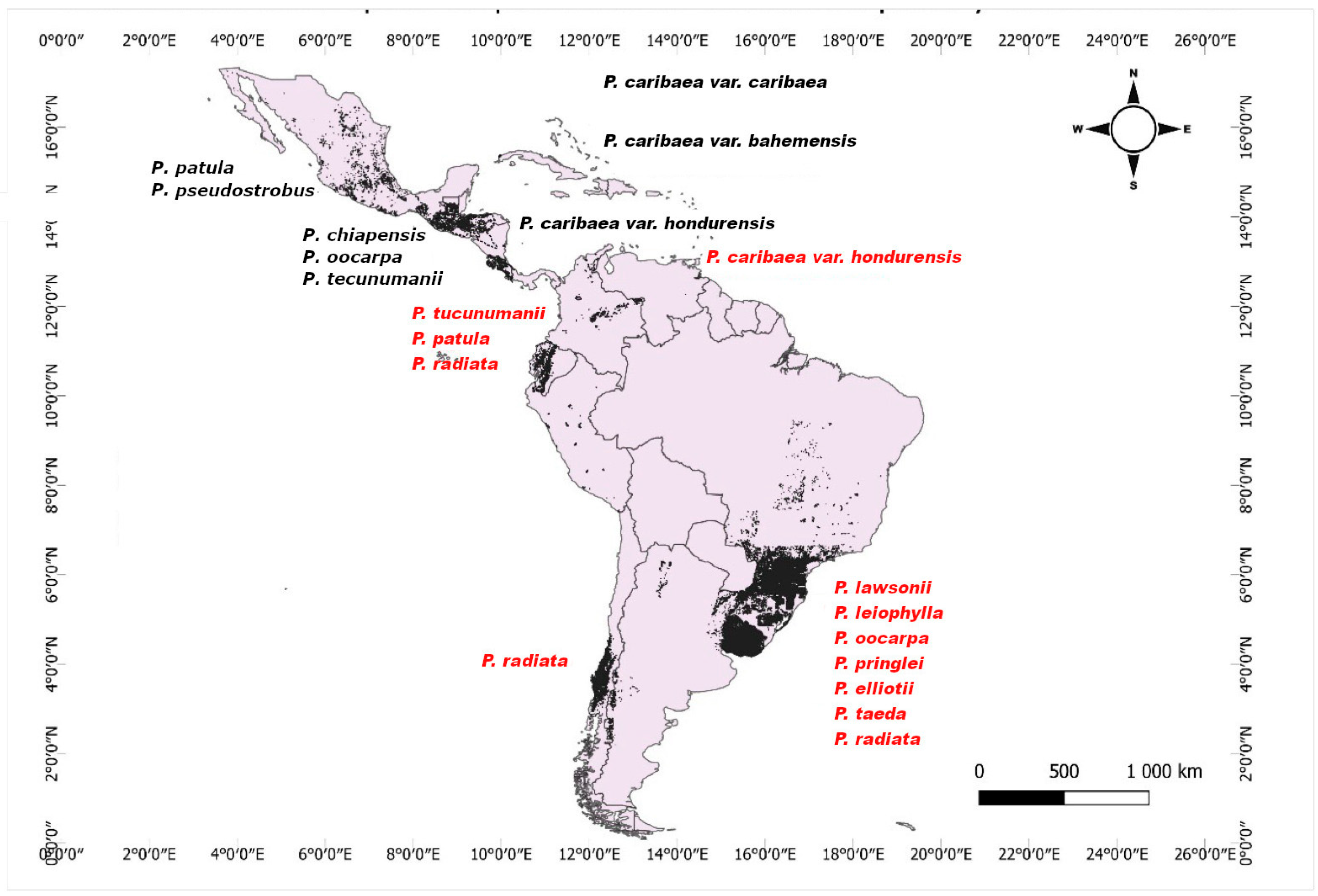
3.1. Distribution of Pine Species Used in Forest Plantations in the Neotropical Region
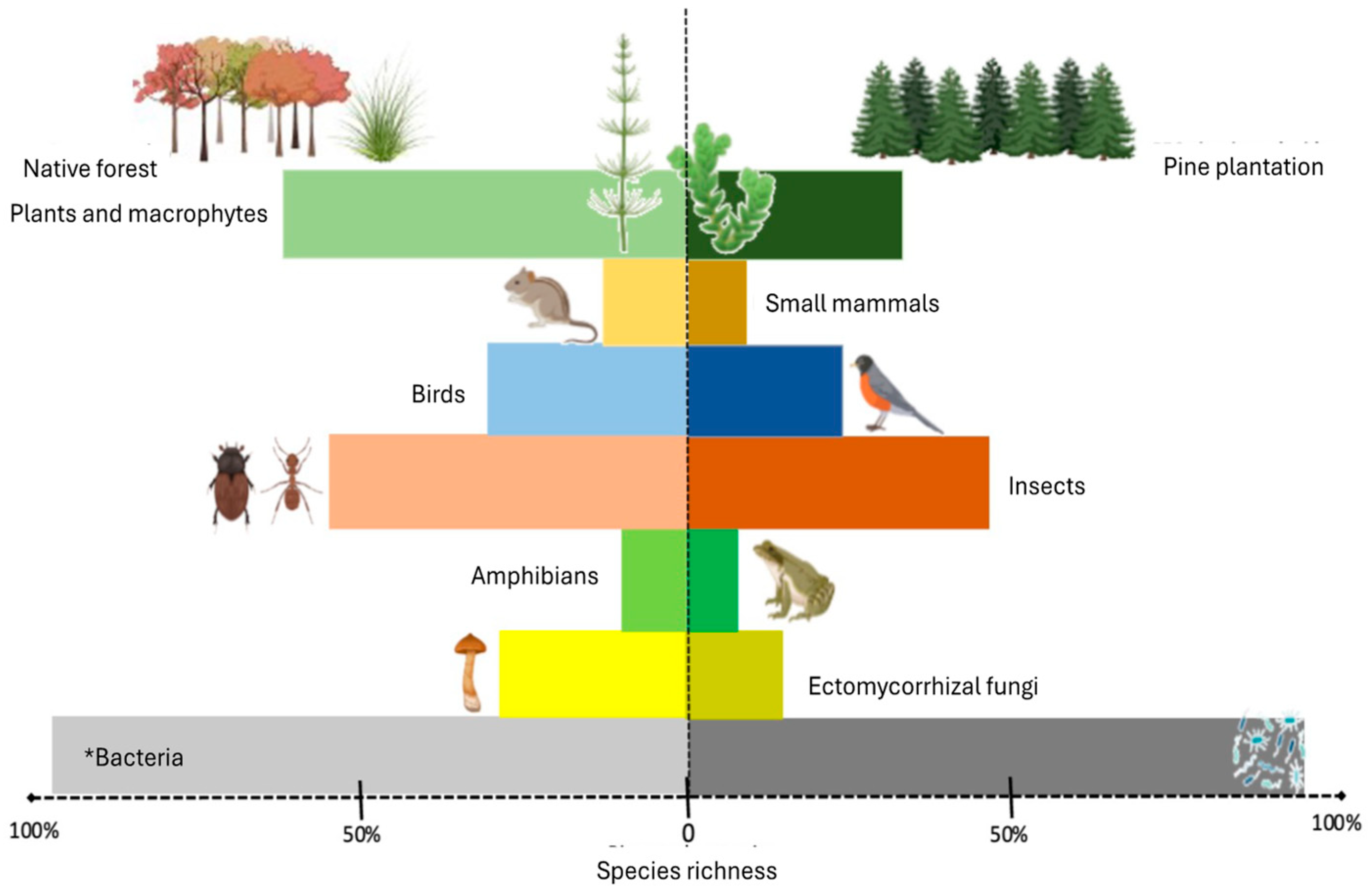
3.2. Effect of Pine Species Introduction on Native Ecosystems in South America
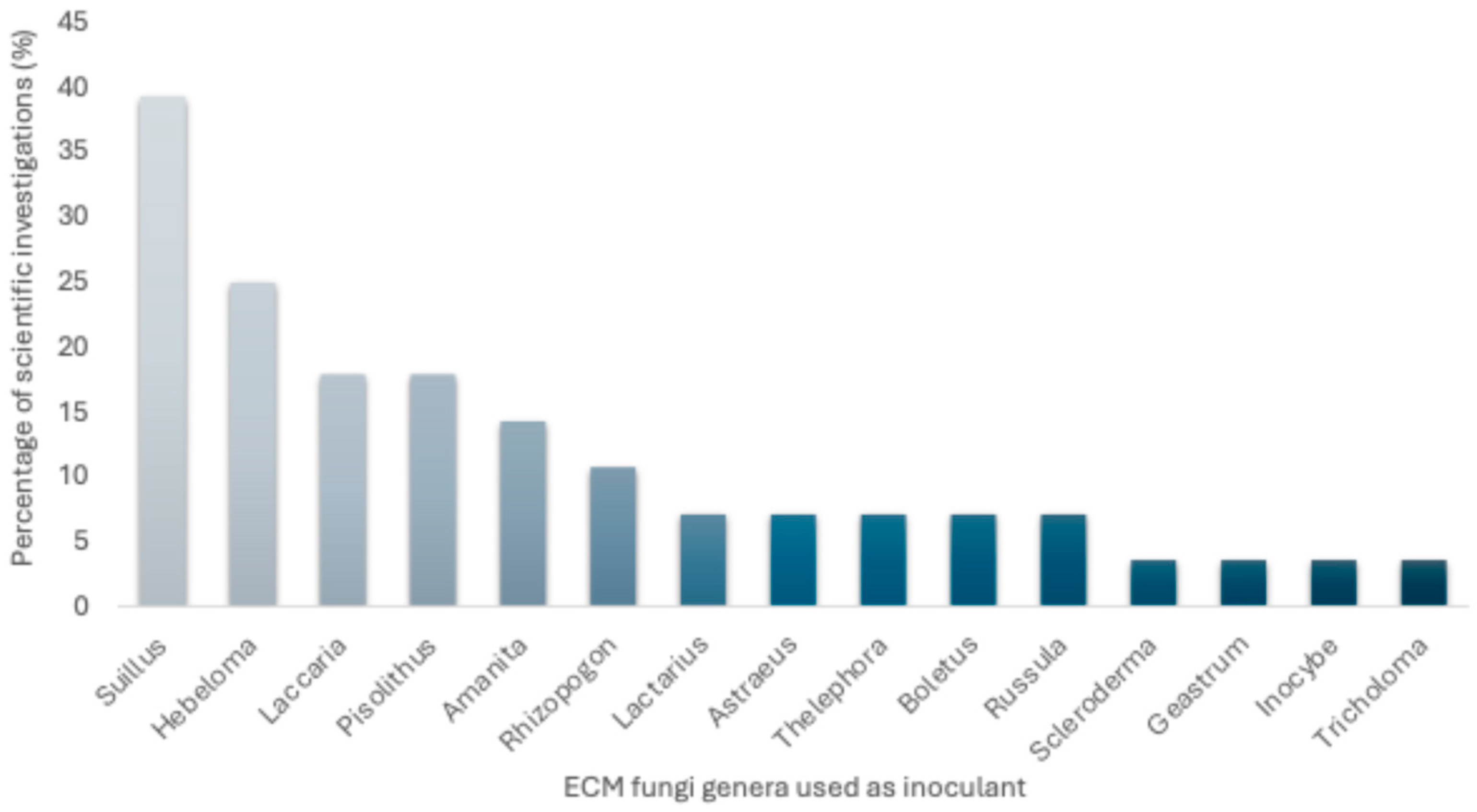
3.3. Importance of Mycorrhization and the Use of Bacteria in Pine Seedling Production
3.4. Response of Pine Seedlings to Mycorrhizal Inoculation
3.5. Cost and Composition of Commercial Microbial Inoculants in Mexico
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | ectomycorrhizal fungi |
| PGPR | plant growth-promoting rhizobacteria |
| PGPB | plant growth-promoting bacteria |
| MHB | mycorrhizal helper bacteria |
References
- Pérez De La Rosa, J.A.; Gernandt, D.S. Pinus vallartensis (Pinaceae), a new species from western Jalisco, Mexico. Phytotaxa 2017, 331, 233–242. [Google Scholar] [CrossRef]
- Jin, W.T.; Gernandt, D.S.; Wehenkel, C.; Xia, X.M.; Wei, X.; Wang, X.Q. Phylogenomic and ecological analyses reveal the spatiotemporal evolution of global pines. Proc. Natl. Acad. Sci. USA 2021, 118, e2022302118. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, M.A.; Chiuffo, M.C.; Torres, A.; Paul, T.; Dimarco, R.D.; Raal, P.; Policelli, N.; Moyano, J.; García, R.A.; van Wilgen, B.W.; et al. Ecology and management of invasive Pinaceae around the world: Progress and challenges. Biol. Invasions 2017, 19, 3099–3120. [Google Scholar] [CrossRef]
- Morrone, J.J.; Escalante, T.; Rodríguez-Tapia, G.; Carmona, A.; Arana, M.; Mercado-Gómez, J.D. Biogeographic regionalization of the Neotropical region: New map and shapefile. An. Acad. Bras. Ciências 2022, 94, e20211167. [Google Scholar] [CrossRef]
- Secretaría de Economía. Norma Mexicana NMX-AA-170-SCFI-2016. Certificación de la Operación de Viveros Forestales. Available online: https://biblioteca.semarnat.gob.mx/janium/Documentos/Ciga/agenda/DOFsr/DO3430.pdf (accessed on 3 December 2023).
- Pauchard, A.; García, R.; Zalba, S.; Sarasola, M.; Zenni, R.; Ziller, S.; Nuñez, M.A. Pine invasions in South America: Reducing their ecological impacts through active management. In Biological Invasions in Changing Ecosystems; De Gruyter Open Ltd.: Berlin, Germany, 2015; pp. 318–342. [Google Scholar] [CrossRef]
- Bâ, A.M.; Diédhiou, A.G.; Prin, Y.; Galiana, A.; Duponnois, R. Management of ectomycorrhizal symbionts associated to useful exotic tree species to improve reforestation performances in tropical Africa. Ann. For. Sci. 2010, 67, 301. [Google Scholar] [CrossRef]
- Salcido-Ruiz, S.; Prieto-Ruíz, J.A.; García-Rodríguez, J.L.; Santana-Aispuro, E.; Chavez-Simental, J.A. Micorrizas y fertilización: Efecto en la producción de Pinus engelmannii Carr. en vivero. Rev. Chapingo Ser. Cienc. For. Amb. 2020, 26, 327–342. [Google Scholar] [CrossRef]
- Barroetaveña, C.; Cázares, E.; Rajchenberg, M. Ectomycorrhizal fungi associated with ponderosa pine and Douglas-fir: A comparison of species richness in native western North American forests and Patagonian plantations from Argentina. Mycorrhiza 2007, 17, 355–373. [Google Scholar] [CrossRef]
- Gonthier, P.; Giordano, L.; Zampieri, E.; Lione, G.; Vizzini, A.; Colpaert, J.V.; Balestrini, R. An ectomycorrhizal symbiosis differently affects host susceptibility to two congeneric fungal pathogens. Fungal Ecol. 2019, 39, 250–256. [Google Scholar] [CrossRef]
- Obase, K. Bacterial community on ectomycorrhizal roots of Laccaria laccata in a chestnut plantation. Mycoscience 2019, 60, 40–44. [Google Scholar] [CrossRef]
- Carrasco-Hernández, V.; Rodríguez Trejo, D.A.; Pérez Moreno, J.; Duarte Zaragoza, V.M.; Navarro Sandoval, J.L.; Quintero Lizaola, R. Evaluación del costo de producción de inoculantes ectomicorrízicos neotropicales a base de esporas. Rev. Mex. Cienc. Agríc. 2018, 9, 417–429. [Google Scholar] [CrossRef][Green Version]
- Barragán-Soriano, J.L.; Pérez-Moreno, J.; Almaraz-Suárez, J.J.; Carcaño-Montiel, M.G.; Medrano-Ortiz, K.I. Inoculation with an edible ectomycorrhizal fungus and bacteria increases growth and improves the physiological quality of Pinus montezumae Lamb. Rev. Chapingo Ser. Cienc. For. Ambient. 2018, 24, 3–16. [Google Scholar] [CrossRef]
- Gardner, T.A.; Hernández, M.I.; Barlow, J.; Peres, C.A. Understanding the biodiversity consequences of habitat change: The value of secondary and plantation forests for neotropical dung beetles. J. Appl. Ecol. 2008, 45, 883–893. [Google Scholar] [CrossRef]
- López-López, M.Á.; Caballero Deloya, M. Análisis financiero de una plantación de Pinus patula Schiede ex Schltdl. et Cham. de pequeña escala. Rev. Mex. Cienc. For. 2018, 9, 186–208. [Google Scholar] [CrossRef][Green Version]
- Comisión Nacional Forestal (CONAFOR). Situación Actual y Perspectivas de las Plantaciones Forestales Comerciales en México; Comisión Nacional Forestal—Colegio de Postgraduados, Montecillo: Texcoco, Estado de México, México, 2009. [Google Scholar]
- Ventura-Ríos, A.; Plascencia-Escalante, F.O.; Hernández de la Rosa, P.; Ángeles-Pérez, G.; Aldrete, A. ¿Es la reforestación una estrategia para la rehabilitación de bosques de pino? Una experiencia en el centro de México. Bosque 2017, 38, 55–66. [Google Scholar] [CrossRef]
- Gómez-Romero, M.; Soto-Correa, J.C.; Blanco-García, J.A.; Sáenz-Romero, C.; Villegas, J.; Lindig-Cisneros, R. Estudio de especies de pino para restauración de sitios degradados. Agrociencia 2012, 46, 795–807. Available online: https://www.scielo.org.mx/pdf/agro/v46n8/v46n8a5.pdf (accessed on 26 November 2023).
- Rebolledo Camacho, V.; Jardón Barbolla, L.; Ramírez Morillo, I.; Vázquez-Lobo, A.; Piñero, D.; Delgado, P. Genetic variation and dispersal patterns in three varieties of Pinus caribaea (Pinaceae) in the Caribbean Basin. Plant Ecol. Evol. 2018, 151, 61–76. [Google Scholar] [CrossRef]
- Instituto Nacional de Bosques (INAB). Plataforma Digital; Instituto Nacional de Bosques: Guatemala City, Guatemala, 2019.
- García, R.A.; Franzese, J.; Policelli, N.; Sasal, Y.; Zenni, R.D.; Nuñez, M.A.; Taylor, K.; Pauchard, A. Non-native pines are homogenizing the ecosystems of South America. In From Biocultural Homogenization to Biocultural Conservation; Rozzi, R., May, R.H., Jr., Chapin, F.S., III, Massardo, F., Gavin, M.C., Klaver, I.J., Pauchard, A., Nuñez, M.A., Simberloff, D., Eds.; Springer: Cham, Switzerland, 2018; Volume 3, pp. 227–243. [Google Scholar] [CrossRef]
- Corley, J.C.; Lantschner, M.V.; Martínez, A.S.; Fischbein, D.; Villacide, J.M. Management of Sirex noctilio populations in exotic pine plantations: Critical issues explaining invasion success and damage levels in South America. J. Pest Sci. 2019, 92, 131–142. [Google Scholar] [CrossRef]
- Baruch, Z.; Nozawa, S.; Johnson, E.; Yerena, E. Ecosystem dynamics and services of a paired Neotropical montane forest and pine plantation. Rev. Biol. Trop. 2019, 67, 24–35. [Google Scholar] [CrossRef]
- Lantschner, M.V.; Atkinson, T.H.; Corley, J.C.; Liebhold, A.M. Predicting North American Scolytinae invasions in the Southern Hemisphere. Ecol. Appl. 2017, 27, 66–77. [Google Scholar] [CrossRef]
- Simberloff, D.; Nuñez, M.A.; Ledgard, N.J.; Pauchard, A.; Richardson, D.M.; Sarasola, M.; Ziller, S.R. Spread and impact of introduced conifers in South America: Lessons from other southern hemisphere regions. Austral Ecol. 2010, 35, 489–504. [Google Scholar] [CrossRef]
- Paritsis, J.; Landesmann, J.B.; Kitzberger, T.; Tiribelli, F.; Sasal, Y.; Quintero, C.; Dimarco, R.D.; Barrios-García, M.N.; Iglesias, A.L.; Diez, J.P.; et al. Pine Plantations and Invasion Alter Fuel Structure and Potential Fire Behavior in a Patagonian Forest-Steppe Ecotone. Forests 2018, 9, 117. [Google Scholar] [CrossRef]
- Uribe, S.V.; Estades, C.F.; Radeloff, V.C. Pine plantations and five decades of land use change in central Chile. PLoS ONE 2020, 15, e0230193. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, C.; Coomes, D.; Salas, J.; Rey-Benayas, J.M.; Lara, A.; Newton, A. Rapid deforestation and fragmentation of Chilean Temperate Forests. Biol. Conserv. 2006, 130, 481–494. [Google Scholar] [CrossRef]
- Bravo-Monasterio, P.; Pauchard, A.; Fajardo, A. Pinus contorta invasion into treeless steppe reduces species richness and alters species traits of the local community. Biol. Invasions 2016, 18, 1883–1894. [Google Scholar] [CrossRef]
- Becerra, P.I.; Simonetti, J.A. Patterns of Exotic Species Richness of Different Taxonomic Groups in a Fragmented Landscape of Central Chile. Bosque 2013, 34, 45–51. [Google Scholar] [CrossRef]
- Becerra, P.I.; Simonetti, J.A. Native and Exotic Plant Species Diversity in Forest Fragments and Forestry Plantations of a Coastal Landscape of Central Chile. Bosque 2020, 41, 125–136. [Google Scholar] [CrossRef]
- Braun, A.C.; Troeger, D.; Garcia, R.; Aguayo, M.; Barra, R.; Vogt, J. Assessing the Impact of Plantation Forestry on Plant Biodiversity: A Comparison of Sites in Central Chile and Chilean Patagonia. Glob. Ecol. Conserv. 2017, 10, 159–172. [Google Scholar] [CrossRef]
- Hernandes-Volpato, G.; Miranda Prado, V.; Dos Anjos, L. What can tree plantations do for forest birds in fragmented forest landscapes? A case study in southern Brazil. For. Ecol. Manag. 2010, 260, 1156–1163. [Google Scholar] [CrossRef]
- Rubio, A.V.; Fredes, F.; Simonetti, J.A. Exotic Pinus radiata plantations do not increase Andes Hantavirus prevalence in rodents. EcoHealth 2019, 16, 659–670. [Google Scholar] [CrossRef]
- Renner, S.; Périco, E.; Sahlén, G. Effects of exotic tree plantations on the richness of dragonflies (Odonata) in Atlantic Forest, Rio Grande do Sul, Brazil. Int. J. Odonatol. 2016, 19, 207–219. [Google Scholar] [CrossRef]
- Policelli, N.; Horton, T.R.; García, R.A.; Naour, M.; Pauchard, A.; Nuñez, M.A. Native and non-native trees can find compatible mycorrhizal partners in each other’s dominated areas. Plant Soil 2020, 454, 285–297. [Google Scholar] [CrossRef]
- Pildain, M.B.; Visnovsky, S.B.; Barroetaveña, C. Diversity of Exotic Ectomycorrhizal Rhizopogon from Pine Plantations in Patagonia. Mycologia 2019, 111, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, N.A.; Zacarias, L.K.E.; Salvador-Montoya, C.A.; Tasselli, M.; Popoff, O.F.; Niveiro, N. Russula (Russulales, Agaricomycetes) associated with Pinus spp. Plantations from Northeastern Argentina. Rodriguésia 2022, 73, e02372020. [Google Scholar] [CrossRef]
- Chapela, I.H.; Osher, L.J.; Horton, T.R.; Henn, M.R. Ectomycorrhizal fungi introduced with exotic pine plantations induce soil carbon depletion. Soil Biol. Biochem. 2001, 33, 1733–1740. [Google Scholar] [CrossRef]
- Vellinga, E.C.; Wolfe, B.E.; Pringle, A. Global patterns of ectomycorrhizal introductions. New Phytol. 2009, 181, 960–973. [Google Scholar] [CrossRef]
- Ángeles-Argáiz, R.E.; Flores-García, A.; Ulloa, M.; Garibay-Orijel, R. Commercial Sphagnum peat moss is a vector for exotic ectomycorrhizal mushrooms. Biol. Invasions 2016, 18, 89–101. [Google Scholar] [CrossRef]
- Gundale, M.J.; Almeida, J.P.; Wallander, H.; Wardle, D.A.; Kardol, P.; Nilsson, M.C.; Fajardo, A.; Pauchard, A.; Peltzer, D.; Ruotsalainen, S.; et al. Differences in endophyte communities of introduced trees depend on the phylogenetic relatedness of the receiving forest. J. Ecol. 2016, 104, 1219–1232. [Google Scholar] [CrossRef]
- Almonacid-Muñoz, L.H.; Herrera, A.; Fuentes-Ramírez, A.; Vargas-Gaete, R.; Larama, G.; Jara, R.; Fernández, C.; da Silva Valadares, R.B. Tree cover species modify the diversity of rhizosphere-associated microorganisms in Nothofagus obliqua (Mirb.) Oerst temperate forests in South-Central Chile. Forests 2022, 13, 756. [Google Scholar] [CrossRef]
- Dickie, I.A.; Bolstridge, N.; Cooper, J.A.; Peltzer, D.A. Co-invasion by Pinus and its mycorrhizal fungi. New Phytol. 2010, 187, 475–484. [Google Scholar] [CrossRef]
- López-Gutiérrez, A.; Pérez-Moreno, J.; Hernández-Santiago, F.; Uscanga-Mortera, E.; García-Esteva, A.; Cetina-Alcalá, V.M.; Cardoso-Villanueva, M.; Xoconostle-Cázares, B. Nutrient mobilization, growth and field survival of Pinus pringlei inoculated with three ectomycorrhizal mushrooms. Bot. Sci. 2018, 96, 286–304. [Google Scholar] [CrossRef]
- Xu, H.; Zwiazek, J.J. Fungal aquaporins in ectomycorrhizal root water transport. Front. Plant Sci. 2020, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Wang, H.; Zhang, Y.; Li, Z.; Wang, C.; Dai, D.; Tang, M. Inoculation with ectomycorrhizal fungi and dark septate endophytes contributes to the resistance of Pinus spp. to pine wilt disease. Front. Microbiol. 2021, 12, 687304. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Gao, J.; Zhang, Y.; Liu, Y.; Tang, M. Effects of ectomycorrhizal fungi (Suillus variegatus) on the growth, hydraulic function, and non-structural carbohydrates of Pinus tabulaeformis under drought stress. BMC Plant Biol. 2021, 21, 171. [Google Scholar] [CrossRef]
- Bastías, D.A.; Johnson, L.J.; Card, S.D. Symbiotic bacteria of plant-associated fungi: Friends or foes? Curr. Opin. Plant Biol. 2020, 56, 1–8. [Google Scholar] [CrossRef]
- Keller, S.; Schneider, K.; Süssmuth, R.D. Structure elucidation of auxofuran, a metabolite involved in stimulating growth of fly agaric, produced by the mycorrhiza helper bacterium Streptomyces AcH 505. J. Antibiot. 2006, 59, 801–803. [Google Scholar] [CrossRef]
- Rigamonte, T.A.; Pylro, V.S.; Duarte, G.F. The role of mycorrhization helper bacteria in the establishment and action of ectomycorrhizae associations. Braz. J. Microbiol. 2010, 41, 832–840. [Google Scholar] [CrossRef]
- Heredia-Acuña, C.; Almaraz-Suarez, J.J.; Arteaga-Garibay, R.; Ferrera-Cerrato, R.; Pineda-Mendoza, D.Y. Isolation, characterization, and effect of plant-growth-promoting rhizobacteria on pine seedlings (Pinus pseudostrobus Lindl.). J. For. Res. 2019, 30, 1727–1734. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, I.; Ramírez-Martínez, D.; Garibay-Orijel, R.; Jacob-Cervantes, V.; Pérez-Moreno, J.; Ortega-Larrocea, M.P.; Arellano-Torres, E. Sympatric species develop more efficient ectomycorrhizae in the Pinus–Laccaria symbiosis. Rev. Mex. Biodivers. 2019, 90, e902868. [Google Scholar] [CrossRef]
- Domínguez-Castillo, C.; Alatorre-Cruz, J.M.; Castañeda-Antonio, D.; Munive, J.A.; Guo, X.; López-Olguín, J.F.; Fuentes-Ramírez, L.E.; Carreño-López, R. Potential seed germination-enhancing plant growth-promoting rhizobacteria for restoration of Pinus chiapensis ecosystems. J. For. Res. 2020, 32, 2143–2153. [Google Scholar] [CrossRef]
- Zuñiga-Cruz, A.J. Inoculación de Pinus cembroides Zucc. con un Hongo Ectomicorrízico Comestible y una Bacteria Auxiliadora de la Micorrización en dos Sustratos. Master’s Thesis, Colegio de Postgraduados, Texcoco, Estado de México, México, 2018. [Google Scholar]
- Sangwan, S.; Prasanna, R. Mycorrhizae Helper Bacteria: Unlocking Their Potential as Bioenhancers of Plant–Arbuscular Mycorrhizal Fungal Associations. Microb. Ecol. 2022, 84, 1–10. [Google Scholar] [CrossRef]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth-promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef]
- Moreno-Valencia, F.D.; Plascencia-Espinosa, M.Á.; Morales-García, Y.E.; Muñoz-Rojas, J. Selection and Effect of Plant Growth-Promoting Bacteria on Pine Seedlings (Pinus montezumae and Pinus patula). Life 2024, 14, 1320. [Google Scholar] [CrossRef] [PubMed]
- van Loon, L.C. Plant responses to plant growth-promoting rhizobacteria. In New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research; Bakker, P.H.M., Raaijmakers, J.M., Bloemberg, G., Höfte, M., Lemanceau, P., Cooke, M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 79–93. [Google Scholar] [CrossRef]
- Fernandes dos Santos, R.; da Cruz, S.P.; Botelho, G.R.; Flores, A.V. Inoculation of Pinus taeda Seedlings with Plant Growth-promoting Rhizobacteria. Floresta Ambient. 2018, 25, e20160056. [Google Scholar] [CrossRef]
- Cacique-, R. Efecto de Ectomicorrizas en el Crecimiento y Extracción de Nutrientes de Pinus greggii var. greggii Engelm Bajo Invernadero; Universidad Autónoma Agraria Antonio Narro: Coahuila, México, 2018; 74p. [Google Scholar]
- Barroetaveña, C.; Bassani, V.N.; Monges, J.I.; Rajchenberg, M. Field performance of Pinus ponderosa seedlings inoculated with ectomycorrhizal fungi planted in steppe-grasslands of Andean Patagonia, Argentina. Bosque 2016, 37, 307–316. [Google Scholar] [CrossRef]
- Arteaga-León, C.; Pérez-Moreno, J.; Espinosa-Victoria, D.; Almaraz-Suárez, J.J.; Silva-Rojas, H.; Delgado-Alvarado, A. Ectomycorrhizal Inoculation with Edible Fungi Increases Plant Growth and Nutrient Contents of Pinus ayacahuite. Rev. Mex. Biodivers. 2018, 89, 1089–1099. [Google Scholar] [CrossRef]
- García, K.; Delaux, P.M.; Cope, K.R.; Ané, J.M. Molecular signals required for the establishment and maintenance of ectomycorrhizal symbioses. New Phytol. 2015, 208, 79–87. [Google Scholar] [CrossRef]
- Rentería, D.; Barradas, V.L.; Álvarez-Sánchez, J. Ectomycorrhizal pre-inoculation of Pinus hartwegii and Abies religiosa is replaced by native fungi in a temperate forest of central Mexico. Symbiosis 2018, 74, 131–144. [Google Scholar] [CrossRef]
- Baeza-Guzmán, Y.; Trejo Aguilar, D.; Montaño, N.M.; Camargo-Ricalde, S.L. Synergistic effects of Amanita stranella and Suillus decipiens inoculation on morphological features and phenolic compounds of Pinus pseudostrobus var. coatepecensis, a narrow endemic Mexican variety. New For. 2023, 55, 1033–1048. [Google Scholar] [CrossRef]
- Restrepo-Llano, M.F.; Osorio-Vega, N.W.; León-Peláez, J.D. Plant growth response of Pinus patula and Pinus maximinoi seedlings at nursery to three types of ectomycorrhizal inocula. Appl. Environ. Soil Sci. 2018, 2018, 6027351. [Google Scholar] [CrossRef]
- Barroetaveña, C.; Bassani, V.N.; Rajchenberg, M. Inoculación micorrícica de Pinus ponderosa en la Patagonia Argentina: Colonización de las raíces, descripción de morfotipos y crecimiento de las plántulas en vivero. Bosque 2012, 33, 163–169. [Google Scholar] [CrossRef]
- Rentería-Chávez, M.C.; Pérez-Moreno, J.; Cetina-Alcalá, V.M.; Ferrera-Cerrato, R.; Xoconostle-Cázares, B. Transferencia de nutrientes y crecimiento de Pinus greggii Engelm. inoculado con hongos comestibles ectomicorrícicos en dos sustratos. Rev. Argent. Microbiol. 2017, 49, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Hernández, V.; Pérez-Moreno, J.; Espinosa-Hernández, V.; Almaraz-Suárez, J.J.; Quintero-Lizaola, R.; Torres Aquino, M. Contenido de nutrientes e inoculación con hongos ectomicorrízicos comestibles en dos pinos neotropicales. Rev. Chil. Hist. Nat. 2011, 84, 83–96. [Google Scholar] [CrossRef]
- Valdés-Ramírez, M.; Ambriz Parra, E.; Camacho Vera, A.; Fierros González, A. Inoculación de plántulas de pinos con diferentes hongos e identificación visual de la ectomicorriza. Rev. Mex. Cienc. For. 2010, 1, 53–63. Available online: https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-11322010000200005&lng=es (accessed on 16 March 2024).
- Hayward, J.; Horton, T.R.; Pauchard, A.; Nuñez, M.A. A Single Ectomycorrhizal Fungal Species Can Enable a Pinus Invasion. Ecology 2015, 96, 1438–1444. [Google Scholar] [CrossRef]
- van Wesenbeeck, B.K.; van Mourik, T.; Duivenvoorden, J.F.; Cleef, A.M. Strong Effects of a Plantation with Pinus patula on Andean Subpáramo Vegetation: A Case Study from Colombia. Biol. Conserv. 2003, 114, 207–218. [Google Scholar] [CrossRef]
- Casas-Pinilla, L.C.; Iserhard, C.A.; Richter, A.; Gawlinski, K.; Cavalheiro, L.B.D.; Romanowski, H.P.; Kaminski, L.A. Different-Aged Pinus Afforestation Does Not Support Typical Atlantic Forest Fruit-Feeding Butterfly Assemblages. For. Ecol. Manag. 2022, 518, 120279. [Google Scholar] [CrossRef]
- Grazzini, G.; Gatto-Almeida, F.; Tiepolo, L.M. Small Mammals from the Lasting Fragments of Araucaria Forest in Southern Brazil: A Study about Richness and Diversity. Iheringia Sér. Zool. 2021, 111, e2021015. [Google Scholar] [CrossRef]
- Pacheco, R.; Silva, R.R.; Morini, M.S.C.; Brandão, C.R.F. A Comparison of the Leaf-Litter Ant Fauna in a Secondary Atlantic Forest with an Adjacent Pine Plantation in Southeastern Brazil. Neotrop. Entomol. 2009, 38, 55–65. [Google Scholar] [CrossRef]
- Rolon, A.S.; Rocha, O.; Maltchik, L. Does pine occurrence influence the macrophyte assemblage in Southern Brazil ponds? Hydrobiologia 2011, 675, 157–165. [Google Scholar] [CrossRef]
- Cicheleiro, J.; Santos-Pereira, M.; Luza, A.L.; Huning, D.S.; Zanella, N. Effects of Natural Forest and Tree Plantations on Leaf-Litter Frog Assemblages in Southern Brazil. Austral Ecol. 2021, 46, 1023–1034. [Google Scholar] [CrossRef]
- Milani, T.; Hoeksema, J.D.; Jobbágy, E.G.; Rojas, J.A.; Vilgalys, R.; Teste, F.P. Co-Invading Ectomycorrhizal Fungal Succession in Pine-Invaded Mountain Grasslands. Fungal Ecol. 2022, 60, 101176. [Google Scholar] [CrossRef]
- Jadán, O.; Cedillo, H.; Pillacela, P.; Guallpa, D.; Gordillo, A.; Zea, P.; Díaz, L.; Bermúdez, F.; Arciniegas, A.; Quizhpe, W.; et al. Regeneration of Pinus patula (Pinaceae) in Natural Ecosystems and Plantations, in an Andean Altitudinal Gradient, Azuay, Ecuador. Rev. Biol. Trop. 2019, 67, 182–195. [Google Scholar] [CrossRef]
- Quiroz Dahik, C.; Marín, F.; Arias, R.; Crespo, P.; Weber, M.; Palomeque, X. Comparison of Natural Regeneration in Natural Grassland and Pine Plantations across an Elevational Gradient in the Páramo Ecosystem of Southern Ecuador. Forests 2019, 10, 745. [Google Scholar] [CrossRef]
- Cavelier, J.; Santos, C. Efectos de Plantaciones Abandonadas de Especies Exóticas y Nativas sobre la Regeneración Natural de un Bosque Montano en Colombia. Rev. Biol. Trop. 1999, 47, 639–646. [Google Scholar] [CrossRef]
- Lantschner, M.V.; Rusch, V.; Peyrou, C. Bird Assemblages in Pine Plantations Replacing Native Ecosystems in NW Patagonia. Biodivers. Conserv. 2008, 17, 969–989. [Google Scholar] [CrossRef]
- Lantschner, M.V.; Rusch, V.; Hayes, J.P. Influences of Pine Plantations on Small Mammal Assemblages of the Patagonian Forest-Steppe Ecotone. Mammalia 2011, 75, 249–255. [Google Scholar]
- Muñoz, F.; Huerta, A.; Curkovic, T. Diversidad de Coleópteros Epigeos en Bosques de Nothofagus glauca y Plantaciones de Pinus radiata en Chile Central. Rev. Colomb. Entomol. 2021, 47, e7522. [Google Scholar] [CrossRef]
- Cifuentes-Croquevielle, C.; Stanton, D.E.; Armesto, J.J. Soil Invertebrate Diversity Loss and Functional Changes in Temperate Forest Soils Replaced by Exotic Pine Plantations. Sci. Rep. 2020, 10, 7762. [Google Scholar] [CrossRef]
- Infante, J.; Riquelme, M.; Huerta, N.; Oettinger, S.; Fredes, F.; Simonetti, J.A.; Rubio, A.V. Cryptosporidium spp. and Giardia spp. in Wild Rodents: Using Occupancy Models to Estimate Drivers of Occurrence and Prevalence in Native Forest and Exotic Pinus radiata Plantations from Central Chile. Acta Trop. 2022, 235, 106635. [Google Scholar] [CrossRef]
- Saavedra, B.; Simonetti, J.A. Small Mammals of Maulino Forest Remnants, a Vanishing Ecosystem of South-Central Chile. Mammalia 2005, 69, 337–348. [Google Scholar] [CrossRef]
- Gutierrez Flores, I.R.; Osses, P.I. The Effect of Native Forest Replacement by Pinus radiata Plantations on Riparian Plant Communities in Chile. Plant Ecol. Divers. 2017, 10, 65–75. [Google Scholar] [CrossRef]
- Heinrichs, S.; Pauchard, A.; Schall, P. Native Plant Diversity and Composition Across a Pinus radiata D.Don Plantation Landscape in South-Central Chile—The Impact of Plantation Age, Logging Roads and Alien Species. Forests 2018, 9, 567. [Google Scholar] [CrossRef]
- Fierro, A.; Grez, A.A.; Vergara, P.M.; Ramírez-Hernández, A.; Micó, E. How Does the Replacement of Native Forest by Exotic Forest Plantations Affect the Diversity, Abundance, and Trophic Structure of Saproxylic Beetle Assemblages? For. Ecol. Manag. 2017, 405, 246–256. [Google Scholar] [CrossRef]
- Paritsis, J.; Aizen, M.A. Effects of Exotic Conifer Plantations on the Biodiversity of Understory Plants, Epigeal Beetles, and Birds in Nothofagus dombeyi Forests. For. Ecol. Manag. 2008, 255, 1575–1583. [Google Scholar] [CrossRef]
- Iezzi, M.E.; Cruz, P.; Varela, D.; De Angelo, C.; Di Bitetti, M.S. Tree Monocultures in a Biodiversity Hotspot: Impact of Pine Plantations on Mammal and Bird Assemblages in the Atlantic Forest. For. Ecol. Manag. 2018, 424, 216–227. [Google Scholar] [CrossRef]
- Gómez-Cifuentes, A.; Munevar, A.; Gimenez, V.C.; Gatti, M.G.; Zurita, G.A. Influence of Land Use on the Taxonomic and Functional Diversity of Dung Beetles (Coleoptera: Scarabaeinae) in the Southern Atlantic Forest of Argentina. J. Insect Conserv. 2017, 21, 147–156. [Google Scholar] [CrossRef]
- Barroetaveña, C.; Pildain, M.B.; Salgado Salomón, M.E.; Eberhart, J.L. Molecular Identification of Ectomycorrhizas Associated with Ponderosa Pine Seedlings in Patagonian Nurseries (Argentina). Can. J. For. Res. 2010, 40, 1940–1950. [Google Scholar] [CrossRef]
- Santoandré, S.; Filloy, J.; Zurita, G.A.; Bellocq, M.I. Ant Taxonomic and Functional Diversity Show Differential Response to Plantation Age in Two Contrasting Biomes. For. Ecol. Manag. 2019, 437, 304–313. [Google Scholar] [CrossRef]
- de Souza Vieira, M.; Overbeck, G.E. Small Seed Bank in Grasslands and Tree Plantations in Former Grassland Sites in the South Brazilian Highlands. Biotropica 2020, 52, 775–782. [Google Scholar] [CrossRef]
- Flores, R.; Díaz, G.; Honrubia, M. Mycorrhizal synthesis of Lactarius indigo (Schw.) Fr. with five Neotropical pine species. Mycorrhiza 2005, 15, 563–570. [Google Scholar] [CrossRef]
- Quiñónez-Martínez, M.; Gómez-Flores, L.d.J.; Garza-Ocañas, F.; Valero-Galván, J.; Nájera-Medellín, J.A. Crecimiento de plantas de Pinus arizonica Engelm. inoculadas con Pisolithus tinctorius y Astraeus hygrometricus en invernadero. Rev. Chapingo Ser. Cienc. For. Y Del Ambiente 2023, 29, 99–118. [Google Scholar] [CrossRef]
- Gross, E.; Thomazini Casagrande, L.I.; Caetano, F.H. Ultrastructural study of ectomycorrhizas on Pinus caribaea Morelet var. hondurensis Barr. & Golf. seedlings. Acta Bot. Bras. 2004, 18, 1–7. [Google Scholar]
- Gómez-Romero, M.; Lindig-Cisneros, R.; Saenz-Romero, C.; Villegas, J. Effect of inoculation and fertilization with phosphorus on the survival and growth of Pinus pseudostrobus in eroded acrisols. Ecological Engineering 2015, 82, 400–403. [Google Scholar] [CrossRef]
- Villegas-Olivera, J.-A.; Pérez-Moreno, J.; Mata, G.; Almaraz-Suárez, J.-J.; Ojeda-Trejo, E.; Espinosa-Hernández, V. Type of Light and Formation of Basidiomata of Two Species of Edible Ectomycorrhizal Mushrooms Associated with Neotropical Pines and the Description of Basidiomata Development. Rev. Fitotec. Mex. 2017, 40, 324–330. Available online: https://www.redalyc.org/articulo.oa?id=61054247005 (accessed on 23 November 2024).
- de Souza Kulmann, M.S.; Aguilar, M.V.M.; Tassinari, A.; Schwalbert, R.; Tabaldi, L.A.; Araujo, M.M.; Antoniolli, Z.I.; Nicoloso, F.T.; Brunetto, G.; Schumacher, M.V. Effects of Increasing Soil Phosphorus and Association with Ectomycorrhizal Fungi (Pisolithus microcarpus) on Morphological, Nutritional, Biochemical, and Physiological Parameters of Pinus taeda L. For. Ecol. Manag. 2023, 544, 121207. [Google Scholar] [CrossRef]
- Chung Guin-po, P.; Pinilla Suárez, J.C.; Casanova del Río, K.; Soto Guevara, H. Incorporación de Boletus edulis y Boletus pinicola en plantaciones de Pinus radiata en Chile. Cienc. Investig. For. 2007, 13, 335–347. [Google Scholar] [CrossRef]
- Chávez, D.; Pereira, G.; Machuca, Á. Estimulación del crecimiento en plántulas de Pinus radiata utilizando hongos ectomicorrícicos y saprobios como biofertilizantes. Bosque 2014, 35, 57–63. [Google Scholar] [CrossRef]
- Castrillón, M.; León, J.D.; Carvajal, D.; Osorio, N.W. Effectiveness of single and combined ectomycorrhizal inocula on three species of Pinus at nursery. Commun. Soil Sci. Plant Anal. 2015, 46, 169–179. [Google Scholar] [CrossRef]
- Carrasco-Hernández, V.; Pérez-Moreno, J.; Quintero-Lizaola, R.; Espinosa-Solares, T.; Lorenzana-Fernández, A.; Espinosa Hernández, V. Edible species of the fungal genus Hebeloma and two Neotropical pines. Pak. J. Bot. 2015, 47, 319–326. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baeza-Guzmán, Y.; Camargo-Ricalde, S.L.; Trejo-Aguilar, D.; Montaño, N.M. Pine Forest Plantations in the Neotropics: Challenges and Potential Use of Ectomycorrhizal Fungi and Bacteria as Inoculants. J. Fungi 2025, 11, 393. https://doi.org/10.3390/jof11050393
Baeza-Guzmán Y, Camargo-Ricalde SL, Trejo-Aguilar D, Montaño NM. Pine Forest Plantations in the Neotropics: Challenges and Potential Use of Ectomycorrhizal Fungi and Bacteria as Inoculants. Journal of Fungi. 2025; 11(5):393. https://doi.org/10.3390/jof11050393
Chicago/Turabian StyleBaeza-Guzmán, Yajaira, Sara Lucía Camargo-Ricalde, Dora Trejo-Aguilar, and Noé Manuel Montaño. 2025. "Pine Forest Plantations in the Neotropics: Challenges and Potential Use of Ectomycorrhizal Fungi and Bacteria as Inoculants" Journal of Fungi 11, no. 5: 393. https://doi.org/10.3390/jof11050393
APA StyleBaeza-Guzmán, Y., Camargo-Ricalde, S. L., Trejo-Aguilar, D., & Montaño, N. M. (2025). Pine Forest Plantations in the Neotropics: Challenges and Potential Use of Ectomycorrhizal Fungi and Bacteria as Inoculants. Journal of Fungi, 11(5), 393. https://doi.org/10.3390/jof11050393





