Unveiling the Hidden Diversity of Termitomyces (Lyophyllaceae, Agaricales) in Northern Thailand: Identification of Five New Species and the First Report of Termitomyces acriumbonatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Morphological Study
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Sequence Alignment and Phylogenetic Analyses
3. Results and Discussion
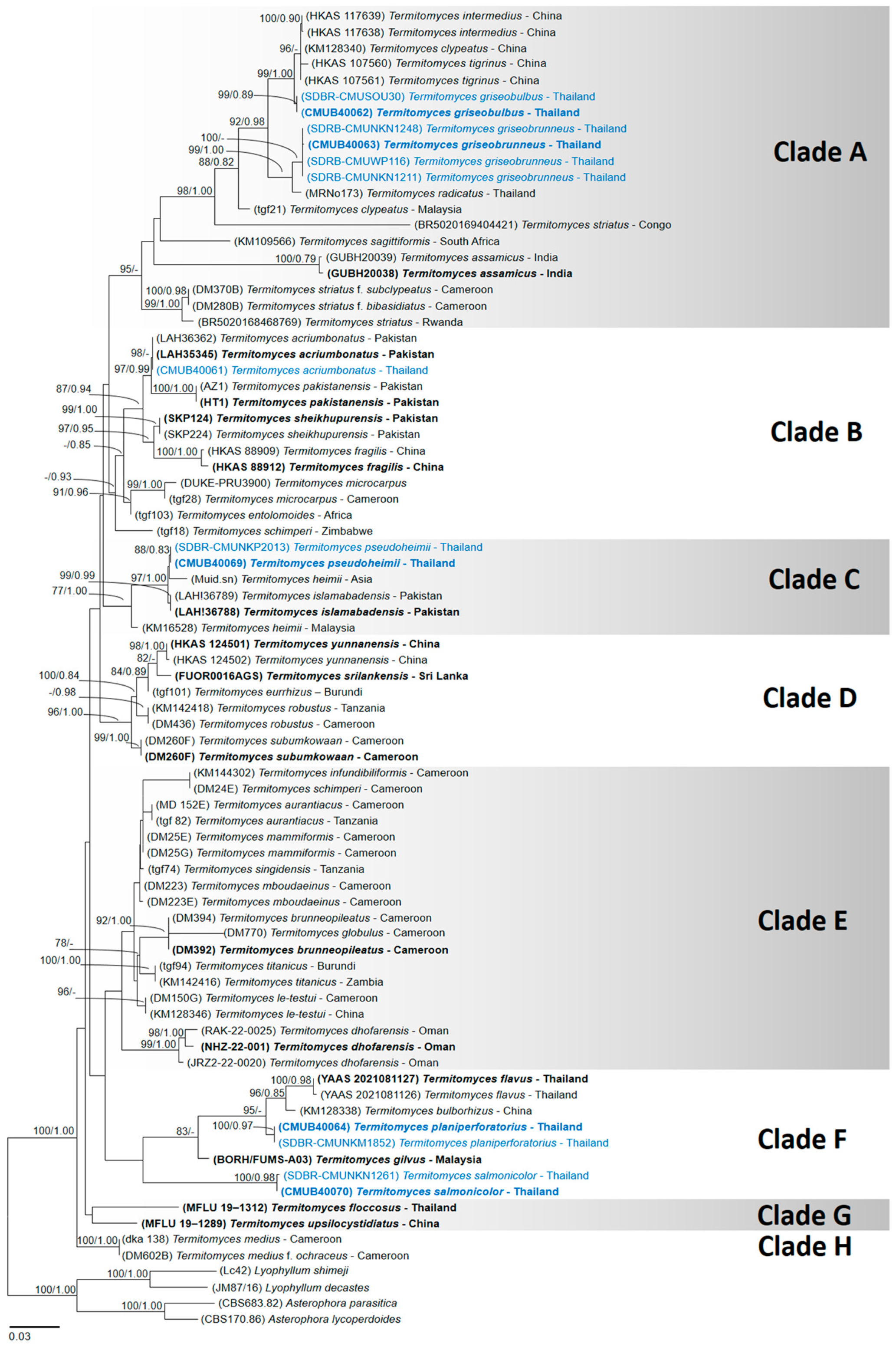
3.1. Phylogenetic Analysis
3.2. Taxonomy
3.2.1. Termitomyces acriumbonatus Usman & Khalid, Phytotaxa 477: 221 (2020)
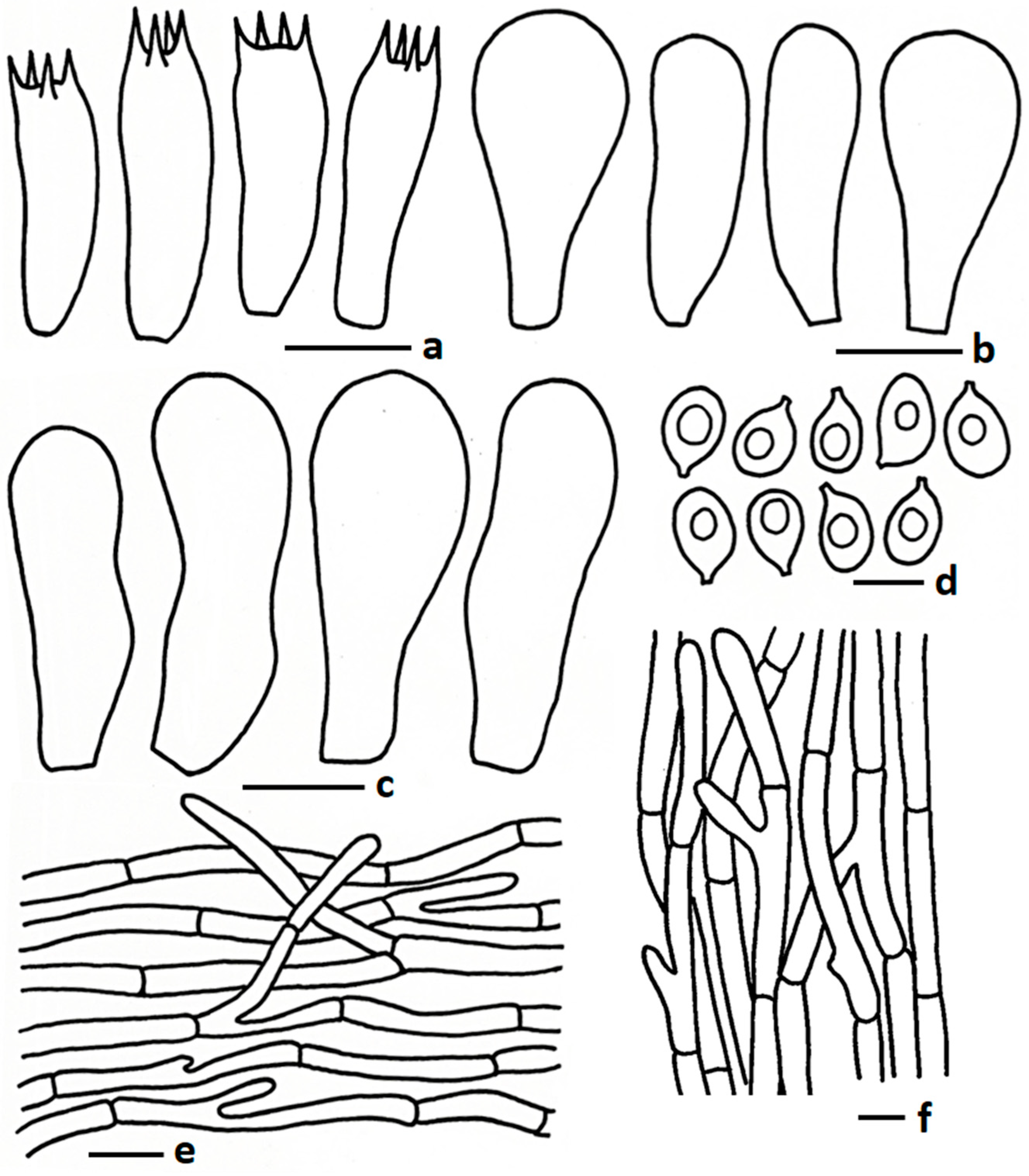
3.2.2. Termitomyces griseobulbus Paloi & N. Suwannar., sp. nov.
3.2.3. Termitomyces griseobrunneus Paloi, W. Phonrob & N. Suwannar., sp. nov.
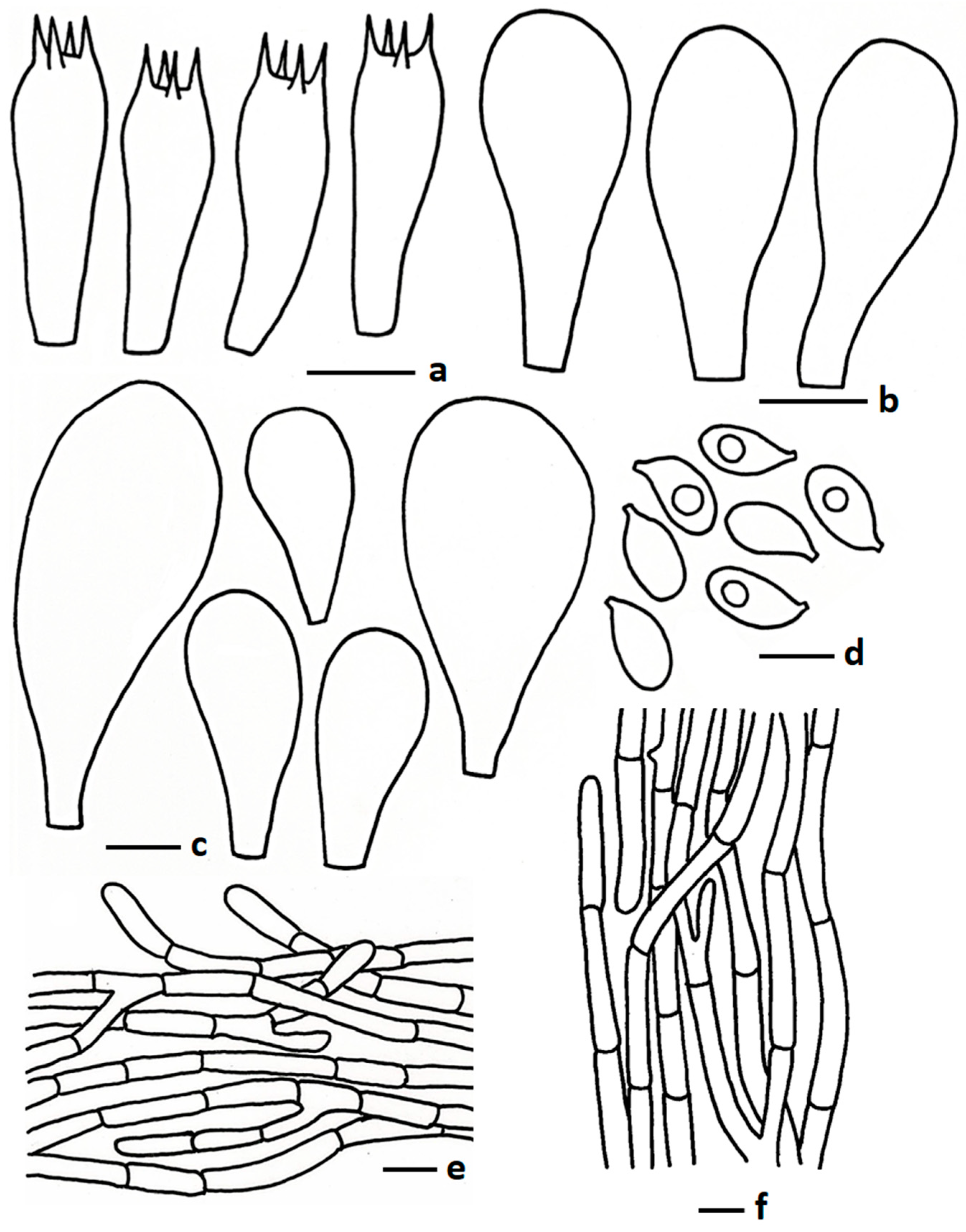
3.2.4. Termitomyces planiperforatorius Paloi & N. Suwannar., sp. nov.
3.2.5. Termitomyces pseudoheimii Paloi, N. Suwannar. & J. Kumla sp. nov.
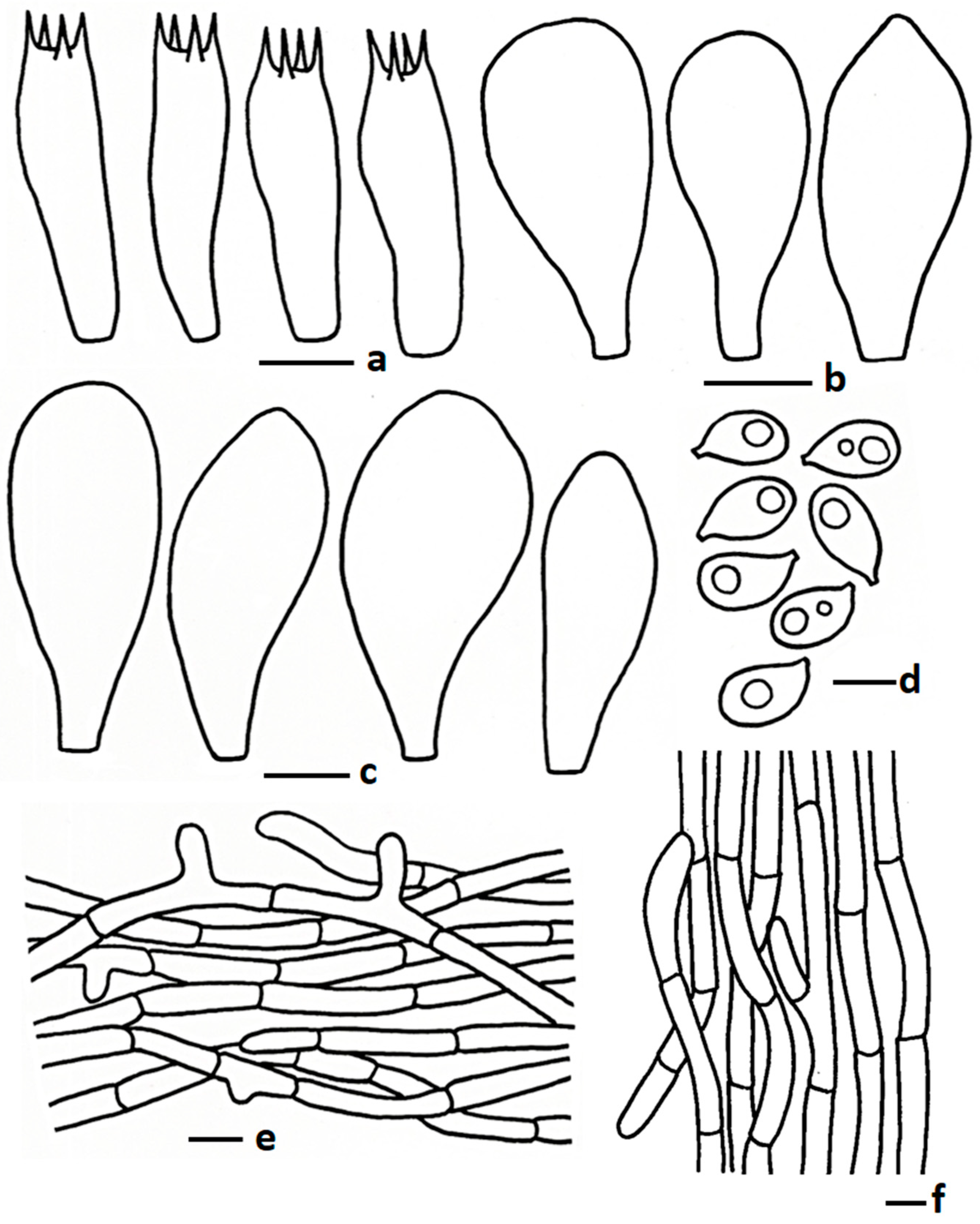
3.2.6. Termitomyces salmonicolor Paloi, W. Phonrob & N. Suwannar., sp. nov.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonelli, A.; Fry, C.; Smith, R.J.; Eden, J.; Govaerts, R.H.A.; Kersey, P.J.; Lughadha, E.M.N.; Onstein, R.E.; Simmonds, M.S.J.; Zizka, R.; et al. State of the World’s Plants and Fungi; Royal Botanic Gardens, Kew: Richmond, UK, 2023; pp. 1–96.
- Gopal, J.; Sivanesan, I.; Muthu, M.; Oh, J.-W. Scrutinizing the nutritional aspects of Asian mushrooms, its commercialization and scope for value-added products. Nutrients 2022, 14, 3700. [Google Scholar] [CrossRef]
- Ye, L.; Karunarathna, S.C.; Li, H.; Xu, J.; Hyde, K.D.; Mortimer, P.E. A survey of Termitomyces (Lyophyllaceae, Agaricales), including a new species, from a subtropical forest in Xishuangbanna, China. Mycobiology 2019, 47, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Teke, N.A.; Kinge, T.R.; Bechem, E.; Nji, T.M.; Ndam, L.M.; Mih, A.M. Ethnomycological study in the Kilum-Ijim mountain forest, Northwest Region, Cameroon. J. Ethnobiol. Ethnomed. 2018, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Njue, A.W.; Omolo, J.O.; Cheplogoi, P.K.; Langat, M.K.; Mulholland, D.A. Cytotoxic ergostane derivatives from the edible mushroom Termitomyces microcarpus (Lyophyllaceae). Biochem. Syst. Ecol. 2018, 76, 12–14. [Google Scholar] [CrossRef]
- Manna, S.; Ray, D.; Roy, A. Tribal relation to spatio-temporal variation of wild mushrooms in eastern lateritic part of India. Ethnobot. Res. Appl. 2014, 12, 15–24. [Google Scholar]
- Aryal, H.P.; Budhathoki, U. Ethnomycology of Termitomyces R. Heim in Nepal. J. Yeast Fungal Res. 2016, 7, 28–38. [Google Scholar]
- Sitotaw, R.; Lulekal, E.; Abate, E. Ethnomycological study of edible and medicinal mushrooms in Menge District, Asossa Zone, Benshangul Gumuz Region, Ethiopia. J. Ethnobiol. Ethnomed. 2020, 16, 11. [Google Scholar] [CrossRef]
- Heim, R. Nouvelles études descriptives sur les agarics termitophiles d’Afrique tropicale. Arch. Mus. Natl. d’Hist. Nat. 6ème Sér. 1942, 18, 107–166. [Google Scholar]
- Index Fungorum. Available online: https://www.indexfungorum.org/names/names.asp (accessed on 1 April 2025).
- Paloi, S.; Kumla, J.; Paloi, B.P.; Srinuanpan, S.; Hoijang, S.; Karunarathna, S.C.; Acharya, K.; Suwannarach, N.; Lumyong, S. Termite mushrooms (Termitomyces), a potential source of nutrients and bioactive compounds exhibiting human health benefits: A review. J. Fungi 2023, 9, 112. [Google Scholar] [CrossRef]
- Osiemo, Z.B.; Marten, A.; Kaib, M.; Gitonga, L.M.; Boga, H.I.; Brandl, R. Open relationships in the castles of clay: High diversity and low host specificity of Termitomyces fungi associated with fungus-growing termites in Africa. Insectes Sociaux 2010, 57, 351–363. [Google Scholar] [CrossRef]
- Aanen, D.K.; Eggleton, P.; Rouland-Lefèvre, C.; Guldberg-Frøslev, T.; Rosendahl, S.; Boomsma, J.J. The evolution of fungus growing termites and their mutualistic fungal symbionts. Proc. Natl. Acad. Sci. USA 2002, 99, 14887–14892. [Google Scholar] [CrossRef] [PubMed]
- Koné, N.A.; Yéo, K.; Konaté, S.; Linsenmair, K.E. Socio-economical aspects of the exploitation of Termitomyces fruit bodies in central and southern Côted’Ivoire: Raising awareness for their sustainable use. J. Appl. Biosci. 2013, 70, 5580–5590. [Google Scholar] [CrossRef]
- Tang, S.M.; He, M.Q.; Raspé, O.; Luo, X.; Zhang, X.L.; Li, Y.; Su, K.M.; Li, S.H.; Thongklang, N.; Hyde, K.D. Two new species of Termitomyces (Agaricales, Lyophyllaceae) from China and Thailand. Phytotaxa 2020, 439, 231–242. [Google Scholar] [CrossRef]
- Tang, S.M.; Vadthanarat, S.; He, J.; Raghoonundon, B.; Yu, F.M.; Karunarathna, S.C.; Li, S.H.; Raspé, O. Morphological and molecular analyses reveal two new species of Termitomyces (Agaricales, Lyophyllaceae) and morphological variability of T. intermedius. MycoKeys 2023, 95, 61–82. [Google Scholar] [CrossRef]
- Izhar, A.; Khalid, A.N.; Bashir, H. Termitomyces sheikhupurensis sp. nov. (Lyophyllaceae, Agaricales) from Pakistan, evidence from morphology and DNA sequences data. Turk. J. Bot. 2022, 44, 694–704. [Google Scholar] [CrossRef]
- Usman, M.; Khalid, A.N. Termitomyces acriumbonatus sp. nov. (Lyophyllaceae, Agaricales) from Pakistan. Phytotaxa 2020, 477, 217–228. [Google Scholar] [CrossRef]
- Ashraf, S.; Usman, M.; Khalid, A.N. Termitomyces islamabadensis sp. nov. (Lyophyllaceae, Agaricales) from the Foothills of the Pakistani Himalayas. Biol. Bull. Russ. Acad. Sci. 2022, 49, S66–S76. [Google Scholar] [CrossRef]
- Razaq, A.; Ishaq, A.; Ilyas, S.; Sadia, S. Termitomyces pakistanensis, a new mushroom species from Pakistan based on scanning electron microscopy and ITS-rDNA barcoding. Microsc. Res. Tech. 2023, 86, 115–121. [Google Scholar] [CrossRef]
- Seelan, J.S.S.; Yee, C.S.; Fui, F.S.; Dawood, M.; Tan, Y.S.; Kim, M.J.; Park, M.S.; Lim, Y.W. New species of Termitomyces (Lyophyllaceae, Basidiomycota) from Sabah (Northern Borneo), Malaysia. Mycobiology 2020, 48, 95–103. [Google Scholar] [CrossRef]
- Ediriweera, A.N.; Voto, P.; Karunarathna, S.C.; Dilshan, B.C. Termitomyces srilankensis sp. nov. (Lyophyllaceae, Agaricales), a new species from Sri Lanka. MycolObs 2023, 6, 47–53. [Google Scholar]
- Das, L.R.; Chattopadhyay, P.; Dutta, A.K.; Narzary, D.; Rana, T.S. Termitomyces assamicus (Lyophyllaceae)—A new species of Termitomyces from India. Phytotaxa 2023, 599, 126–136. [Google Scholar] [CrossRef]
- Hussain, S.; Al-Kharousi, M.; Al-Owaisi, A.A.; Al-Maqbali, D.; Al-Muharabi, M.A.; Al-Shabibi, Z.; Al-Balushi, A.H.; Al Saady, N.; Velazhahan, R.; Rashan, L.; et al. The genus Termitomyces: Outline, phylogeny, and divergence times estimation with description of a new edible species from Arabian Peninsula. Sydowia 2024, 76, 187–200. [Google Scholar]
- Sangvichien, E.; Taylor-Hawksworth, P.A. Termitomyces mushrooms: A tropical delicacy. Mycologist 2001, 15, 31–33. [Google Scholar] [CrossRef]
- Sawhasan, P.; Worapong, J.; Vinijsanun, T. Morphological and molecular studies of selected Termitomyces species collected from 8 districts of Kanchanaburi Province, Thailand. Thai J. Agric. Sci. 2011, 44, 183–196. [Google Scholar]
- Jannual, N.; Nipitwattanaphon, M.; Hasin, S.; Kaewgrajang, T. Morphological and molecular characterization of Termitomyces (Lyophyllaceae, Agaricales) in Thailand. Biodiversitas 2020, 22, 2481–2491. [Google Scholar] [CrossRef]
- Srikram, A.; Supapvanich, S. Proximate compositions and bioactive compounds of edible wild and cultivated mushrooms from Northeast Thailand. Agric. Nat. Resour. 2016, 50, 432–436. [Google Scholar] [CrossRef]
- Tang, S.-M.; Li, E.-X.; Luo, H.-M.; Ao, C.-C.; Lv, T.; Li, S.-H. Termitomyces flavus sp. nov. (Lyophyllaceae, Agaricales), a new species from northern Thailand. Chiang Mai J. Sci. 2024, 51, e2024060. [Google Scholar] [CrossRef]
- Hughes, A.C. Mapping priorities for conservation in Southeast Asia. Biol. Conserv. 2017, 209, 395–405. [Google Scholar] [CrossRef]
- Liengsiri, C.; Luangjame, J.; Ponoy, B.; Sukhotanang, A.; Visuthitepkul, S.; Thienhirun, S.; Vacharangkura, T.; Luangviriyasaeng, V.; Semsuntud, N.; Wichiennopparat, W.; et al. Forestry in Thailand; Royal Forest Department, Ministry of Natural Resources and Environment: Bangkok, Thailand, 2009; pp. 1–48.
- Lertlumnaphakul, W.; Ngoen-Klan, R.; Vongkaluang, C.; Chareonviriyaphap, T. A review of termite species and their distribution in Thailand. Insects 2022, 13, 186. [Google Scholar] [CrossRef]
- Kornerup, A.; Wanscher, J.H. Taschenlexikon der Farben, 3rd ed.; Musterschmidt: Northeim-Sudheim, Germany, 1981. [Google Scholar]
- Mossebo, D.C.; Essouman, E.; Machouart, M.; Gueidan, C. Phylogenetic relationships, taxonomic revision and new taxa of Termitomyces (Lyophyllaceae, Basidiomycota) inferred from combined nLSU and mtSSU rDNA sequences. Phytotaxa 2017, 321, 71–102. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Paloi, S.; Suwannarach, N.; Kumla, J.; Phonrob, W.; Karunarathna, S.C.; Lumyong, S. An update on species diversity, distribution and sequence data of Tulostoma in Asia with the addition of Tulostoma exasperatum, a new record for Thailand. Chiang Mai J. Sci. 2023, 50, e2023018. [Google Scholar] [CrossRef]
- Moncalvo, J.M.; Lutzoni, F.M.; Rehner, S.A.; Johnson, J.; Vilgalys, R. Phylogenetic relationships of agaric fungi based on nuclear large subunit ribosomal DNA sequences. Syst. Biol. 2000, 49, 278–305. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, V.; Clémençon, H.; Vilgalys, R.; Moncalvo, J.M. Phylogenetic analyses of the Lyophylleae (Agaricales, Basidiomycota) based on nuclear and mitochondrial rDNA sequences. Mycol. Res. 2002, 106, 1043–1059. [Google Scholar] [CrossRef]
- Frøslev, T.G.; Aanen, D.K.; Læssøe, T.; Rosendahl, S. Phylogenetic relationships of Termitomyces and related taxa. Mycol. Res. 2003, 107, 1277–1286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Willis, E. The Taxonomic Identity of Termitomyces aurantiacus Using the Internal Transcribed Spacer Regions 1 & 2 (ITS1 & ITS2) of the Ribosomal DNA (rDNA). Ph.D. Thesis, University of Putra, Serdang, Malaysia, 2007. [Google Scholar]
- Hofstetter, V.; Redhead, S.A.; Kauff, F.; Moncalvo, J.M.; Matheny, P.B.; Vilgalys, R. Taxonomic revision and examination of ecological transitions of the Lyophyllaceae (Basidiomycota, Agaricales) based on a multigene phylogeny. Cryptogam. Mycol. 2014, 35, 399–425. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, e772. [Google Scholar] [CrossRef]
- Hillis, D.M.; Bull, J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 1993, 42, 182–192. [Google Scholar] [CrossRef]
- Alfaro, M.E.; Zoller, S.; Lutzoni, F. Bayes or bootstrap? A simulation study comparing the performance of bayesian markov chain monte carlo sampling and bootstrapping in assessing phylogenetic confidence. Mol. Biol. Evol. 2003, 20, 255–266. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree, v1.3.1. 2009. Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 10 February 2025).
- Natarajan, K. A new species of Termitomyces from India. Curr. Sci. 1977, 46, 679–680. [Google Scholar]
- Heim, R. Les Termitomyces du Congo Belge recueillis par Madame M. Goossens-Fontana. Bull. Jard. Bot. État. Brux. 1951, 21, 205–222. [Google Scholar] [CrossRef]
- Wei, T.Z.; Tang, B.H.; Yao, Y.J. Revision of Termitomyces in China. Mycotaxon 2009, 108, 257–285. [Google Scholar] [CrossRef]
- Wei, T.Z.; Yao, Y.J.; Wang, B.; Pegler, D.N. Termitomyces bulborhizus sp. nov. from China, with a key to allied species. Mycol. Res. 2004, 108, 1458–1462. [Google Scholar] [CrossRef]
- Westhuizen, G.C.A.; Eicker, A. Species of Termitomyces occurring in South Africa. Mycol. Res. 1990, 94, 923–937. [Google Scholar] [CrossRef]
- Natarajan, K. South Indian Agaricales V: Termitomyces heimii. Mycologia 1979, 71, 853–855. [Google Scholar] [CrossRef]
- Wei, T.Z.; Tang, B.H.; Yao, Y.J.; Pegler, D.N. A revision of Sinotermitomyces, a synonym of Termitomyces (Agaricales). Fungal Divers. 2006, 21, 225–237. [Google Scholar]

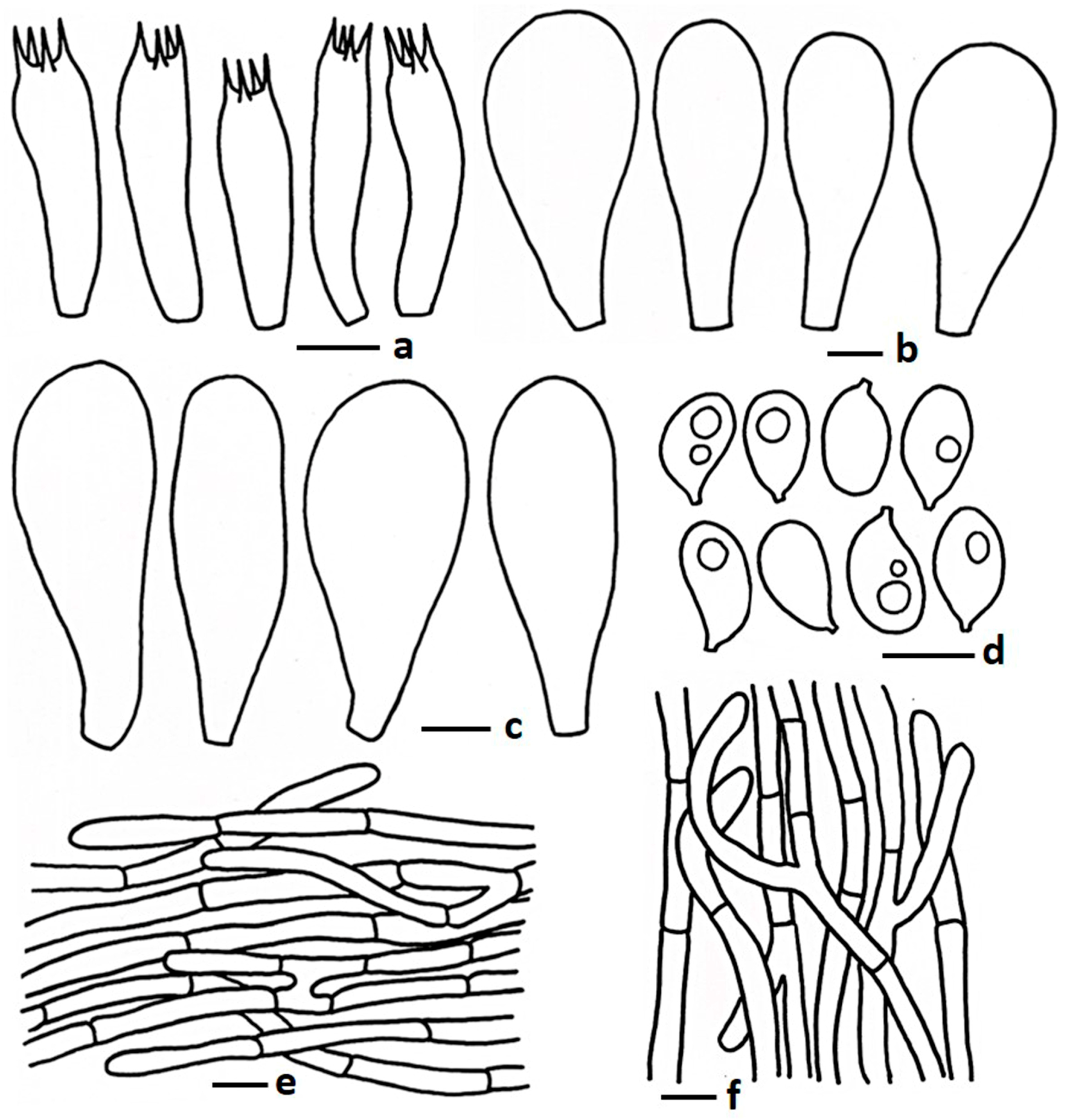
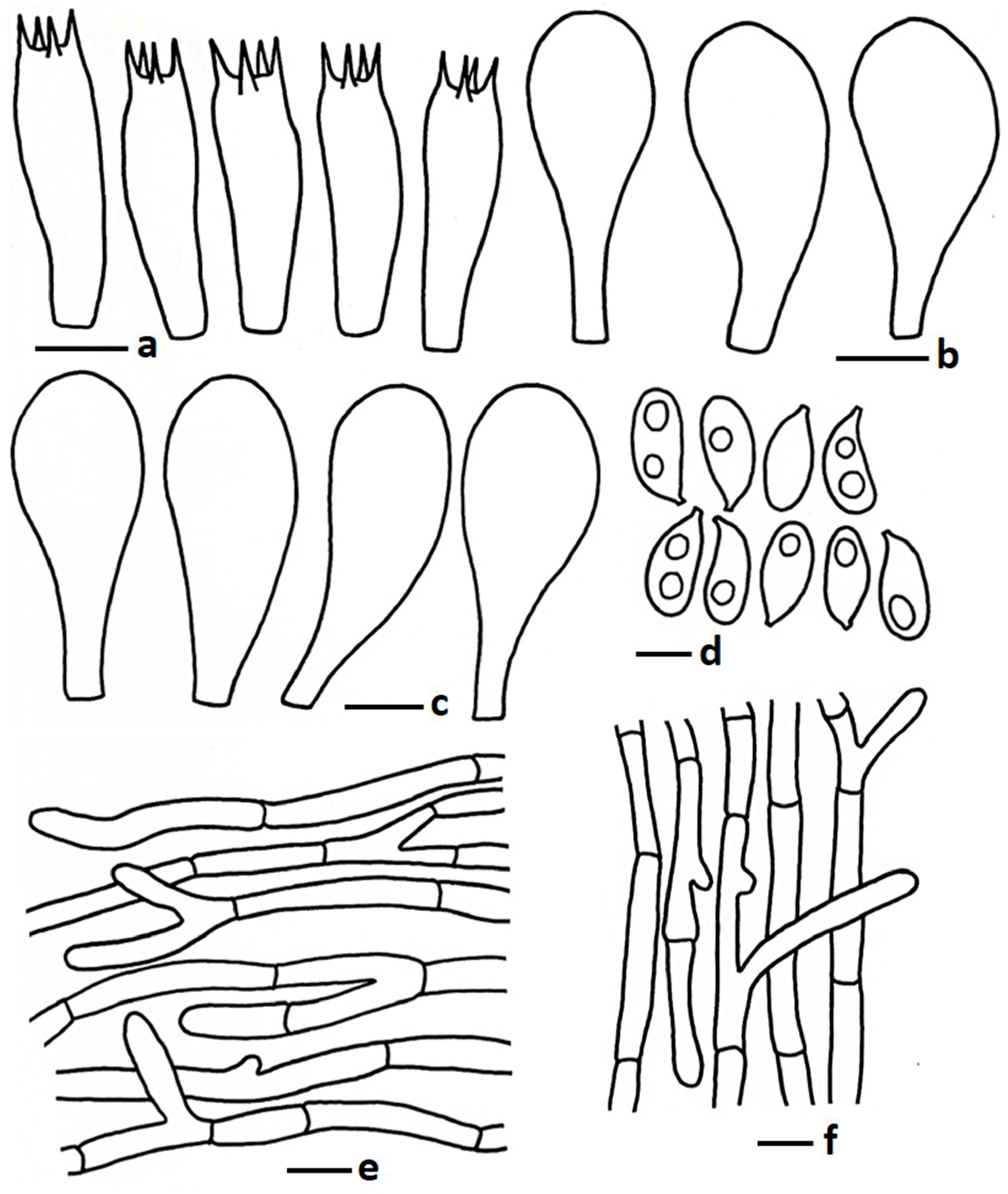
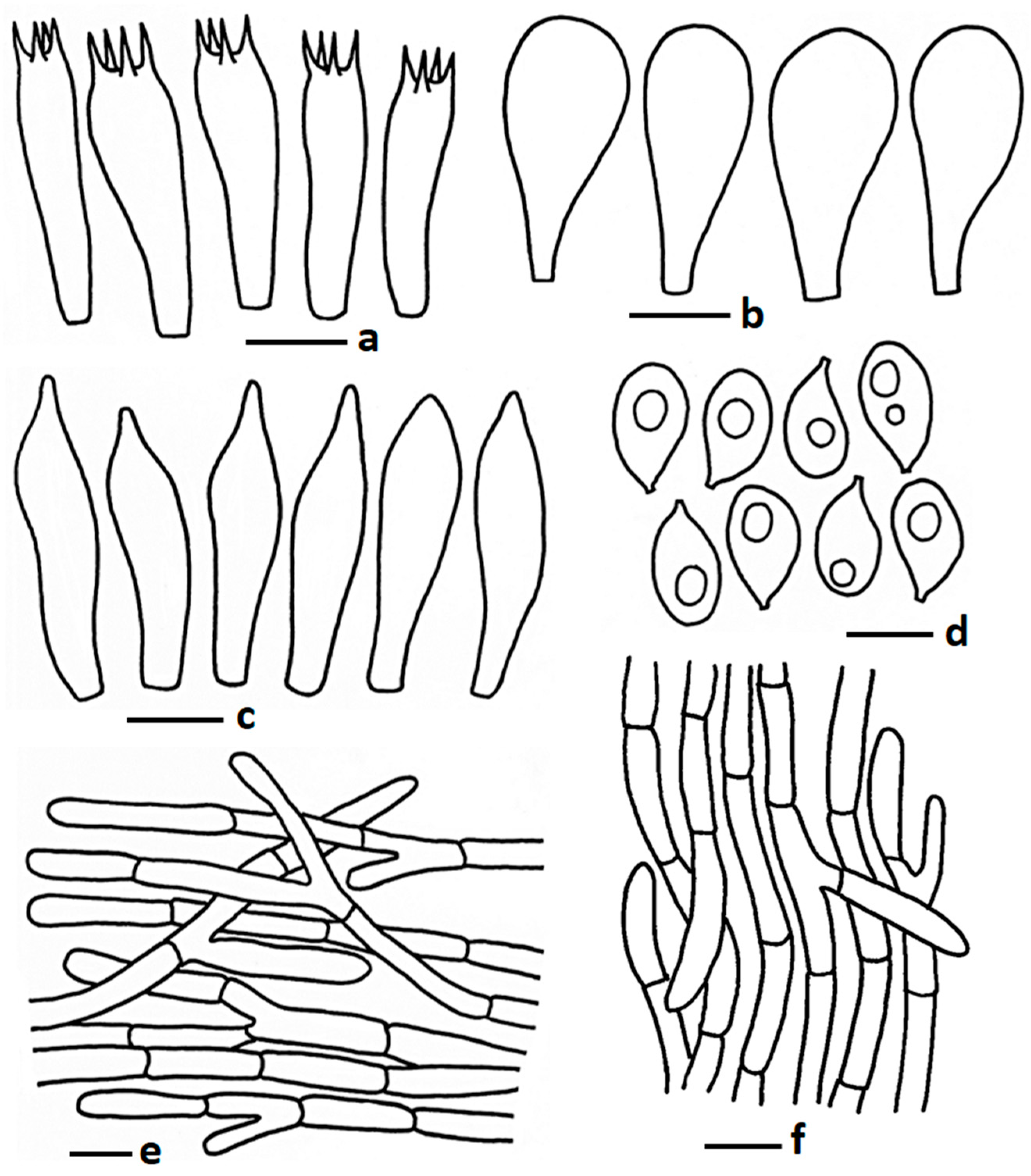
| Taxa | Voucher Number | Country | GenBank Accession Number | Reference | ||
|---|---|---|---|---|---|---|
| nrITS | nrLSU | mtSSU | ||||
| Termitomyces acriumbonatus T | LAH35345 | Pakistan | MT179688 | MT179689 | NA | [17] |
| T. acriumbonatus | LAH36362 | Pakistan | MT179687 | MT179690 | NA | [17] |
| T. acriumbonatus | CMUB40061 | Thailand | PQ896627 | PQ896626 | PV020670 | This study |
| T. assamicus T | GUBH20038 | India | OQ346313 | NA | NA | [23] |
| T. assamicus | GUBH20039 | India | OQ976999 | NA | NA | [23] |
| T. aurantiacus | DM 152E | Cameroon | NA | KY809234 | KY809186 | [41] |
| T. aurantiacus | tgf 82 | Tanzania | NA | AY127804 | AY127852 | [13] |
| T. brunneopileatus T | DM392 | Cameroon | NA | KY809273 | KY809225 | [34] |
| T. brunneopileatus | DM394 | Cameroon | NA | KY809244 | KY809197 | [34] |
| T. bulborhizus | KM128338 | China | NA | KY809261 | KY809213 | [34] |
| T. clypeatus | KM128340 | China | NA | KY809262 | KY809214 | [34] |
| T. clypeatus | tgf21 | Malaysia | NA | AY127802 | AY127850 | [13] |
| T. dhofarensis | JRZ2-22-0020 | Oman | OR297695 | OR338598 | NA | [24] |
| T. dhofarensis | RAK-22-0025 | Oman | OR297696 | OR338597 | NA | [24] |
| T. dhofarensis T | NHZ-22-001 | Oman | OR297694 | NA | NA | [24] |
| T. entolomoides | tgf103 | Africa | NA | AY232693 | AY232680 | [40] |
| T. eurrhizus | tgf101 | Burundi | NA | AY232694 | NA | [40] |
| T. floccosus T | MFLU 19–1312 | Thailand | MN633305 | MN701029 | [15] | |
| T. fragilis T | HKAS 88912 | China | KY214475 | NA | NA | [3] |
| T. fragilis | HKAS 88909 | China | KY214476 | NA | NA | [3] |
| T. gilvus T | BORH/FUMS-A03 | Malaysia | NA | MK472701 | MK478904 | [21] |
| T. globulus | DM770 | Cameroon | NA | KY809252 | KY809204 | [39] |
| T. griseobulbus T | CMUB40062 | Thailand | PQ900075 | PQ896628 | PV020671 | This study |
| T. griseobulbus | SDBR–CMUSOU30 | Thailand | PQ900076 | PQ896629 | PV020672 | This study |
| T. griseobrunneus T | CMUB40063 | Thailand | PQ899488 | PQ896878 | PV020673 | This study |
| T. griseobrunneus | SDBR–CMUNKN1248 | Thailand | PQ899489 | PQ899490 | PV020676 | This study |
| T. griseobrunneus | SDBR–CMUWP116 | Thailand | NA | PQ896901 | PV020674 | This study |
| T. griseobrunneus | SDBR–CMUNKN1211 | Thailand | NA | PQ899491 | PV020675 | This study |
| T. heimii | KM16528 | Malaysia | NA | KY809253 | KY809205 | [34] |
| T. heimii | Muid.sn | Asia | NA | AF042586 | AF357091 | [38] |
| T. infundibiliformis | KM144302 | Cameroon | NA | KY809245 | NA | [34] |
| T. intermedius | HKAS 117638 | China | ON557369 | ON556484 | ON557367 | [16] |
| T. intermedius | HKAS 117639 | China | ON557370 | ON556485 | ON557368 | [16] |
| T. islamabadensis T | LAH!36788 | Pakistan | MW520178 | OM100949 | NA | [18] |
| T. islamabadensis | LAH!36789 | Pakistan | MW520179 | OM100950 | NA | [18] |
| T. le-testui | DM150G | Cameroon | NA | KY809231 | KY809184 | [34] |
| T. le-testui | KM128346 | China | NA | KY809263 | KY809215 | [34] |
| T. mammiformis | DM25E | Cameroon | NA | KY809229 | KY809182 | [34] |
| T. mammiformis | DM25G | Cameroon | NA | KY809230 | KY809183 | [34] |
| T. mboudaeinus | DM223 | Cameroon | NA | KY809274 | KY809226 | [34] |
| T. mboudaeinus | DM223E | Cameroon | NA | KY809237 | KY809189 | [34] |
| T. medius | dka 138 | Cameroon | NA | AY127796 | AY127844 | [13] |
| T. medius f. ochraceus | DM602B | Cameroon | NA | KY809246 | KY809198 | [34] |
| T. microcarpus | tgf28 | Cameroon | NA | AY127799 | AY127847 | [13] |
| T. microcarpus | DUKE-PRU3900 | NA | AF357023 | AF042587 | AF357092 | [39] |
| T. pakistanensis | HT1 | Pakistan | OP688120 | NA | NA | [20] |
| T. pakistanensis | AZ1 | Pakistan | OP688121 | NA | NA | [20] |
| T. planiperforatorius T | CMUB40064 | Thailand | NA | PQ896983 | PV020677 | This study |
| T. planiperforatorius | SDBR–CMUNKM1852 | Thailand | NA | PQ896985 | PV020678 | This study |
| T. pseudoheimii T | CMUB40069 | Thailand | PQ897224 | PQ897223 | PV020679 | This study |
| T. pseudoheimii | SDBR–CMUNKP2013 | Thailand | PQ897225 | PQ897226 | PV020680 | This study |
| T. radicatus | MRNo173 | Thailand | LC068787 | NA | NA | UnP |
| T. robustus | KM142418 | Tanzania | NA | KY809265 | KY809217 | [34] |
| T. robustus | DM436 | Cameroon | NA | KY809271 | KY809223 | [34] |
| T. sagittiformis | KM109566 | South Africa | NA | KY809260 | KY809212 | [34] |
| T. salmonicolor T | CMUB40070 | Thailand | NA | PQ897227 | PV020681 | This study |
| T. salmonicolor | SDBR–CMUNKN1261 | Thailand | NA | PQ897229 | PV020682 | This study |
| T. schimperi | DM24E | Cameroon | NA | KY809228 | KY809181 | [34] |
| T. schimperi | tgf18 | Zimbabwe | NA | AY232712 | AY232686 | [40] |
| T. sheikhupurensis T | SKP124 | Pakistan | MT192217 | MT192228 | NA | [19] |
| T. sheikhupurensis | SKP224 | Pakistan | MT192218 | NA | NA | [19] |
| T. singidensis | tgf74 | Tanzania | NA | AY232713 | AY232687 | [40] |
| T. srilankensis T | FUOR0016AGS | Sri Lanka | ON685313 | NA | NA | [22] |
| T. striatus | BR5020169404421 | Congo | OP179299 | OP168080 | OP179293 | [16] |
| T. striatus | BR5020168468769 | Rwanda | OP179297 | OP168081 | OP179294 | [16] |
| T. striatus f. bibasidiatus | DM280B | Cameroon | NA | KY809241 | KY809193 | [34] |
| T. striatus f. subclypeatus | DM370B | Cameroon | NA | KY809268 | KY809220 | [34] |
| T. subumkowaan | DM260F | Cameroon | NA | KY809239 | KY809190 | [34] |
| T. subumkowaan T | DM260B | Cameroon | NA | KY809275 | KY809227 | [34] |
| T. tigrinus | HKAS 107560 | China | MT683156 | MT679729 | MT683152 | [16] |
| T. tigrinus T | HKAS 107561 | China | MT683157 | MT679730 | MT683153 | [16] |
| T. titanicus | tgf94 | Burundi | NA | AY127801 | AY127849 | [13] |
| T. titanicus | KM142416 | Zambia | NA | KY809264 | KY809216 | [34] |
| T. upsilocystidiatus T | MFLU 19–1289 | China | NA | MN636637 | MN636642 | [15] |
| T. yunnanensis T | HKAS 124501 | China | OP179295 | OP168083 | OP179290 | [16] |
| T. yunnanensis | HKAS 124502 | China | OP179296 | OP168084 | OP179291 | [16] |
| Termitomyces flavus T | YAAS 2021081127 | Thailand | PP264695 | PP264703 | PP264701 | [29] |
| Termitomyces flavus | YAAS 2021081126 | Thailand | PP264696 | PP264704 | PP264702 | [29] |
| Asterophora lycoperdoides | CBS170.86 | NA | AF357037 | AF223190 | AF357109 | [42] |
| A. parasitica | CBS683.82 | NA | AF357038 | AF223191 | AF357110 | [42] |
| Lyophyllum shimeji | Lc42 | NA | AF357060 | AF357078 | AF357137 | [42] |
| L. decastes | JM87/16 | NA | AF357059 | AF042583 | AF357136 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paloi, S.; Kumla, J.; Phonrob, W.; Paloi, B.P.; Suwannarach, N. Unveiling the Hidden Diversity of Termitomyces (Lyophyllaceae, Agaricales) in Northern Thailand: Identification of Five New Species and the First Report of Termitomyces acriumbonatus. J. Fungi 2025, 11, 830. https://doi.org/10.3390/jof11120830
Paloi S, Kumla J, Phonrob W, Paloi BP, Suwannarach N. Unveiling the Hidden Diversity of Termitomyces (Lyophyllaceae, Agaricales) in Northern Thailand: Identification of Five New Species and the First Report of Termitomyces acriumbonatus. Journal of Fungi. 2025; 11(12):830. https://doi.org/10.3390/jof11120830
Chicago/Turabian StylePaloi, Soumitra, Jaturong Kumla, Wiphawanee Phonrob, Barsha Pratiher Paloi, and Nakarin Suwannarach. 2025. "Unveiling the Hidden Diversity of Termitomyces (Lyophyllaceae, Agaricales) in Northern Thailand: Identification of Five New Species and the First Report of Termitomyces acriumbonatus" Journal of Fungi 11, no. 12: 830. https://doi.org/10.3390/jof11120830
APA StylePaloi, S., Kumla, J., Phonrob, W., Paloi, B. P., & Suwannarach, N. (2025). Unveiling the Hidden Diversity of Termitomyces (Lyophyllaceae, Agaricales) in Northern Thailand: Identification of Five New Species and the First Report of Termitomyces acriumbonatus. Journal of Fungi, 11(12), 830. https://doi.org/10.3390/jof11120830







