Co-Culture of Monascus purpureus and Aspergillus niger Isolated from Wuyi Hongqu to Enhance Monascus Pigments Production While Inhibiting Citrinin Production
Abstract
1. Introduction
2. Materials and Methods
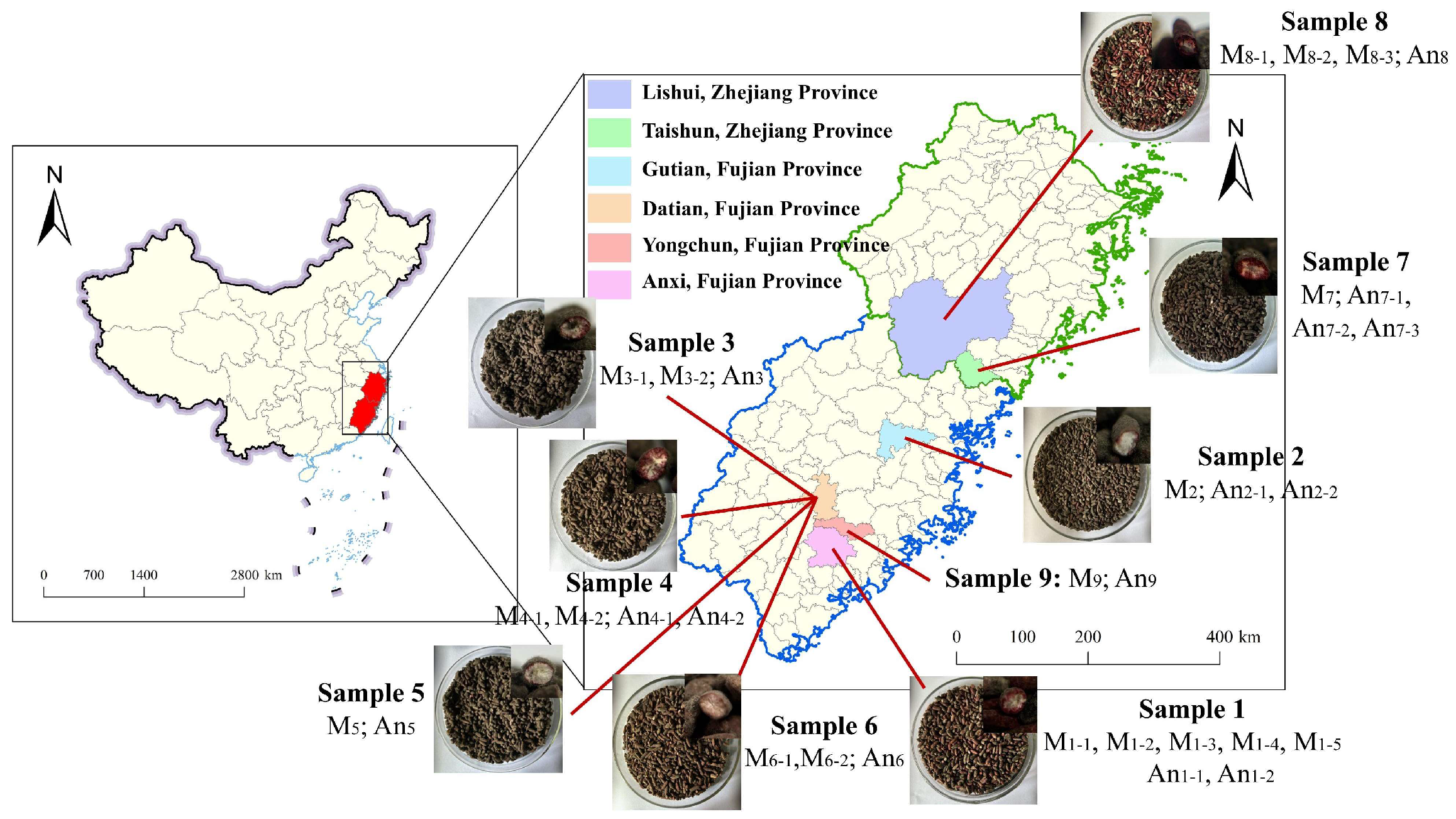
2.1. Samples
2.2. Media
2.3. Strain Isolation
2.4. Colonial and Microscopic Morphologies of Strains Isolated from WYH
2.5. DNA Extraction, Sequencing, and Microbial Classification
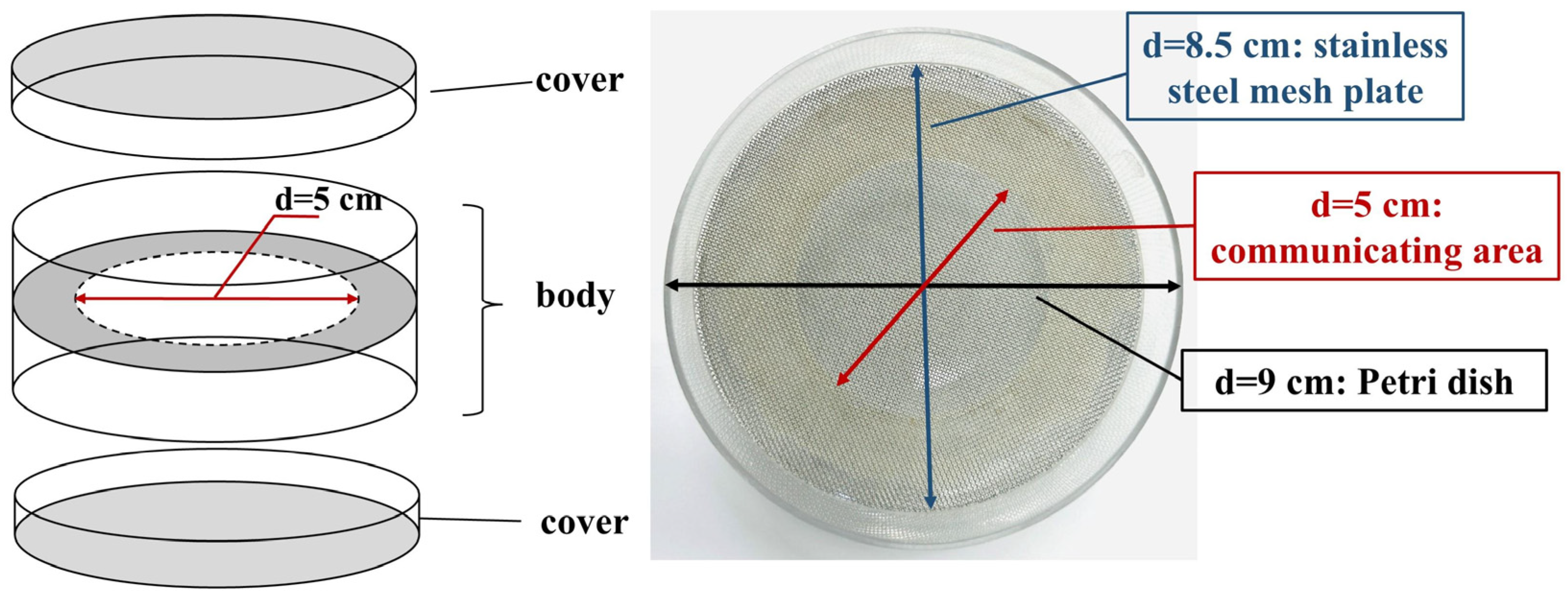
2.6. Preparation for Double-Sided Petri Dish
2.7. Co-Culture Methods for Fungi
2.8. Detection of Secondary Metabolites
2.9. RT-qPCR
3. Results
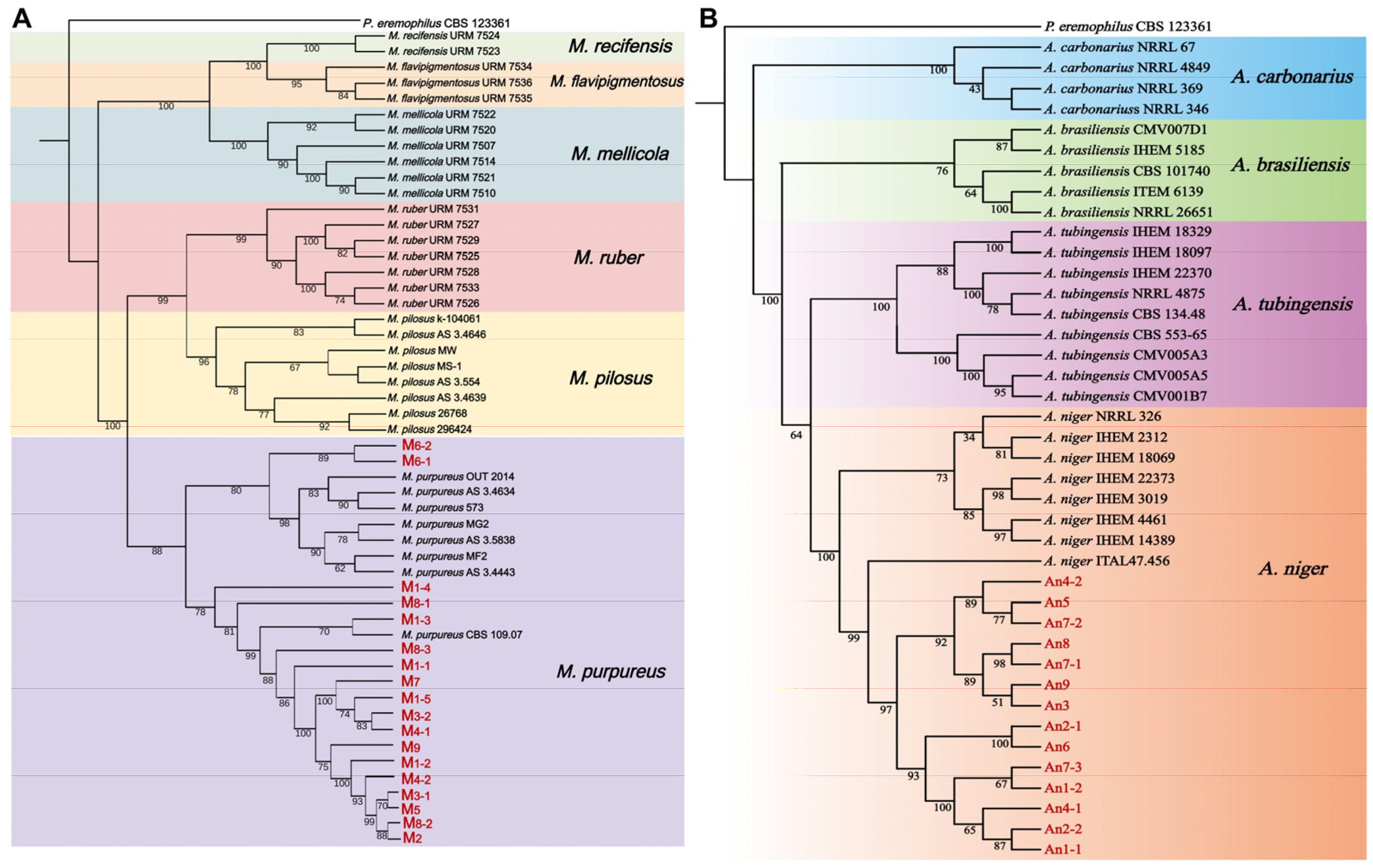
3.1. Isolation and Classification of Fungal Strains from WYH Samples
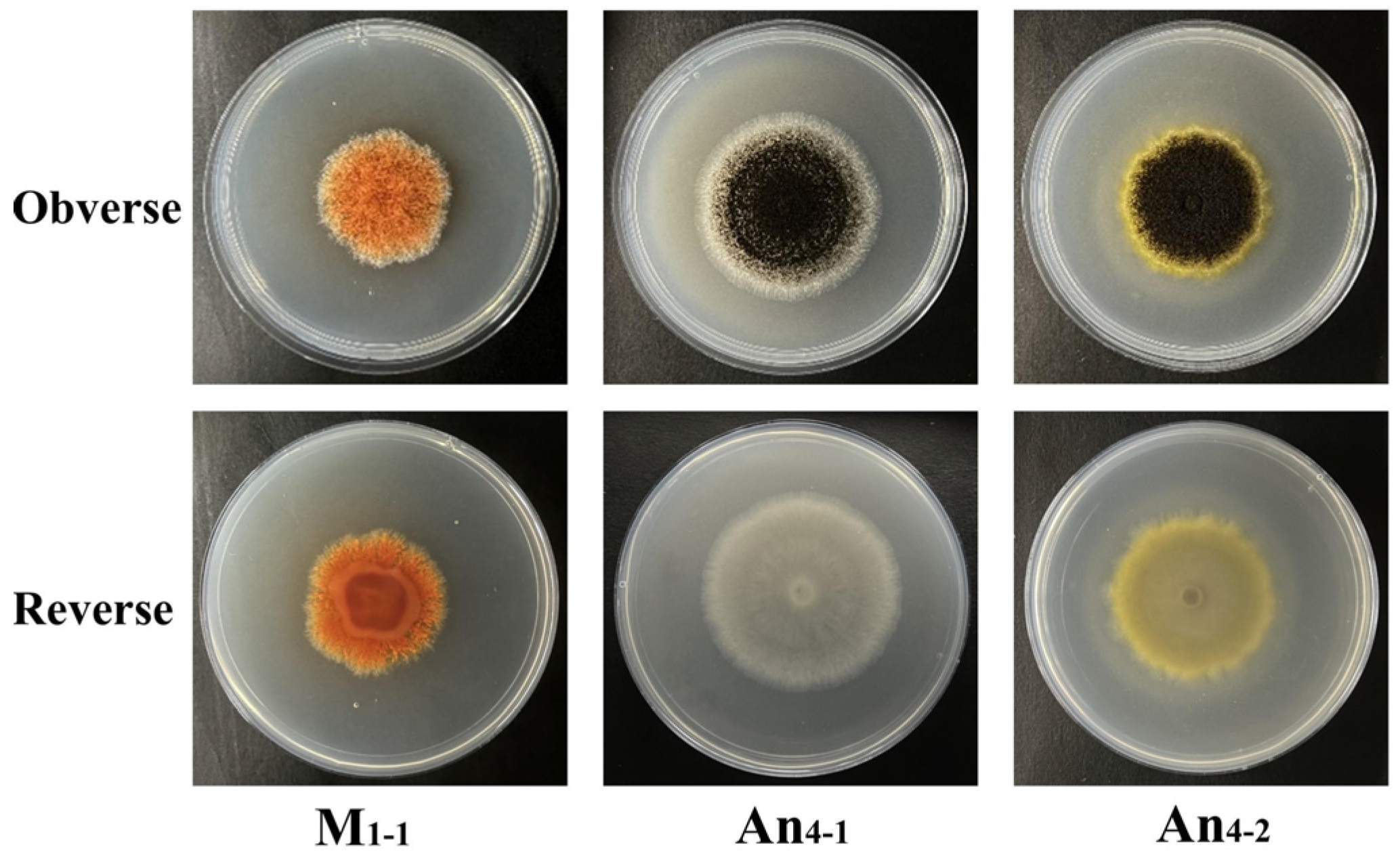
3.2. Morphological Properties and Biomass in Co-Culture of M. purpureus and A. niger
3.3. Co-Cultivation Effects on M. purpureus Secondary Metabolites
3.4. RT-qPCR for Analyzing Expression Levels of Genes Relative MPs and CIT Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, W.; Qian, M.; Dong, H.; Liu, X.; Bai, W.; Liu, G.; Lv, X. Effect of Hong Qu on the flavor and quality of Hakka yellow rice wine (Huangjiu) produced in Southern China. LWT 2022, 160, 113264. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, C.; Gao, X.; Kang, Y.; Huang, M.; Wu, J.; Liu, Y.; Zhang, J.; Li, H.; Zhang, Y. Characterization of key aroma compounds in Huangjiu from northern China by sensory-directed flavor analysis. Food Res. INT 2020, 134, 109238. [Google Scholar] [CrossRef]
- Mao, Q.; Tu, X.; Jia, X.; Zhou, L. The fermentationtechnology of black-skin-red-koji and the brewing of indica rice wine. Jiangsu Condiment Subsid. Food 2011, 28, 28–31, 40. (In Chinese) [Google Scholar]
- Huang, Y.Y.; Liang, Z.C.; Lin, X.Z.; He, Z.G.; Ren, X.Y.; Li, W.X.; Molnár, I. Fungal community diversity and fermentation characteristics in regional varieties of traditional fermentation starters for Hong Qu glutinous rice wine. Food Res. Int. 2021, 141, 110146. [Google Scholar] [CrossRef]
- Lv, X.C.; Huang, R.L.; Chen, F.; Zhang, W.; Rao, P.F.; Ni, L. Bacterial community dynamics during the traditional brewing of Wuyi Hong Qu glutinous rice wine as determined by culture-independent methods. Food Control 2013, 34, 300–306. [Google Scholar] [CrossRef]
- Park, K.H.; Liu, Z.; Park, C.S.; Ni, L. Microbiota associated with the starter cultures and brewing process of traditional Hong Qu glutinous rice wine. Food Sci. Biotechnol. 2016, 25, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.C.; Huang, Z.Q.; Zhang, W.; Rao, P.F.; Ni, L. Identification and characterization of filamentous fungi isolated from fermentation starters for Hong Qu glutinous rice wine brewing. J. Gen. Appl. Microbiol. 2012, 58, 33–42. [Google Scholar] [CrossRef]
- Arruda, G.L.; Reis, W.S.M.; Raymundo, M.T.F.R.; Shibukawa, V.P.; Cruz-Santos, M.M.; Silos, N.O.; Prado, C.A.; Marcelino, P.R.F.; Silva, S.S.; Santos, J.C. Biotechnological potential of Monascus: Biological aspects, metabolites of interest, and opportunities for new products. Microbiol. Res. 2025, 297, 128177. [Google Scholar] [CrossRef] [PubMed]
- Farawahida, A.H.; Palmer, J.; Flint, S. Monascus spp. and citrinin: Identification, selection of Monascus spp. isolates, occurrence, detection and reduction of citrinin during the fermentation of red fermented rice. Int. J. Food Microbiol. 2022, 379, 109829. [Google Scholar] [CrossRef]
- Feng, Y.; Shao, Y.; Chen, F. Monascus pigments. Appl. Microbiol. Biot. 2012, 96, 1421–1440. [Google Scholar] [CrossRef]
- Husakova, M.; Patakova, P. Purified Monascus pigments: Biological activities and mechanisms of action. J. Nat. Prod. 2025, 88, 607–615. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Y.; Molnár, I.; Chen, F. Nature and nurture: Confluence of pathway determinism with metabolic and chemical serendipity diversifies Monascus azaphilone pigments. Nat. Prod. Rep. 2019, 36, 561–572. [Google Scholar] [CrossRef]
- Dai, W.; Shao, Y.; Chen, F. Production of Monacolin K in Monascus pilosus: Comparison between industrial strains and analysis of its gene klusters. Microorganisms 2021, 9, 747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Wen, Q.; Xiong, Z.; Cao, X.; Zheng, Z.; Zhang, Y.; Huang, Z. An overview on the biosynthesis and metabolic regulation of monacolin K/lovastatin. Food Func. 2020, 11, 5738–5748. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Tang, G.; Man, H.; Wang, J.; Han, J.; Zhao, J. Transportation of citrinin is regulated by the CtnC gene in the medicinal fungus Monascus purpureus. J. Zhejiang Univ. Sci. B 2023, 24, 543–548. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, W.; Lu, J.; Wang, W.; Yu, X.; Feng, Y. Insight into Monascus pigments production promoted by glycerol based on physiological and transcriptome analyses. Process Biochem. 2021, 102, 141–149. [Google Scholar] [CrossRef]
- Zhen, Z.; Xiong, X.; Liu, Y.; Zhang, J.; Wang, S.; Li, L.; Gao, M. NaCl inhibits citrinin and stimulates Monascus pigments and Monacolin K production. Toxins 2019, 11, 118. [Google Scholar] [CrossRef]
- Yang, X.; Xiang, L.; Zhang, C.; Cao, Y.; Wang, C. Promotion of monacolin K production in Monascus extractive fermentation: The variation in fungal morphology and in the expression levels of biosynthetic gene clusters. J. Sci. Food Agric. 2021, 101, 5652–5659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liang, J.; Yang, L.; Chai, S.; Zhang, C.; Sun, B.; Wang, C. Glutamic acid promotes monacolin K production and monacolin K biosynthetic gene cluster expression in Monascus. AMB Express 2017, 7, 22. [Google Scholar] [CrossRef]
- Zhang, H.; Ahima, J.; Yang, Q.; Zhao, L.; Zhang, X.; Zheng, X. A review on citrinin: Its occurrence, risk implications, analytical techniques, biosynthesis, physiochemical properties and control. Food Res. Int. 2021, 141, 110075. [Google Scholar] [CrossRef]
- Li, Y.Z.; Zhang, W.Q.; Hu, P.F.; Yang, Q.; Molnár, I.; Xu, P.; Zhang, B. Harnessing microbial co-culture to increase the production of known secondary metabolites. Nat. Prod. Rep. 2025, 42, 623–637. [Google Scholar] [CrossRef]
- Xu, S.; Li, M.; Hu, Z.; Shao, Y.; Ying, J.; Zhang, H. The potential use of fungal co-culture strategy for discovery of new secondary metabolites. Microorganisms 2023, 11, 464. [Google Scholar] [CrossRef]
- Knowles, S.L.; Raja, H.A.; Roberts, C.D.; Oberlies, N.H. Fungal–fungal co-culture: A primer for generating chemical diversity. Nat. Prod. Rep. 2022, 39, 1557–1573. [Google Scholar] [CrossRef]
- Tomm, H.A.; Ucciferri, L.; Ross, A.C. Advances in microbial culturing conditions to activate silent biosynthetic gene clusters for novel metabolite production. J. Ind. Microbiol. Biot. 2019, 46, 1381–1400. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Tan, Y.; Duan, Y.L.; Li, M. Monaspin B a novel cyclohexyl-furan from cocultivation of Monascus purpureus and Aspergillus oryzae, exhibits potent antileukemic activity. J. Agric. Food Chem. 2024, 72, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- El-sayed, A.S.A.; Shindia, A.A.; Abouzeid, A.; Koura, A.; Hassanein, S.E.; Ahmed, R.M. Triggering the biosynthetic machinery of Taxol by Aspergillus flavipes via cocultivation with Bacillus subtilis: Proteomic analyses emphasize the chromatin remodeling upon fungal-bacterial interaction. Environ. Sci. Pollut. Res. 2021, 28, 39866–39881. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.; Zhang, J.; Mai, T.; Li, L.; Gu, T.; Liu, Y.; Gao, M. Co-culture of Rhodotorula mucilaginosa and Monascus purpureus increased the yield of carotenoids and Monascus pigments. LWT 2023, 183, 114949. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, F. Cocultivation study of Monascus spp. and Aspergillus niger inspired from black-skin-red-koji by a double-sided petri dish. Front. Microbiol. 2021, 12, 670684. [Google Scholar] [CrossRef]
- Zheng, C. Isolation of High-Yield Esterase and Non-Citrinin Monascus Strain and Its Esterase Production Condition Optimation. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2017. (In Chinese). [Google Scholar]
- Feng, Y.; Shao, Y.; Zhou, Y.; Chen, F. Monacolin K production by citrinin-free Monascus pilosus MS-1 and fermentation process monitoring. Eng. Life Sci. 2014, 14, 538–545. [Google Scholar] [CrossRef]
- Bian, C.; Kusuya, Y.; Sklenář, F.; D’hooge, E.; Yaguchi, T.; Ban, S.; Visagie, C.M.; Houbraken, J.; Takahashi, H.; Hubka, V. Reducing the number of accepted species in Aspergillus series. Nigri. Stud. Mycol. 2022, 102, 95–132. [Google Scholar] [CrossRef]
- Barbosa, R.N.; Leong, S.L.; Vinnere-Pettersson, O.; Chen, A.J.; Souza-Motta, C.M.; Frisvad, J.C.; Samson, R.A.; Oliveira, N.T.; Houbraken, J. Phylogenetic analysis of Monascus and new species from honey, pollen and nests of stingless bees. Stud. Mycol. 2017, 86, 29–51. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zhou, H.; Zhang, J.; Li, W.; Wang, G. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Wang, X.; Wei, J.; Tang, F.; Chen, F. Effects of blue light on pigment and citrinin production in Monascus ruber M7 via MrcreD, encoding an arrestin-like protein. Int. J. Biol. Macromol. 2025, 288, 138604. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, S.; Gao, M.; Ding, B.; Zhang, J.; Zhou, Y.; Liu, Y.; Yang, H.; Wu, Q.; Chen, F. Acidic conditions induce the accumulation of orange Monascus pigments during liquid-state fermentation of Monascus ruber M7. Appl. Microbiol. Biot. 2019, 103, 8393–8402. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, Y.; Mao, Z.; Shao, Y. Histone deacetylase MrRpd3 plays a major regulational role in the mycotoxin production of Monascus ruber. Food Control 2022, 132, 108457. [Google Scholar] [CrossRef]
- Yao, T.; Wang, X.; Chen, F. The role of enoyl reductase in the Monacolin K biosynthesis pathway in Monascus spp. J. Fungi 2025, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tang, F.; Zhao, Y.; Mao, Z.; Huang, Y.; Hu, Y.; Liu, B.; Huang, Y.; Shao, Y. Consistency of gene expression and protein acetylation modification with physiological and biochemical changes responding to the deficiency of histone deacetylase 1 homolog in Monascus ruber. Int. J. Biol. Macromol. 2025, 308, 142485. [Google Scholar] [CrossRef]
- Chen, W.; Chen, R.; Liu, Q.; He, Y.; He, K.; Ding, X.; Kang, L.; Guo, X.; Xie, N.; Zhou, Y.; et al. Orange, red, yellow: Biosynthesis of azaphilone pigments in Monascus fungi. Chem. Sci. 2017, 8, 4917–4925. [Google Scholar] [CrossRef]
- He, Y.; Cox, R.J. The molecular steps of citrinin biosynthesis in fungi. Chem. Sci. 2016, 7, 2119–2127. [Google Scholar] [CrossRef]
- Okada, B.K.; Seyedsayamdost, M.R. Antibiotic dialogues: Induction of silent biosynthetic gene clusters by exogenous small molecules. FEMS Microbiol. Rev. 2017, 41, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Chen, A.J.; Liu, B.; Wei, Q.; Bai, J.; Hu, Y.C. Investigation of citrinin and monacolin K gene clusters variation among pigment producer Monascus species. Fungal Genet. Biol. 2022, 160, 103687. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.S.; Chiu, S.H.; Chen, C.C.; Lin, C.H. Investigation of monacolin K, yellow pigments, and citrinin production capabilities of Monascus purpureus and Monascus ruber (Monascus pilosus). J. Food Drug Anal. 2023, 31, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Higa, Y.; Kim, Y.S.; Amin, A.U.; Huang, M.; Ono, N.; Kanaya, S. Divergence of metabolites in three phylogenetically close Monascus species (M. pilosus, M. ruber, and M. purpureus) based on secondary metabolite biosynthetic gene clusters. BMC Genom. 2020, 21, 679. [Google Scholar] [CrossRef]
- Kwon, H.J.; Balakrishnan, B.; Kim, Y.K. Some Monascus purpureus genomes lack the Monacolin K biosynthesis locus. J. Appl. Biol. Chem. 2016, 59, 45–47. [Google Scholar] [CrossRef]
- Chen, Y.P.; Yuan, G.F.; Hsieh, S.Y.; Lin, Y.S.; Wang, W.Y.; Liaw, L.L.; Tseng, C.P. Identification of the mokH gene encoding transcription factor for the upregulation of monacolin K biosynthesis in Monascus pilosus. J. Agric. Food Chem. 2010, 58, 287–293. [Google Scholar] [CrossRef]
- Alanzi, A.; Elhawary, E.A.; Ashour, M.L.; Moussa, A.Y. Aspergillus co-cultures: A recent insight into their secondary metabolites and microbial interactions. Arch. Pharm. Res. 2023, 46, 273–298. [Google Scholar] [CrossRef]
- Yu, R.; Liu, J.; Wang, Y.; Wang, H.; Zhang, H. Aspergillus niger as a secondary metabolite factory. Front. Chem. 2021, 9, 701022. [Google Scholar] [CrossRef]
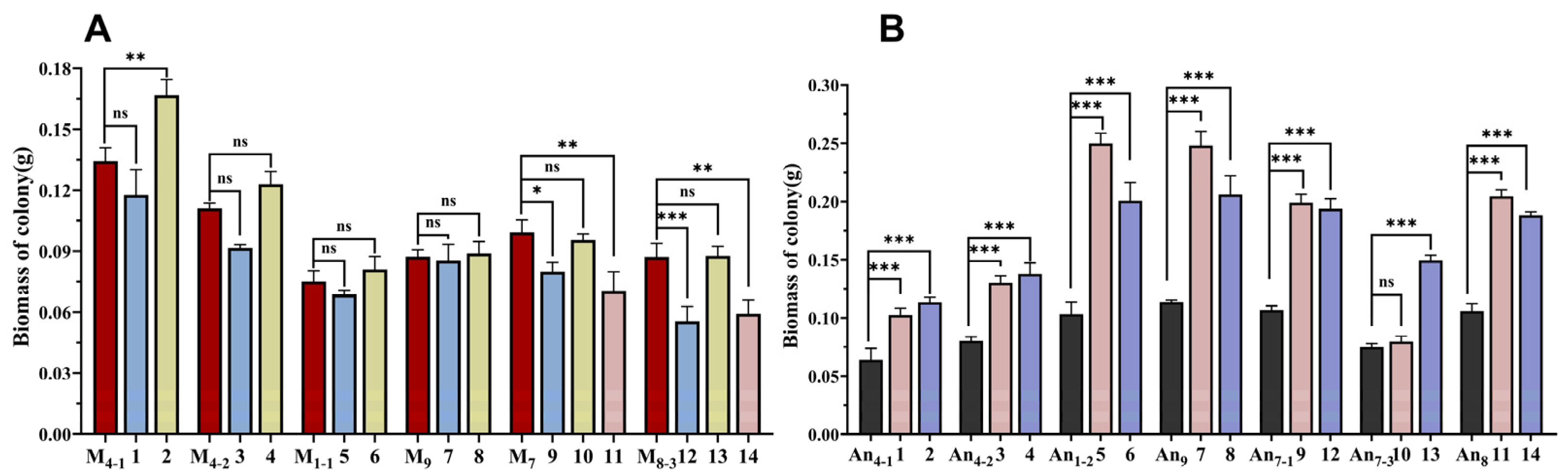
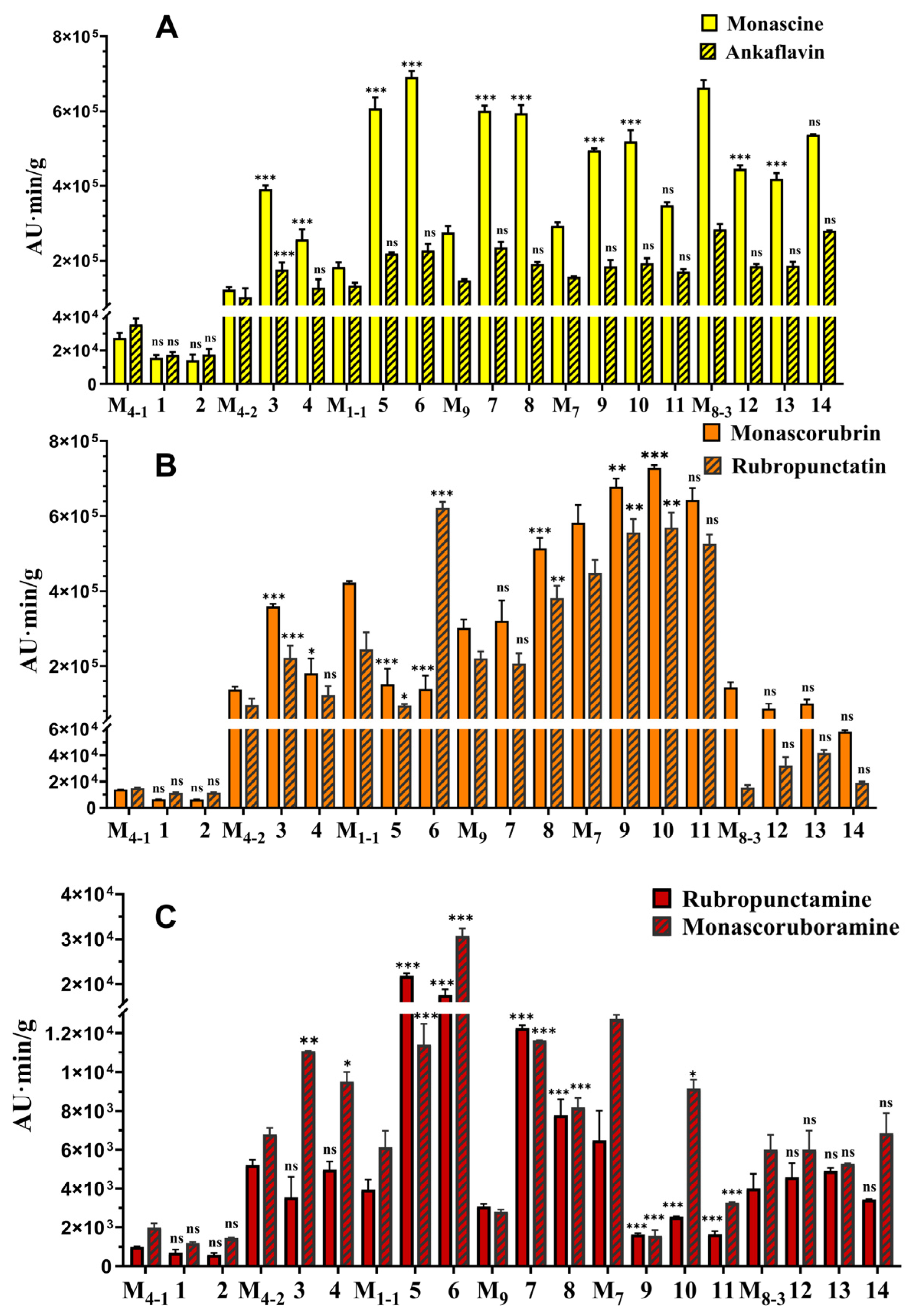
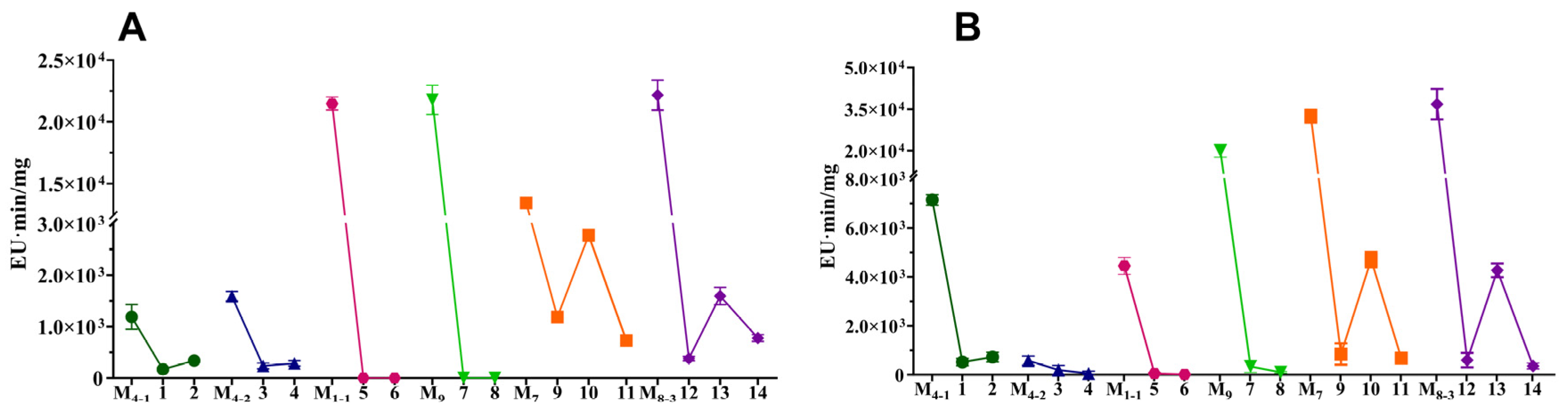
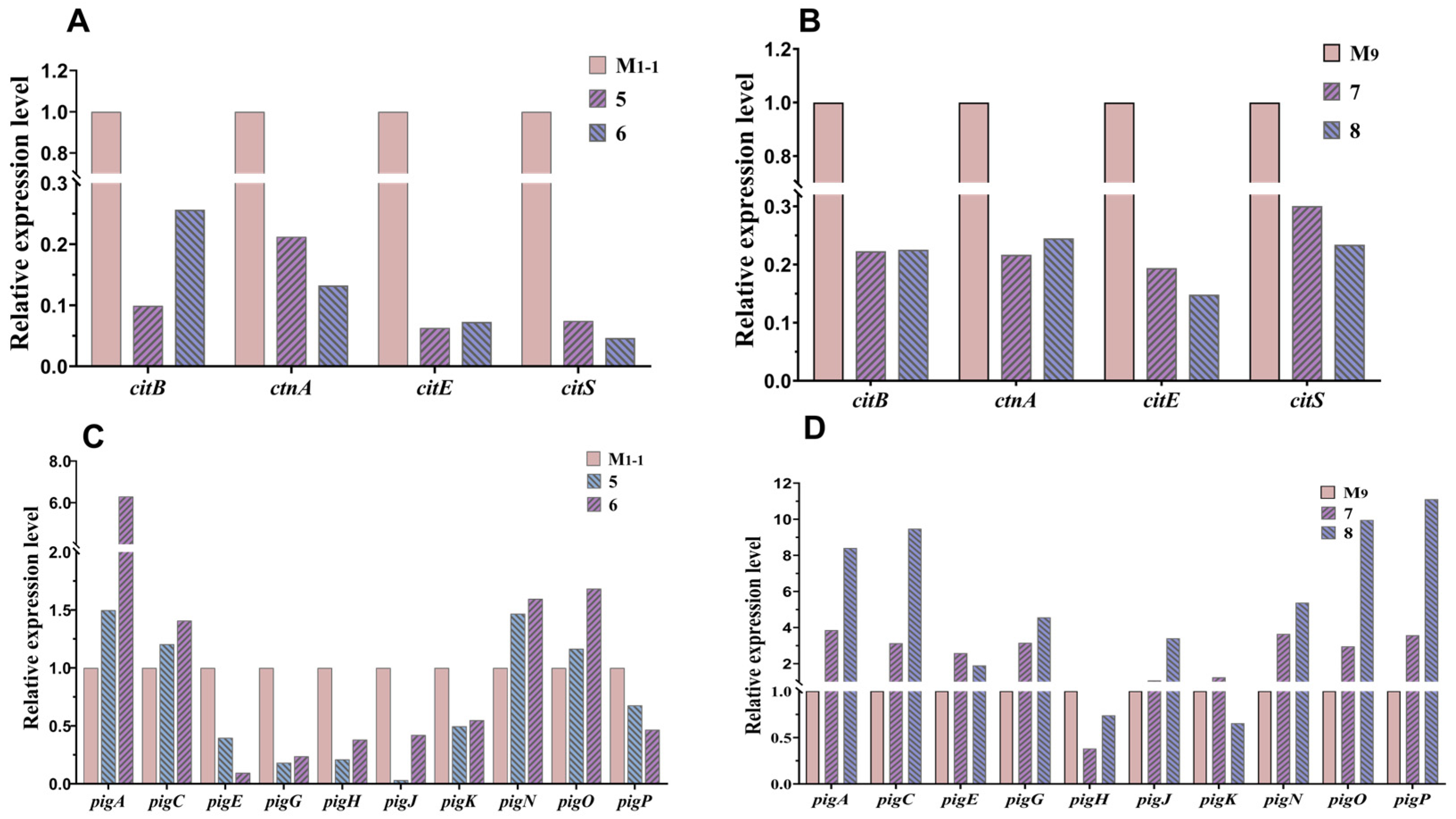
| No. of Co-Culture | Strain Names | Strain Sources | Pigment-Production Abilities | Growth Abilities |
|---|---|---|---|---|
| 1 | M4-1 & An4-1 | Sample 4 | M4-1(+) | M4-1(+) |
| 2 | M4-1 & An4-2 | M4-2(++) | M4-2(++) | |
| 3 | M4-2 & An4-1 | An4-1(−) | An4-1(+++) | |
| 4 | M4-1 & An4-2 | An4-2(+) | An4-2(++) | |
| 5 | M1-1 & An1-2 | Sample 1 and Sample 9 | M1-1(++) | M1-1(++) |
| 6 | M1-1 & An9 | M9(++) | M9(++) | |
| 7 | M9 & An1-2 | An1-2(−) | An1-2(+++) | |
| 8 | M9 & An9 | An9(−) | An9(+++) | |
| 9 | M7 & An7-1 | Sample 7 and Sample 8 | M7(++) M8-3(+) An7-1(−) An7-3(+) An8(−) | M7(++) M8-3(+) An7-1(+++) An7-3(++) An8(+++) |
| 10 | M7 & An7-3 | |||
| 11 | M7 & An8 | |||
| 12 | M8-3 & An7-1 | |||
| 13 | M8-3 & An7-3 | |||
| 14 | M8-3 & An8 |
| Strain Types | Culture Types | Colonial Sizes | Colonies Colors | Aerial Hyphae | Pigment Granules Shapes | Cleistothecia Number | Conidia Number |
|---|---|---|---|---|---|---|---|
| M. purpureus | Monoculture | ++ | red | + | Combs. 1, 2, 10, 11: block-like to needle-like; Comb. 5: needle -like to block -like | Combs. 2, 12, 13: increased. | Combs. 7, 8: increased; Com3,4: decreased. |
| Co-culture | + | orange or yellow | ++ | ||||
| A. niger | Monoculture | ++ | black | / | / | / | / |
| Co-culture | +++ | black | / | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Yuan, X.; Chen, F. Co-Culture of Monascus purpureus and Aspergillus niger Isolated from Wuyi Hongqu to Enhance Monascus Pigments Production While Inhibiting Citrinin Production. J. Fungi 2025, 11, 829. https://doi.org/10.3390/jof11120829
Yu Q, Yuan X, Chen F. Co-Culture of Monascus purpureus and Aspergillus niger Isolated from Wuyi Hongqu to Enhance Monascus Pigments Production While Inhibiting Citrinin Production. Journal of Fungi. 2025; 11(12):829. https://doi.org/10.3390/jof11120829
Chicago/Turabian StyleYu, Qin, Xi Yuan, and Fusheng Chen. 2025. "Co-Culture of Monascus purpureus and Aspergillus niger Isolated from Wuyi Hongqu to Enhance Monascus Pigments Production While Inhibiting Citrinin Production" Journal of Fungi 11, no. 12: 829. https://doi.org/10.3390/jof11120829
APA StyleYu, Q., Yuan, X., & Chen, F. (2025). Co-Culture of Monascus purpureus and Aspergillus niger Isolated from Wuyi Hongqu to Enhance Monascus Pigments Production While Inhibiting Citrinin Production. Journal of Fungi, 11(12), 829. https://doi.org/10.3390/jof11120829







