From the Metabolic Effects and Mechanism of Monovalent Cation Transport to the Actual Measurement of the Plasma Membrane Potential in Yeast
Abstract
1. Introduction
2. Historical Background
2.1. From the Effects of K+ to Its Transport Mechanism
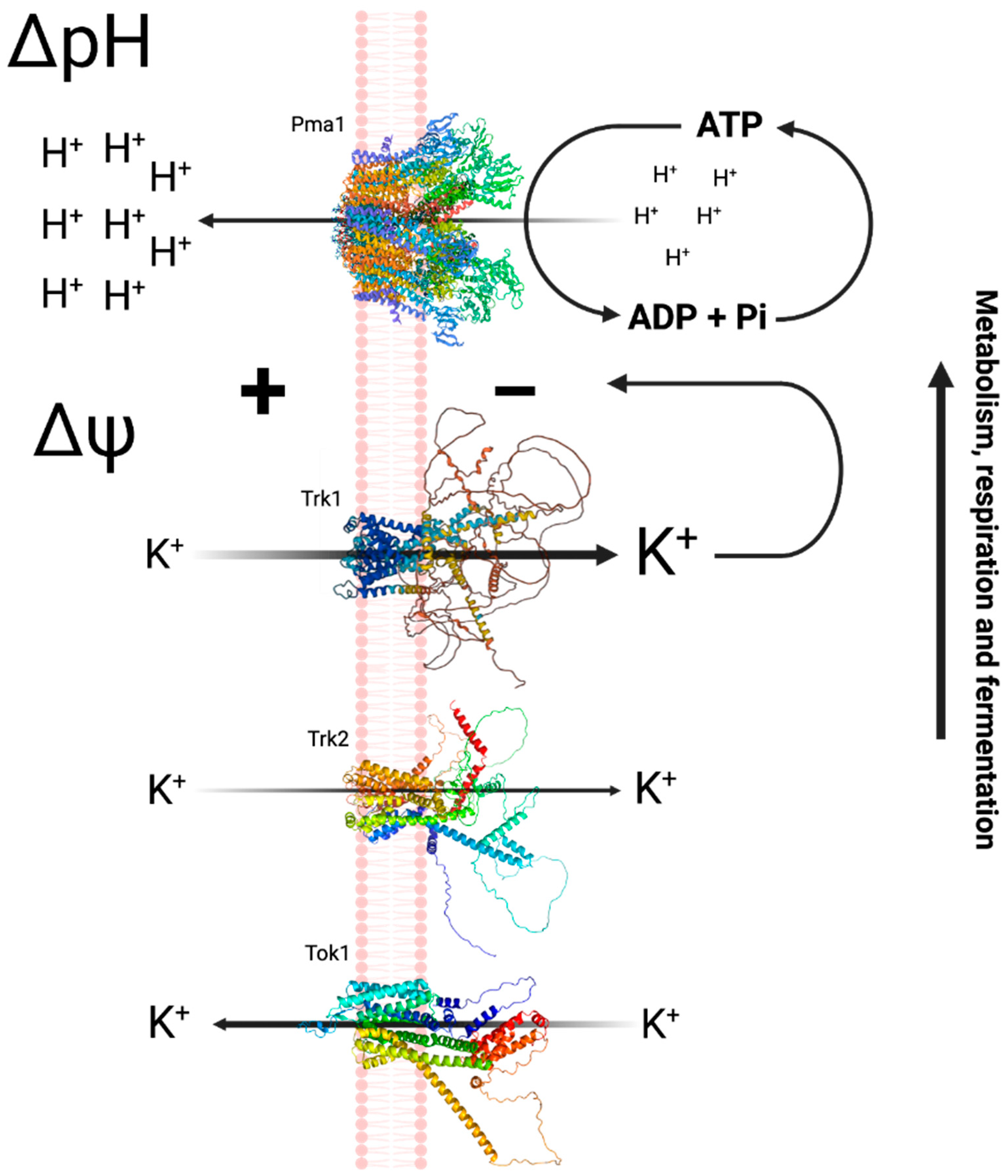
- The plasma membrane contains a H+-ATPase (Pma1p) which generates an electric potential difference by pumping protons out, resulting in a negative potential inside the membrane.
- Two transporters (Trk1p and Trk2p), with different affinities driven by the PMP, are responsible for the uptake of monovalent cations, thereby maintaining the electrical and ionic homeostasis in the cell.
2.2. Regulation of the Internal pH of Yeast
3. Estimating the Value of the Plasma Membrane Potential (PMP)
3.1. The Estimation of the Yeast PMP by Using Fluorescent Dyes
- Ethidium Bromide(EtBr)
- DiSC3(3)
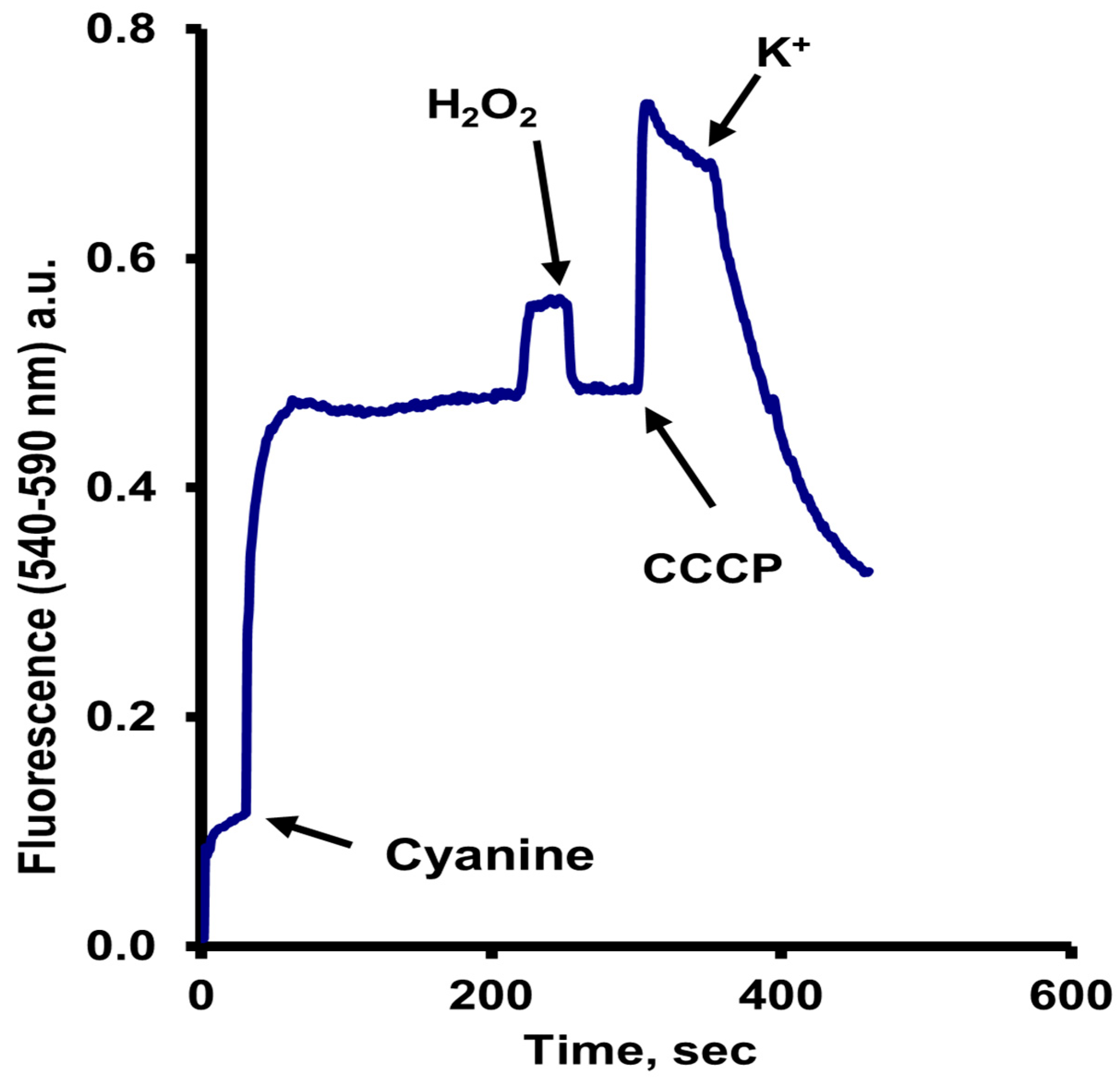
- (a)
- An increase in fluorescence upon the addition of the dye;
- (b)
- After three or four minutes, another slight increase took place, coincident with the exhaustion of oxygen;
- (c)
- As expected, the addition of a small concentration of H2O2, which, by the action of the catalase, re-established the oxygen levels in the sample buffer, resulted in the return of the fluorescence to the previous level;
- (d)
- The addition of a low concentration of the uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP) (10 µM) resulted in a much larger increase in fluorescence, and finally;
- (e)
- (a)
- The fluorescence changes in starved cells required the addition of glucose to provide energy, and the dye was taken up but mostly accumulated in the mitochondria, where due to the negative internal membrane potential of these organelles and the high concentration reached, its fluorescence was partially quenched.
- (b)
- The slight increase in fluorescence upon oxygen exhaustion was due to an efflux of the dye from the mitochondria to the cytoplasm, where, owed to a higher relative volume, fluorescence increased, but not completely, because the mitochondria could still be partially energized by the ATP produced by fermentation.
- (c)
- The former was confirmed by the fluorescence diminution after the addition of hydrogen peroxide.
- (d)
- The rather high increase in the fluorescence level after adding CCCP was due to the efflux of the dye from the mitochondria to the cytoplasm, where it increases due to its distribution into a larger volume, the cytoplasm. Here it is important to note that a concentration of CCCP low enough to uncouple mitochondria, but not the plasma membrane, was used.
- (e)
- Finally, the decrease after the addition of KCl was due to the efflux of the dye, owing to the also partial decrease in the PMP resulting from the uptake of K+.
Changes in Fluorescence Depend on the Concentration of the Dyes
- Recent research using DiSC3(3) molecules to estimate the PMP
- Fluorescent dyes different from DiSC3(3)
- Thioflavin T (ThT)
3.2. The Actual Measurement of the Yeast PMP with Thioflavin T (ThT), an Old Dye
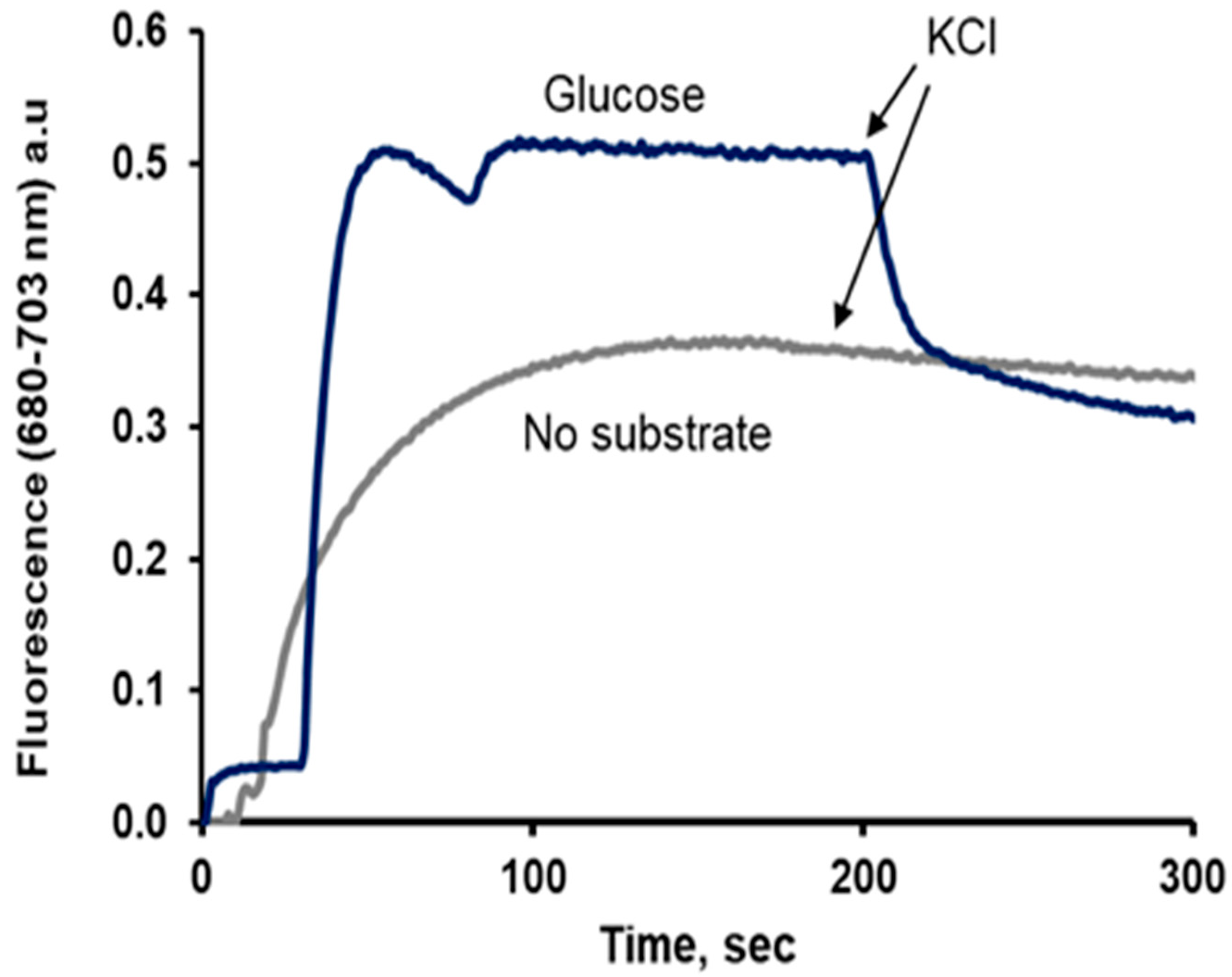
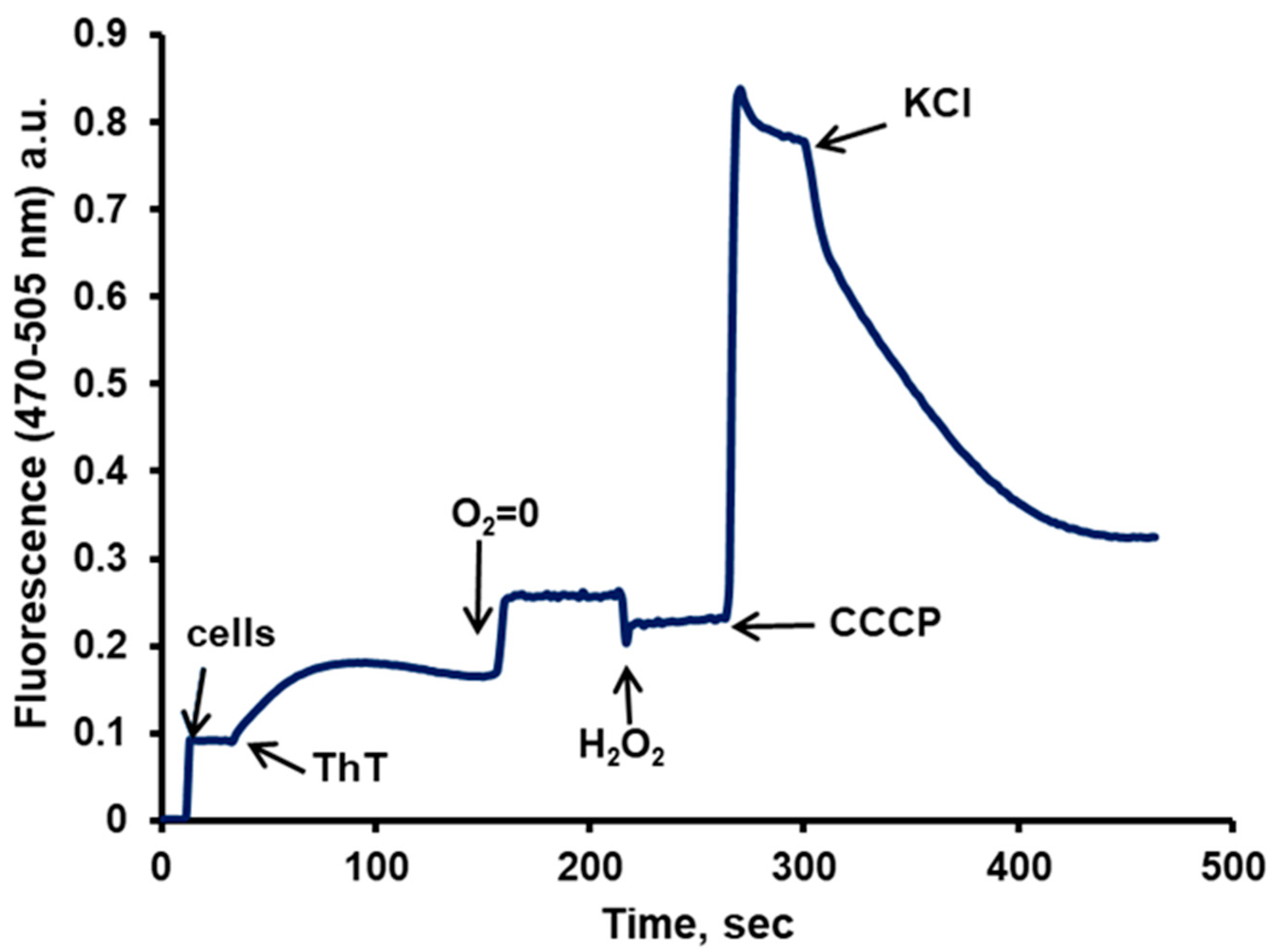
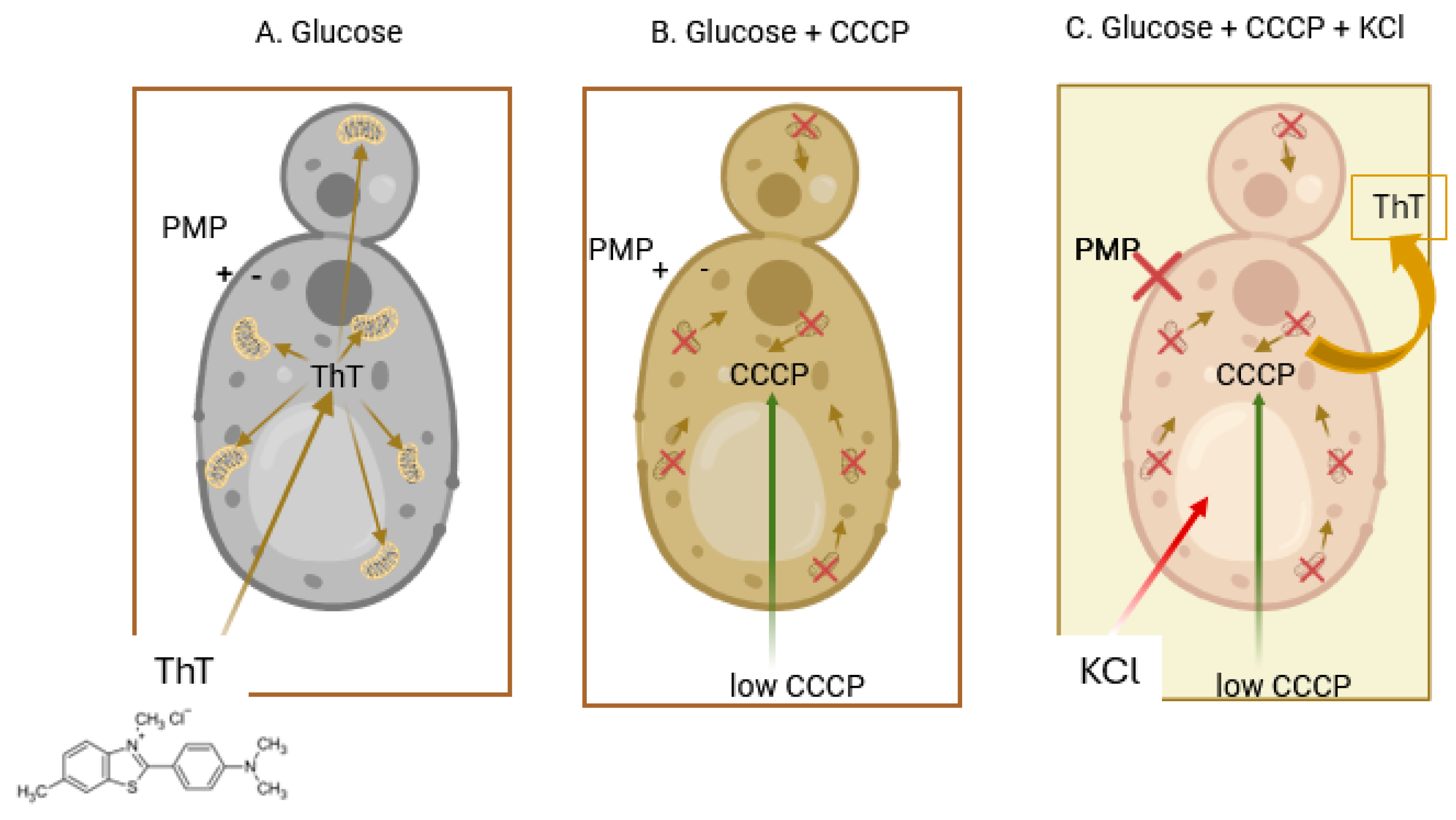
- Driven by the PMP, the dye enters the cells, but once inside, it is accumulated by the mitochondria, where due to the high concentration reached, its fluorescence is greatly quenched; this accumulation can be reversed by the addition of a low concentration (10 µM) of CCCP that uncouples the membrane potential of the organelles without affecting the PMP of the cell [42] (Figure 5, panels A and B). A low concentration of KCl (5–10 mM) is capable of depolarizing the cellular plasma membrane, resulting in the release of the dye, lowering the total fluorescence in the sample (Figure 5, panel C). This had been previously seen also for the cyanine DiSC3(3) [38].
- The microscopic images of the cells showed that the dye did not enter the vacuole; therefore, it was necessary to obtain the actual value of the cytoplasm volume, which was calculated from the total volume of the cells obtained with 14C-inulin and with the image analysis of the cells, subtracting the vacuolar from the total volume. This gave the actual concentration of the dye in the cytoplasm in the presence of the uncoupler.
- The accumulation of this dye is not only attributable to the PMP; part of it is due to its hydrophobic and cationic nature, which results from its binding to the internal molecules of the cell. To avoid dragging this error into the PMP calculation by means of the Nernst equation, chitosan was used to permeabilize the cells and allow all the ThT to leave the cell. Any remaining ThT inside is bound to hydrophobic and/or anionic molecules. By adding a high concentration of KCl (200 mM), the ThT bound to anionic molecules is displaced, leaving only that bound due to its hydrophobic character. Consequently, the dye bound solely due to its cationic nature is calculated. Subsequently, the actual concentration of ThT within the cytoplasm can be obtained by subtracting these values from the raw ones. The actual concentration of the dye inside the cytoplasm can be obtained after the cells are incubated with glucose plus CCCP, in the absence, as well as in the presence of K+. With the corrected concentration values in the presence of CCCP, both in the absence and in the presence of KCl, the actual values of the PMP can be obtained by utilizing the Nernst equation. A report with different strains further confirmed the validity of the procedure employed [26].
- However, to calculate the values of the PMP, it is necessary that the strains used present a sensitivity of the plasma membrane potential lower than that of the mitochondria. When this sensitivity is similar, low concentrations of an uncoupler affect both the mitochondrial and plasma membrane electric potentials, turning uncertain the values of the PMP. Such is the case of Debaryomyces hansenii, Meyerozyma guilliermondii, and Rhodotorula mucilaginosa in which only approximate values can be obtained with this method [26].
3.3. A New and Promising Option to Estimate PMP: The Genetically Encoded Voltage Indicator (GEVI) Proteins
4. Conclusions
5. An Additional Consideration
Tributes
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCCP | Carbonyl cyanide m-chlorophenylhydrazone |
| FCCP | Carbonyl cyanide p-trifluoromethoxyphenylhydrazone |
| PMP | Plasma membrane potential |
References
- Pulver, R.; Verzár, F. Connexion between Carbohydrate and Potassium Metabolism in the Yeast Cell. Nature 1940, 145, 823–824. [Google Scholar] [CrossRef]
- Conway, E.J.; Brady, T. Mechanism of High Acid Production by Yeast and Its Bearing on Hydrochloric Acid Formation in the Stomach. Nature 1947, 159, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.J.; Downey, M. pH Values of the Yeast Cell. Biochem. J. 1950, 47, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.J.; Brady, T.G.; Carton, E. Biological Production of Acid and Alkali. 2. A Redox Theory for the Process in Yeast with Application to the Production of Gastric Acidity. Biochem. J. 1950, 47, 369–374. [Google Scholar] [CrossRef]
- Conway, E.J.; Kernan, R.P. The Effect of Redox Dyes on the Active Transport of Hydrogen, Potassium and Sodium Ions across the Yeast Cell Membrane. Biochem. J. 1955, 61, 32. [Google Scholar] [CrossRef]
- Rothstein, A.; Demis, C. The Relationship of the Cell Surface to Metabolism. The Stimulation of Fermentation by Extracellular Potassium. Arch. Biochem. Biophys. 1953, 44, 18–29. [Google Scholar] [CrossRef]
- Rothstein, A.; Bruce, M. The Potassium Efflux and Influx in Yeast at Different Potassium Concentrations. J. Cell Comp. Physiol. 1958, 51, 145–159. [Google Scholar] [CrossRef]
- Peña, A.; Cinco, G.; Gómez Puyou, A.; Tuena, M. Studies on the Mechanism of the Stimulation of Glycolysis and Respiration by K+ in Saccharomyces cerevisiae. Biochim. Biophys. Acta (BBA) Bioenerg. 1969, 180, 1–8. [Google Scholar] [CrossRef]
- Peña, A.; Cinco, G.; Gómez-Puyou, A.; Tuena, M. Effect of the pH of the Incubation Medium on Glycolysis and Respiration in Saccharomyces cerevisiae. Arch. Biochem. Biophys. 1972, 153, 413–425. [Google Scholar] [CrossRef]
- Mitchell, P. Coupling of Phosphorylation to Electron and Hydrogen Transfer by a Chemi-Osmotic Type of Mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef]
- Skou, J.C. Enzymatic Basis for Active Transport of Na+ and K+ Across Cell Membrane. Physiol. Rev. 1965, 45, 596–617. [Google Scholar] [CrossRef] [PubMed]
- Sachs, G.; Mitch, W.E.; Hirschowitz, B.I. Frog Gastric Mucosal ATPase. Exp. Biol. Med. 1965, 119, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Slayman, C.W.; Slayman, C.L. Potassium Transport in Neurospora: Evidence for a Multisite Carrier at High pH. J. Gen. Physiol. 1970, 55, 758–786. [Google Scholar] [CrossRef] [PubMed]
- Peña, A. Studies on the Mechanism of K+ Transport in Yeast. Arch. Biochem. Biophys. 1975, 167, 397–409. [Google Scholar] [CrossRef]
- Gaber, R.F. Molecular Genetics of Yeast Ion Transport. Int. Rev. Citol. 1992, 137A, 299–353. [Google Scholar]
- Serrano, R.; Kielland-Brandt, M.C.; Fink, G.R. Yeast Plasma Membrane ATPase Is Essential for Growth and Has Homology with (Na+ + K+), K+-and Ca2+-ATPases. Nature 1986, 319, 689–693. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, A.; Ramos, J. Dual System for Potassium Transport in Saccharomyces cerevisiae. J. Bacteriol. 1984, 159, 940–945. [Google Scholar] [CrossRef]
- Gaber, R.F.; Styles, C.A.; Fink, G.R. TRK1 Encodes a Plasma Membrane Protein Required for High-Affinity Potassium Transport in Saccharomyces cerevisiae. Mol. Cell Biol. 1988, 8, 2848–2859. [Google Scholar] [CrossRef]
- Ko, C.H.; Buckley, A.M.; Gaber, R.F. TRK2 Is Required for Low Affinity K+ Transport in Saccharomyces cerevisiae. Genetics 1990, 125, 305–312. [Google Scholar] [CrossRef]
- Ko, C.H.; Gaber, R.F. TRK1 and TRK2 Encode Structurally Related K+ Transporters in Saccharomyces cerevisiae. Mol. Cell Biol. 1991, 11, 4266–4273. [Google Scholar] [CrossRef]
- Foury, F.; Boutry, M.; Goffeau, A. Transport Functions of Plasmic ATPase in Schizosaccharomyces pombe. Arch. Int. Physiol. Biochim. 1976, 84, 618–619. [Google Scholar] [PubMed]
- Zhou, X.L.; Vaillant, B.; Loukin, S.H.; Kung, C.; Saimi, Y. YKC1 Encodes the Depolarization-Activated K+ Channel in the Plasma Membrane of Yeast. FEBS Lett. 1995, 373, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Ketchum, K.A.; Joiner, W.J.; Sellers, A.J.; Kaczmarek, L.K.; Goldstein, S.A.N. A New Family of Outwardly Rectifying Potassium Channel Proteins with Two Pore Domains in Tandem. Nature 1995, 376, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Gustin, M.C.; Martinac, B.; Saimi, Y.; Culbertson, M.R.; Kung, C. Ion Channels in Yeast. Science 1986, 233, 1195–1197. [Google Scholar] [CrossRef]
- Bertl, A.; Slayman, C.L.; Gradmann, D. Gating and Conductance in an Outward-Rectifying K+ Channel from the Plasma Membrane of Saccharomyces cerevisiae. J. Membr. Biol. 1993, 132, 183–199. [Google Scholar] [CrossRef]
- Peña, A.; Sánchez, N.S.; Padilla-Garfias, F.; Ramiro-Cortés, Y.; Araiza-Villanueva, M.; Calahorra, M. The Use of Thioflavin T for the Estimation and Measurement of the Plasma Membrane Electric Potential Difference in Different Yeast Strains. J. Fungi 2023, 9, 948. [Google Scholar] [CrossRef]
- Calahorra, M.; Martínez, G.A.; Hernández-Cruz, A.; Peña, A. Influence of Monovalent Cations on Yeast Cytoplasmic and Vacuolar pH. Yeast 1998, 14, 501–515. [Google Scholar] [CrossRef]
- Hauer, R.; Höfer, M. Evidence for Interactions between the Energy-Dependent Transport of Sugars and the Membrane Potential in the Yeast Rhodotorula gracilis (Rhodosporidium toruloides). J. Membr. Biol. 1978, 43, 335–349. [Google Scholar] [CrossRef]
- Borst-Pauwels, G.W.F.H. Ion Transport in Yeast. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 1981, 650, 88–127. [Google Scholar] [CrossRef]
- Vacata, V.; Kotyk, A.; Sigler, K. Membrane Potential in Yeast Cells Measured by Direct and Indirect Methods. Biochim. Biophys. Acta (BBA) Biomembr. 1981, 643, 265–268. [Google Scholar] [CrossRef]
- de la Peña, P.; Barros, F.; Gascón, S.; Ramos, S.; Lazo, P.S. The Electrochemical Proton Gradient of Saccharomyces. The Role of Potassium. Eur. J. Biochem. 1982, 123, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Gitler, C.; Rubalcava, B.; Caswell, A. Fluorescence Changes of Ethidium Bromide on Binding to Erythrocyte and Mitochondrial Membranes. Biochim. Biophys. Acta (BBA) Biomembr. 1969, 193, 479–481. [Google Scholar] [CrossRef]
- Sims, P.J.; Waggoner, A.S.; Wang, C.H.; Hoffman, J.F. Studies on the Mechanism by Which Cyanine Dyes Measure Membrane Potential in Red Blood Cells and Phosphatidylcholine Vesicles. Biochemistry 1974, 13, 3315–3330. [Google Scholar] [CrossRef]
- Hoffman, J.F.; Laris, P.C. Determination of Membrane Potentials in Human and Amphiuma Red Blood Cells by Means of a Fluorescent Probe. J. Physiol. 1974, 239, 519–552. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, A.S.; Wang, C.H.; Tolles, R.L. Mechanism of Potential-Dependent Light Absorption Changes of Lipid Bilayer Membranes in the Presence of Cyanine and Oxonol Dyes. J. Membr. Biol. 1977, 33, 109–140. [Google Scholar] [CrossRef] [PubMed]
- Peña, A.; Clemente, S.M.; Borbolla, M.; Carrasco, N.; Uribe, S. Multiple Interactions of Ethidium Bromide with Yeast Cells. Arch. Biochem. Biophys. 1980, 201, 420–428. [Google Scholar] [CrossRef]
- Brunner, A.; Carrasco, N.; Peña, A. Correlation between Resistance to Ethidium Bromide and Changes in Monovalent Cation Uptake in Yeast. Arch. Biochem. Biophys. 1982, 217, 30–36. [Google Scholar] [CrossRef]
- Peña, A.; Uribe, S.; Pardo, J.P.; Borbolla, M. The Use of a Cyanine Dye in Measuring Membrane Potential in Yeast. Arch. Biochem. Biophys. 1984, 231, 217–225. [Google Scholar] [CrossRef]
- Plášek, J.; Dale, R.E.; Sigler, K.; Laskay, G. Transmembrane Potentials in Cells: A DiS-C3(3) Assay for Relative Potentials as an Indicator of Real Changes. Biochim. Biophys. Acta 1994, 1196, 181–190. [Google Scholar] [CrossRef]
- Plášek, J.; Sigler, K. Slow Fluorescent Indicators of Membrane Potential: A Survey of Different Approaches to Probe Response Analysis. J. Photochem. Photobiol. B 1996, 33, 101–124. [Google Scholar] [CrossRef]
- Plášek, J.; Gášková, D.; Lichtenberg-Fraté, H.; Ludwig, J.; Höfer, M. Monitoring of Real Changes of Plasma Membrane Potential by DiS-C3(3) Fluorescence in Yeast Cell Suspensions. J. Bioenerg. Biomembr. 2012, 44, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Peña, A.; Sánchez, N.S.; Calahorra, M. Estimation of the Electric Plasma Membrane Potential Difference in Yeast with Fluorescent Dyes: Comparative Study of Methods. J. Bioenerg. Biomembr. 2010, 42, 419–432. [Google Scholar] [CrossRef]
- Plášek, J.; Gášková, D. Complementary Methods of Processing DiS-C3(3) Fluorescence Spectra Used for Monitoring the Plasma Membrane Potential of Yeast: Their Pros and Cons. J. Fluoresc. 2014, 24, 541–547. [Google Scholar] [CrossRef]
- Peña, A.; Ramírez, G. Interaction of Ethidium Bromide with the Transport System for Monovalent Cations in Yeast. J. Membr. Biol. 1975, 22, 369–384. [Google Scholar] [CrossRef]
- Hou, J.; Daniels, P.N.; Burke, M.D. Small Molecule Channels Harness Membrane Potential to Concentrate Potassium in trk1Δtrk2Δ Yeast. ACS Chem. Biol. 2020, 15, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Kodedová, M.; Valachovič, M.; Sychrová, H. The Replacement of Ergosterol with Alternative Sterols Affects the Physiological Function of the Yeast Plasma Membrane, Including Its H+-ATPase Activity and Resistance to Antifungal Drugs. Microbes Infect. 2025, 27, 105409. [Google Scholar] [CrossRef]
- Vázquez-Carrada, M.; Feldbrügge, M.; Olicón-Hernández, D.R.; Guerra-Sánchez, G.; Pardo, J.P. Functional Analysis of the Plasma Membrane H+-ATPases of Ustilago maydis. J. Fungi 2022, 8, 550. [Google Scholar] [CrossRef]
- Bidiuk, V.A.; Alexandrov, A.I.; Valiakhmetov, A.Y. Extracellular pH and High Concentration of Potassium Regulate the Primary Necrosis in the Yeast Saccharomyces cerevisiae. Arch. Microbiol. 2022, 204, 35. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Zhao, J.; Shi, Z.; Ma, C.; Yu, Q.; Li, M. Deletion of PMP3 Increases Ketoconazole Resistance by Affecting Plasma Membrane Potential in Candida albicans. Microbiol. Res. 2024, 289, 127918. [Google Scholar] [CrossRef]
- de Souza-Guerreiro, T.C.; Huan Bacellar, L.; da Costa, T.S.; Edwards, C.L.A.; Tasic, L.; Asally, M. Membrane potential dynamics unveil the promise of bioelectrical antimicrobial susceptibility Testing (BeAST) for anti-fungal screening. mBio 2024, 15, e01302-24. [Google Scholar] [CrossRef]
- Peña, A.; Sánchez, N.S.; Calahorra, M. The Plasma Membrane Electric Potential in Yeast: Probes, Results, Problems, and Solutions: A New Application of an Old Dye? In Old Yeasts; Lucas, C., Pais, C., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Peña, A.; Sánchez, N.S.; Calahorra, M. Estimations and Actual Measurements of the Plasma Membrane Electric Potential Difference in Yeast. In Membrane Potential: An Overview; Milan, M., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2019; pp. 81–104. ISBN 978-1-53616-743-6. [Google Scholar]
- Peña, A.; Sánchez, N.S.; Ramiro-Cortés, Y.; Calahorra, M. Effects of Medium pH on the Yeast Plasma Membrane Potential. Arch. Biochem. Biophys. 2024, 760, 110131. [Google Scholar] [CrossRef] [PubMed]
- Limapichat, W.; Pornthanakasem, W.; Satitthammachart, C.; Chitnumsub, P.; Leartsakulpanich, U. Real-Time Detection of Changes in Yeast Plasma Membrane Potential Using Genetically Encoded Voltage Indicator Proteins. FEMS Yeast Res. 2020, 20, 41. [Google Scholar] [CrossRef]
- Limapichat, W. Genetically Encoded Voltage Indicator Proteins Revealed Differential Effects of Hyperosmotic Stress on Yeast Plasma Membrane Potential Imposed by Different Stress Conditions. FEMS Microbiol. Lett. 2022, 368, 1–8. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Cell Wall Glucans of Fungi. A Review. Cell Surf. 2019, 5, 100022. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Analysis of the Phylogenetic Relationships and Evolution of the Cell Walls from Yeasts and Fungi. FEMS Yeast Res. 2010, 10, 225–243. [Google Scholar] [CrossRef]
- Pardo, J.P.; González-Andrade, M.; Allen, K.; Kuroda, T.; Slayman, C.L.; Rivetta, A. A Structural Model for Facultative Anion Channels in an Oligomeric Membrane Protein: The Yeast TRK (K+) System. Pflugers Arch. 2015, 467, 2447–2460. [Google Scholar] [CrossRef]
- Reyes-Rosario, D.; Pardo, J.P.; Guerra-Sánchez, G.; Vázquez-Meza, H.; López-Hernández, G.; Matus-Ortega, G.; González, J.; Baeza, M.; Romero-Aguilar, L. Analysis of the Respiratory Activity in the Antarctic Yeast Rhodotorula mucilaginosa M94C9 Reveals the Presence of Respiratory Supercomplexes and Alternative Elements. Microorganisms 2024, 12, 1931. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña, A.; Sánchez, N.S.; Calahorra, M. From the Metabolic Effects and Mechanism of Monovalent Cation Transport to the Actual Measurement of the Plasma Membrane Potential in Yeast. J. Fungi 2025, 11, 522. https://doi.org/10.3390/jof11070522
Peña A, Sánchez NS, Calahorra M. From the Metabolic Effects and Mechanism of Monovalent Cation Transport to the Actual Measurement of the Plasma Membrane Potential in Yeast. Journal of Fungi. 2025; 11(7):522. https://doi.org/10.3390/jof11070522
Chicago/Turabian StylePeña, Antonio, Norma Silvia Sánchez, and Martha Calahorra. 2025. "From the Metabolic Effects and Mechanism of Monovalent Cation Transport to the Actual Measurement of the Plasma Membrane Potential in Yeast" Journal of Fungi 11, no. 7: 522. https://doi.org/10.3390/jof11070522
APA StylePeña, A., Sánchez, N. S., & Calahorra, M. (2025). From the Metabolic Effects and Mechanism of Monovalent Cation Transport to the Actual Measurement of the Plasma Membrane Potential in Yeast. Journal of Fungi, 11(7), 522. https://doi.org/10.3390/jof11070522






