A Critical Review of the Effectiveness of Biochar Coupled with Arbuscular Mycorrhizal Fungi in Soil Cadmium Immobilization
Abstract
1. Introduction
2. Research Methodology
3. Geochemical Behavior of Cadmium in the Environment
4. Biochar Effects on Cadmium Behavior in Soil–Plant System
4.1. Biochar Effects on the Migration of Cadmium in the Soil Environment
4.2. Biochar Effects on Cadmium Speciation in the Soil Environment
4.3. Biochar Effects on Cadmium Uptake and Accumulation by Plants
5. Effects of AMF on Cadmium Behavior in the Soil–Plant Systems
5.1. Effects of AMF on Cadmium Behavior in the Soil Environment
5.2. Effects of AMF on the Absorption and Accumulation of Cadmium in the Plants
5.3. Effects of AMF on Cadmium Dynamics in the Soil Environment
6. Effects of Biochar Coupled with AMF on Cadmium Behavior in Soil–Plant Systems
6.1. Effects of Biochar Associated with AMF on the Migration and Transformation of Cadmium in Soil
6.2. Effects of Biochar Associated with AMF on Plant Growth and Physiology
6.3. Effects of Biochar Associated with AMF on Cadmium Enrichment Reduction in Plant
6.4. Mechanisms of Biochar Combined with AMF on the Migration and Transformation of Cadmium
6.5. Non-Synergistic Effects of Biochar Combined with AMF in the Soil–Plant Systems
7. The Synergistic Influences of Biochar Coupled with AMF on Cadmium Immobilization in the Soil
8. Recommendations and Prospect
9. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, S.; Liu, H.; Nie, Z.; Rengel, Z.; Gao, W.; Li, C.; Peng, Z. Toxicity of Cadmium and Its Competition with Mineral Nutrients for Uptake by Plants: A Review. Pedosphere 2020, 30, 168–180. [Google Scholar] [CrossRef]
- Arora, N.K.; Chauhan, R. Heavy Metal Toxicity and Sustainable Interventions for Their Decontamination. Environ. Sustain. 2021, 4, 1–3. [Google Scholar] [CrossRef]
- Twagirayezu, G.; Irumva, O.; Huang, K.; Xia, H.; Uwimana, A.; Nizeyimana, J.C.; Manzi, H.P.; Nambajemariya, F.; Itangishaka, A.C. Environmental Effects of Electrical and Electronic Waste on Water and Soil: A Review. Pol. J. Environ. Stud. 2022, 31, 2507–2525. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Liu, L.; Jin, X.; Duan, C.; Cui, Y.; Wang, J.; Ma, D.; Zhao, W.; Wang, Y.; Fang, L. Co-Inoculation Effect of Plant-Growth-Promoting Rhizobacteria and Rhizobium on EDDS Assisted Phytoremediation of Cu Contaminated Soils. Chemosphere 2020, 254, 126724. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, D.; Luo, Y.; Zhang, Y.; Xu, L.; Chen, P.; Wu, E.; Ma, Q.; Wang, H.; Zhao, L.; et al. Cadmium Tolerance and Accumulation from the Perspective of Metal Ion Absorption and Root Exudates in Broomcorn Millet. Ecotoxicol. Environ. Saf. 2023, 250, 114506. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, H.; Kopittke, P.M.; Zhao, F.J. Cadmium Contamination in Agricultural Soils of China and the Impact on Food Safety. Environ. Pollut. 2019, 249, 1038–1048. [Google Scholar] [CrossRef]
- Zhao, F.-J.; Ma, Y.; Zhu, Y.-G.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- Yang, J.; Hu, R.; Zhao, C.; Wang, L.; Lei, M.; Guo, G.; Shi, H.; Liao, X.; Chen, T. Challenges and Opportunities for Improving the Environmental Quality of Cadmium-Contaminated Soil in China. J. Hazard. Mater. 2023, 445, 130560. [Google Scholar] [CrossRef]
- Hussain, B.; Ashraf, M.N.; Abbas, A.; Li, J.; Farooq, M. Cadmium Stress in Paddy Fields: Effects of Soil Conditions and Remediation Strategies. Sci. Total Environ. 2021, 754, 142188. [Google Scholar] [CrossRef]
- Chuan, L.; Zhao, T.; Zheng, H.; Zhao, J.; Zhang, X.; Tan, C.; Li, G. Research Advances in Remediation of Heavy Metal Contaminated Soils. Environ. Sci. Technol. 2014, 37, 213–222. [Google Scholar]
- Gong, Y.; Zhao, D.; Wang, Q. An Overview of Field-Scale Studies on Remediation of Soil Contaminated with Heavy Metals and Metalloids: Technical Progress over the Last Decade. Water Res. 2018, 147, 440–460. [Google Scholar] [CrossRef]
- Cao, X.D.; Wei, X.X.; Dai, G.L.; Yang, Y.L. Combined Pollution of Multiple Heavy Metals and Their Chemical Immobilization in Contaminated Soils: A Review. Chin. J. Environ. Eng. 2011, 5, 1441–1453. [Google Scholar]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation Techniques for Heavy Metal-Contaminated Soils: Principles and Applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Wang, X.; Fang, L.; Beiyuan, J.; Cui, Y.; Peng, Q.; Zhu, S.; Wang, M.; Zhang, X. Improvement of Alfalfa Resistance against Cd Stress through Rhizobia and Arbuscular Mycorrhiza Fungi Co-Inoculation in Cd-Contaminated Soil. Environ. Pollut. 2021, 277, 116758. [Google Scholar] [CrossRef] [PubMed]
- Saxena, G.; Bharagava, R.N. Bioremediation of Industrial Waste for Environmental Safety; Springer: Singapore, 2020. [Google Scholar]
- Shi, Z.; Li, K.; Zhu, X.; Wang, F. The Worldwide Leaf Economic Spectrum Traits Are Closely Linked with Mycorrhizal Traits. Fungal Ecol. 2020, 43, 100877. [Google Scholar] [CrossRef]
- Wang, F.; Rengel, Z. Disentangling the Contributions of Arbuscular Mycorrhizal Fungi to Soil Multifunctionality. 2024; in press. [Google Scholar]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of Arbuscular Mycorrhizal Fungi on Plant Growth and Performance: Importance in Biotic and Abiotic Stressed Regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, H.; Twagirayezu, G.; Zhang, F.; Shi, Y.; Luo, C.; Yan, F.; Wang, Z.; Xing, D. Arbuscular Mycorrhizal Fungi Adjusts Root Architecture to Promote Leaf Nitrogen Accumulation and Reduce Leaf Carbon–Nitrogen Ratio of Mulberry Seedlings. Forests 2023, 14, 2448. [Google Scholar] [CrossRef]
- Wang, H.R.; Zhao, X.Y.; Zhang, J.M.; Lu, C.; Feng, F.J. Arbuscular Mycorrhizal Fungus Regulates Cadmium Accumulation, Migration, Transport, and Tolerance in Medicago sativa. J. Hazard. Mater. 2022, 435, 129077. [Google Scholar] [CrossRef] [PubMed]
- Gai, J.P.; Fan, J.Q.; Zhang, S.B.; Mi, N.N.; Christie, P.; Li, X.L.; Feng, G. Direct Effects of Soil Cadmium on the Growth and Activity of Arbuscular Mycorrhizal Fungi. Rhizosphere 2018, 7, 43–48. [Google Scholar] [CrossRef]
- Nasiri, K.; Babaeinejad, T.; Ghanavati, N.; Mohsenifar, K. Arbuscular Mycorrhizal Fungi Affecting the Growth, Nutrient Uptake and Phytoremediation Potential of Different Plants in a Cadmium-Polluted Soil. BioMetals 2022, 35, 1243–1253. [Google Scholar] [CrossRef]
- Rask, K.A.; Johansen, J.L.; Kjøller, R.; Ekelund, F. Differences in Arbuscular Mycorrhizal Colonisation Influence Cadmium Uptake in Plants. Environ. Exp. Bot. 2019, 162, 223–229. [Google Scholar] [CrossRef]
- Twagirayezu, G.; Huang, K.; Sang, C.; Guan, M.; Xia, H.; Li, Y. Performance and Mechanisms of Biochar for Promoting the Removal Efficiency of Organic Solids in the Vermi-Wetland during the Recycling of Excess Sludge Performance and Mechanisms of Biochar for Promoting the Removal Efficiency of Organic Solids in the Ver. J. Clean. Prod. 2022, 360, 132172. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, Y.; Li, H.; Hu, W.; Cheng, J.; Lee, X. A Bibliometric Review of Biochar for Soil Carbon Sequestration and Mitigation from 2001 to 2020. Ecotoxicol. Environ. Saf. 2023, 264, 115438. [Google Scholar] [CrossRef] [PubMed]
- Twagirayezu, G.; Cheng, H.; Wu, Y.; Lu, H.; Huang, S.; Fang, X.; Irumva, O. Insights into the Influences of Biochar on the Fate and Transport of Pesticides in the Soil Environment: A Critical Review. Biochar 2024, 6, 9. [Google Scholar] [CrossRef]
- Xu, P.; Gao, Y.; Cui, Z.; Wu, B.; Yan, B.; Wang, Y.; Zaitongguli, K.; Wen, M.; Wang, H.; Jing, N. Research Progress on Effects of Biochar on Soil Environment and Crop Nutrient Absorption and Utilization. Sustainability 2023, 15, 4861. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Feng, Y. The Effects of Biochar Addition on Soil Physicochemical Properties: A Review. Catena 2021, 202, 105284. [Google Scholar] [CrossRef]
- Shi, W.; Ju, Y.; Bian, R.; Li, L.; Joseph, S.; Mitchell, D.R.G.; Munroe, P.; Taherymoosavi, S.; Pan, G. Biochar Bound Urea Boosts Plant Growth and Reduces Nitrogen Leaching. Sci. Total Environ. 2020, 701, 134424. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Xu, H.; Song, F.; Ge, H.; Chen, L.; Yue, S.; Yang, W. Effect of Biochar on Immobilization Remediation of Cd⁃contaminated Soil and Environmental Quality. Environ. Res. 2022, 204, 111840. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Yue, F.; Yan, X.; Wang, F.; Bloszies, S.; Wang, Y. Effects of Arbuscular Mycorrhizal Inoculation and Biochar Amendment on Maize Growth, Cadmium Uptake and Soil Cadmium Speciation in Cd-Contaminated Soil. Chemosphere 2018, 194, 495–503. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in Soils and Groundwater: A Review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Cheng, H.; Ning, Z.; Liu, Y.; Lin, S.; Li, Y.; Wang, X.; Hill, P.; Chadwick, D.; Jones, D.L. Field Aging Declines the Regulatory Effects of Biochar on Cadmium Uptake by Pepper in the Soil. J. Environ. Manag. 2022, 321, 115832. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil Contamination with Cadmium, Consequences and Remediation Using Organic Amendments. Sci. Total Environ. 2017, 601, 1591–1605. [Google Scholar] [CrossRef]
- Ullah, F.; Liqun, C.; Coulter, J.A.; Alam, S.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Ecotoxicology and Environmental Safety Cadmium Toxicity in Plants: Impacts and Remediation Strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P. Cadmium Bioavailability, Uptake, Toxicity and Detoxification in Soil-Plant System. Rev. Environ. Contam. Toxicol. 2016, 241, 73–137. [Google Scholar]
- Huang, X.; Duan, S.; Wu, Q.; Yu, M.; Shabala, S. Reducing Cadmium Accumulation in Plants: Structure--Function Relations and Tissue-Specific Operation of Transporters in the Spotlight. Plants 2020, 9, 223. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, J.T.; Liu, X.; Sun, L.X.; Tian, J.; Wu, N. Cadmium Pollution Impact on the Bacterial Community Structure of Arable Soil and the Isolation of the Cadmium Resistant Bacteria. Front. Microbiol. 2021, 12, 698834. [Google Scholar] [CrossRef]
- Naeem, A.; Zafar, M.; Khalid, H.; Zia-ur-Rehman, M.; Ahmad, Z.; Ayub, M.A.; Qayyum, M.F. Cadmium-Induced Imbalance in Nutrient and Water Uptake by Plants. In Cadmium Toxicity and Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 299–326. [Google Scholar]
- Mahmood, Q.; Asif, M.; Shaheen, S.; Hayat, M.T.; Ali, S. Cadmium Contamination in Water and Soil; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Zhang, H.; Reynolds, M. Science of the Total Environment Cadmium Exposure in Living Organisms: A Short Review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of Cadmium Pollution on Food Safety and Human Health. Curr. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. Trophic Transfer, Bioaccumulation, and Biomagnification of Non-Essential Hazardous Heavy Metals and Metalloids in Food Chains/Webs—Concepts and Implications for Wildlife and Human Health. Hum. Ecol. Risk Assess. An Int. J. 2018, 25, 1353–1376. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The Uptake and Bioaccumulation of Heavy Metals by Food Plants, Their Effects on Plants Nutrients, and Associated Health Risk: A Review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liao, Q.; Chillrud, S.N.; Yang, Q.; Huang, L.; Bi, J.; Yan, B. Environmental Exposure to Cadmium: Health Risk Assessment and Its Associations with Hypertension and Impaired Kidney Function. Sci. Rep. 2016, 6, 29989. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Caporale, A.G.; Violante, A. Chemical Processes Affecting the Mobility of Heavy Metals and Metalloids in Soil Environments. Curr. Pollut. Rep. 2016, 2, 15–27. [Google Scholar] [CrossRef]
- Wei, B.; Peng, Y.; Jeyakumar, P.; Lin, L.; Zhang, D.; Yang, M.; Zhu, J.; Lin, C.S.K.; Wang, H.; Wang, Z. Soil PH Restricts the Ability of Biochar to Passivate Cadmium: A Meta-Analysis. Environ. Res. 2023, 219, 115110. [Google Scholar] [CrossRef]
- Houben, D.; Evrard, L.; Sonnet, P. Mobility, Bioavailability and PH-Dependent Leaching of Cadmium, Zinc and Lead in a Contaminated Soil Amended with Biochar. Chemosphere 2013, 92, 1450–1457. [Google Scholar] [CrossRef]
- Jia, Y.; Li, J.; Zeng, X.; Zhang, N.; Wen, J.; Liu, J.; Jiku, M.A.S.; Wu, C.; Su, S. The Performance and Mechanism of Cadmium Availability Mitigation by Biochars Differ among Soils with Different PH: Hints for the Reasonable Choice of Passivators. J. Environ. Manag. 2022, 312, 114903. [Google Scholar] [CrossRef]
- Han, X.; Cui, L.; Yan, J. Effects of Biochar on Purslane-Mediated Transfer and Uptake of Soil Bioavailable Cadmium. Water Air Soil Pollut. 2022, 233, 479. [Google Scholar] [CrossRef]
- Meng, F.; Huang, Q.; Cai, Y.; Li, F.; Yuan, G. Effects of Biowaste-Derived Biochar on the Dynamic Behavior of Cadmium Fractions in Soils. Environ. Sci. Pollut. Res. 2022, 29, 59043–59051. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential Role of Biochars in Decreasing Soil Acidification-a Critical Review. Sci. Total Environ. 2017, 581, 601–611. [Google Scholar] [CrossRef]
- Bai, S.; Ni, X.; Yang, Y.Y. Immobilization of Soil Cadmium and Zinc by Different Raw Material Derived Biochars. Jiangsu J. Agric. Sci. 2021, 37, 1199–1205. [Google Scholar]
- Hu, Y.; Guo, Z.; Xu, Z.; Chang Lian, P.; Xutong, W. Effects of Biochar from Cd-Containing Rice Straw on Stabilization of Cd in Soils. Trans. Chin. Soc. Agric. Eng. 2022, 38, 204–211. [Google Scholar]
- De Lima Veloso, V.; da Silva, F.B.V.; dos Santos, N.M.; do Nascimento, C.W.A. Phytoattenuation of Cd, Pb, and Zn in a Slag-Contaminated Soil Amended with Rice Straw Biochar and Grown with Energy Maize. Environ. Manag. 2022, 69, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, X.; Tsang, D.C.W.; Cao, X. Contrasting Impacts of Pre-and Post-Application Aging of Biochar on the Immobilization of Cd in Contaminated Soils. Environ. Pollut. 2018, 242, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Marković, J.; Jović, M.; Smičiklas, I.; Šljivić-Ivanović, M.; Onjia, A.; Trivunac, K.; Popović, A. Cadmium Retention and Distribution in Contaminated Soil: Effects and Interactions of Soil Properties, Contamination Level, Aging Time and in Situ Immobilization Agents. Ecotoxicol. Environ. Saf. 2019, 174, 305–314. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Niazi, N.K.; Xu, S.; Yuan, G.; Chen, X.; Zhang, X. Effect of Bamboo and Rice Straw Biochars on the Mobility and Redistribution of Heavy Metals (Cd, Cu, Pb and Zn) in Contaminated Soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar Reduces the Bioavailability and Phytotoxicity of Heavy Metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, J.; Wang, L.; Yang, G.; Zhang, X. Effect of Biochar on Heavy Metal Speciation of Paddy Soil. Water Air Soil Pollut. 2015, 226, 429. [Google Scholar] [CrossRef]
- Ma, Z.; Tao, R.; Hu, J.; Cao, C.; Hu, Z.; Chen, Y.; Hu, H.; Ma, Y. Effects of Formula Fertilizer and Biochar on Cadmium and Plumbum Absorption in Maize (Zea mays L.). Sustainability 2023, 15, 4696. [Google Scholar] [CrossRef]
- Xiaohui, M.; Shiqing, L.; Ruijuan, D. Study of Soil Cd Fractionation and Its Bioavailability on the Loess Plateau. J. Northwest Sci-Tech Univ. Agric. For. 2008, 36, 135–142. [Google Scholar]
- Liu, Y.-L.; Zhu, H.-C.; Peng, O.; Li, D.-Y.; Yang, R.-J.; Peng, J.; Tie, B.-Q. Adsorption of Cadmium and Arsenic by Corn Stalk Biochar Solidified Microorganism. Huan Jing ke Xue Huanjing Kexue 2020, 41, 4322–4332. [Google Scholar]
- Puga, A.P.; Abreu, C.; Melo, L.C.A.; Beesley, L. Biochar Application to a Contaminated Soil Reduces the Availability and Plant Uptake of Zinc, Lead and Cadmium. J. Environ. Manag. 2015, 159, 86–93. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-ur-Rehman, M.; Qayyum, M.F.; Abbas, F.; Hannan, F.; Rinklebe, J.; Ok, Y.S. Effect of Biochar on Cadmium Bioavailability and Uptake in Wheat (Triticum aestivum L.) Grown in a Soil with Aged Contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef]
- Yao, P.; Zhou, H.; Li, X.; Wei, L.; Wang, J.; Zhang, S.; Ye, X. Effect of Biochar on the Accumulation and Distribution of Cadmium in Tobacco (Yunyan 87) at Different Developmental Stages. Ecotoxicol. Environ. Saf. 2021, 207, 111295. [Google Scholar] [CrossRef]
- Ren, T.; Chen, N.; Mahari, W.A.W.; Xu, C.; Feng, H.; Ji, X.; Yin, Q.; Chen, P.; Zhu, S.; Liu, H.; et al. Biochar for Cadmium Pollution Mitigation and Stress Resistance in Tobacco Growth. Environ. Res. 2021, 192, 110273. [Google Scholar] [CrossRef]
- Vilela, L.A.F.; Barbosa, M.V. Contribution of Arbuscular Mycorrhizal Fungi in Promoting Cadmium Tolerance in Plants. In Cadmium Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 553–586. [Google Scholar]
- Wang, X.; Fan, X.; Chang, W.; Li, K.; Zhang, M.; Ping, Y.; Song, F. Combining the Advantages of Rhizophagus Intraradices and Serendipita Indica to Reduce the Risk of Cd on Soybean Safety and Environmental Pollution. Rhizosphere 2023, 25, 100669. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, R.; Jiang, L.; Chen, K.; Zhu, W. Alleviation of Cadmium Toxicity to Medicago Truncatula by AMF Involves the Changes of Cd Speciation in Rhizosphere Soil and Subcellular Distribution. Phyton-Int. J. Exp. Bot. 2021, 90, 403–415. [Google Scholar] [CrossRef]
- Gao, M.Y.; Mo, C.H.; Wong, M.H.; Chen, X.W. Potential Use of Arbuscular Mycorrhizal Fungi for Simultaneous Mitigation of Arsenic and Cadmium Accumulation in Rice. J. Exp. Bot. 2022, 73, 50–67. [Google Scholar]
- Chen, X.W.; Wu, L.; Luo, N.; Mo, C.H.; Wong, M.H.; Li, H. Arbuscular Mycorrhizal Fungi and the Associated Bacterial Community Influence the Uptake of Cadmium in Rice. Geoderma 2019, 337, 749–757. [Google Scholar] [CrossRef]
- González-Chávez, M.C.; Carrillo-González, R.; Wright, S.F.; Nichols, K.A. The Role of Glomalin, a Protein Produced by Arbuscular Mycorrhizal Fungi, in Sequestering Potentially Toxic Elements. Environ. Pollut. 2004, 130, 317–323. [Google Scholar] [CrossRef]
- Gerami, Z.; Lakzian, A.; Hemati, A.; Amirifar, A.; Asgari Lajayer, B.; van Hullebusch, E.D. Effect of Cadmium on Sorghum Root Colonization by Glomeral Fungi and Its Impact on Total and Easily Extractable Glomalin Production. Environ. Sci. Pollut. Res. 2021, 28, 34570–34583. [Google Scholar] [CrossRef]
- Joner, E.J.; Briones, R.; Leyval, C. Metal-Binding Capacity of Arbuscular Mycorrhizal Mycelium. Plant Soil 2000, 226, 227–234. [Google Scholar] [CrossRef]
- Nayuki, K.; Chen, B.; Ohtom, R.; Kuga, Y. Cellular Imaging of Cadmium in Resin Sections of Arbuscular Mycorrhizas Using Synchrotron Micro X-Ray Fluorescence. Microbes Environ. 2014, 29, 60–66. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Melville, L.H.; Ferrol, N.; Lott, J.N.A.; Azcón-Aguilar, C.; Peterson, R.L. Ultrastructural Localization of Heavy Metals in the Extraradical Mycelium and Spores of the Arbuscular Mycorrhizal Fungus Glomus Intraradices. Can. J. Microbiol. 2008, 54, 103–110. [Google Scholar] [CrossRef]
- Ferrol, N.; Tamayo, E.; Vargas, P. The Heavy Metal Paradox in Arbuscular Mycorrhizas: From Mechanisms to Biotechnological Applications. J. Exp. Bot. 2016, 67, 6253–6565. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; George, E.; Marschner, H. Contribution of an Arbuscular Mycorrhizal Fungus to the Uptake of Cadmium and Nickel in Bean and Maize Plants. Plant Soil 1996, 184, 195–205. [Google Scholar] [CrossRef]
- Hutchinson, J.J.; Young, S.D.; Black, C.R.; West, H.M. Determining Uptake of Radio-Labile Soil Cadmium by Arbuscular Mycorrhizal Hyphae Using Isotopic Dilution in a Compartmented-Pot System. New Phytol. 2004, 164, 477–484. [Google Scholar] [CrossRef]
- Joner, E.J.; Leyval, C. Uptake of 109Cd by Roots and Hyphae of a Glomus Mosseae/Trifolium Subterraneum Mycorrhiza from Soil Amended with High and Low Concentrations of Cadmium. New Phytol. 1997, 135, 353–360. [Google Scholar] [CrossRef]
- Chen, B.; Nayuki, K.; Kuga, Y.; Zhang, X.; Wu, S.; Ohtomo, R. Uptake and Intraradical Immobilization of Cadmium by Arbuscular Mycorrhizal Fungi as Revealed by a Stable Isotope Tracer and Synchrotron Radiation µX-ray Fluorescence Analysis. Microbes Environ. 2018, 33, 257–263. [Google Scholar] [CrossRef]
- Gao, M.Y.; Chen, X.W.; Huang, W.X.; Wu, L.; Yu, Z.S.; Xiang, L.; Mo, C.H.; Li, Y.W.; Cai, Q.Y.; Wong, M.H.; et al. Cell Wall Modification Induced by an Arbuscular Mycorrhizal Fungus Enhanced Cadmium Fixation in Rice Root. J. Hazard. Mater. 2021, 416, 125894. [Google Scholar] [CrossRef]
- Lenoir, I.; Fontaine, J.; Sahraoui, A.L.-H. Arbuscular Mycorrhizal Fungal Responses to Abiotic Stresses: A Review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.W.; Wu, L.; Luo, N.; Huang, W.X.; Mo, C.H.; Li, Y.W.; Xiang, L.; Zhao, H.M.; Cai, Q.Y.; et al. Effects of Arbuscular Mycorrhizal Fungi on Redox Homeostasis of Rice under Cd Stress. Plant Soil 2020, 455, 121–138. [Google Scholar] [CrossRef]
- Zhang, X.F.; Hu, Z.H.; Yan, T.X.; Lu, R.R.; Peng, C.L.; Li, S.S.; Jing, Y.X. Arbuscular Mycorrhizal Fungi Alleviate Cd Phytotoxicity by Altering Cd Subcellular Distribution and Chemical Forms in Zea mays. Ecotoxicol. Environ. Saf. 2019, 171, 352–360. [Google Scholar] [CrossRef]
- Li, J.; Yue, F.; Yanfang, W.; Zhang, Y.; Ni, R.; Wang, F.; Fu, G.; Liu, L. Effects of Biochar Amendment and Arbuscular Mycorrhizal Inoculation on Maize Growth and Physiological Biochemistry under Cadmium Stress. J. Penelit. Pendidik. Guru Sekol. Dasar 2016, 6, 128. [Google Scholar]
- Wang, Y.; Huang, J.; Gao, Y. Arbuscular Mycorrhizal Colonization Alters Subcellular Distribution and Chemical Forms of Cadmium in Medicago sativa L. and Resists Cadmium Toxicity. PLoS ONE 2012, 7, e48669. [Google Scholar] [CrossRef]
- Del Val, C.; Barea, J.M.; Azcón-Aguilar, C. Diversity of Arbuscular Mycorrhizal Fungus Populations in Heavy-Metal- Contaminated Soils. Appl. Environ. Microbiol. 1999, 65, 718–723. [Google Scholar] [CrossRef]
- Biró, I.; Németh, T.; Takács, T. Changes of Parameters of Infectivity and Efficiency of Different Glomus Mosseae Arbuscular Mycorrhizal Fungi Strains in Cadmium-Loaded Soils. Commun. Soil Sci. Plant Anal. 2009, 40, 227–239. [Google Scholar] [CrossRef]
- Gunwal, I.; Sharma, K.C.; Mago, P. Spore Density and Root Colonization by Arbuscularmycorrhizal Fungi in Heavy-Metal-Contaminated Soils. IOSR J. Pharm. Biol. Sci. 2014, 9, 49–53. [Google Scholar]
- Johnson, N.C.; Wilson, G.W.T.; Wilson, J.A.; Miller, R.M.; Bowker, M.A. Mycorrhizal Phenotypes and the Law of the Minimum. New Phytol. 2015, 205, 1473–1484. [Google Scholar] [CrossRef]
- You, Y.; Wang, L.; Ju, C.; Wang, X.; Wang, Y. How Does Phosphorus Influence Cd Tolerance Strategy in Arbuscular Mycorrhizal-Phragmites Australis Symbiotic System? J. Hazard. Mater. 2023, 452, 131318. [Google Scholar] [CrossRef]
- Wang, C.; White, P.J.; Li, C. Colonization and Community Structure of Arbuscular Mycorrhizal Fungi in Maize Roots at Different Depths in the Soil Profile Respond Differently to Phosphorus Inputs on a Long-Term Experimental Site. Mycorrhiza 2017, 27, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Jamiołkowska, A.; Ksiȩzniak, A.; Gałązka, A.; Hetman, B.; Kopacki, M.; Skwaryło-Bednarz, B. Impact of Abiotic Factors on Development of the Community of Arbuscular Mycorrhizal Fungi in the Soil: A Review. Int. Agrophysics 2018, 32, 133–140. [Google Scholar] [CrossRef]
- Hart, M.M.; Reader, R.J.; Klironomos, J.N. Plant Coexistence Mediated by Arbuscular Mycorrhizal Fungi. Trends Ecol. Evol. 2003, 18, 418–423. [Google Scholar] [CrossRef]
- Gujre, N.; Soni, A.; Rangan, L.; Tsang, D.C.W.; Mitra, S. Sustainable Improvement of Soil Health Utilizing Biochar and Arbuscular Mycorrhizal Fungi: A Review. Environ. Pollut. 2021, 268, 115549. [Google Scholar] [CrossRef]
- Li, S.; Chi, S.; Lin, C.; Cai, C.; Yang, L.; Peng, K.; Huang, X.; Liu, J. Combination of Biochar and Amf Promotes Phosphorus Utilization by Stimulating Rhizosphere Microbial Co-Occurrence Networks and Lipid Metabolites of Phragmites. Sci. Total Environ. 2022, 845, 157339. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Sohail, M.I.; Cao, X.; Hussain, B.; Aziz, M.Z.; Usman, M.; He, Z.; Yang, X. An Explanation of Soil Amendments to Reduce Cadmium Phytoavailability and Transfer to Food Chain. Sci. Total Environ. 2019, 660, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gong, Z.; Zhang, Y.; Li, P. Growth, Cadmium Uptake and Accumulation of Maize (Zea mays L.) under the Effects of Arbuscular Mycorrhizal Fungi. Ecotoxicology 2014, 23, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I.; et al. Arbuscular Mycorrhizal Fungi-Induced Mitigation of Heavy Metal Phytotoxicity in Metal Contaminated Soils: A Critical Review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, M.; Li, Y.; Che, Y.; Xiao, Y. Effects of Arbuscular Mycorrhizal Fungi, Biochar and Cadmium on the Yield and Element Uptake of Medicago sativa. Sci. Total Environ. 2019, 655, 1150–1158. [Google Scholar] [CrossRef]
- Qiao, Y.; Crowley, D.; Wang, K.; Zhang, H.; Li, H. Effects of Biochar and Arbuscular Mycorrhizae on Bioavailability of Potentially Toxic Elements in an Aged Contaminated Soil. Environ. Pollut. 2015, 206, 636–643. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, Q.; Luo, Q.; Li, H.; Yan, J.; Liao, T. Application of Wetland Waste Plant Biochar in Combination with Arbuscular Mycorrhizal Fungi on Immobilization of Cd in Contaminated Soil. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Pu, D.; Qiu, L.; Zhou, Q.; Liang, W. Effects of Canna Biochar Combined with Arbuscular Mycorrhiza on Soil Cadmium Immobilization. Environ. Sci. Technol. 2023, 46, 56. [Google Scholar]
- Alotibi, M.M.; Alotaibi, N.M.; Hussain, G.S.; Hussain, S.; Shah, S.H.; Ghoneim, A.M.; Dawar, K.; Hareem, M. Use of Zinc Quantum Dot Biochar and AMF for Alleviation of Cd Stress in Maize: Regulation of Physiological and Biochemical Attributes. Plant Stress 2023, 10, 100262. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y. Effects of Biochar and AM Fungi on Growth, Mineral Elements and Cadmium Uptake of Mulberry under Cadmium Stress. IOP Conf. Ser. Earth Environ. Sci. 2021, 687, 012021. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, L.; Xiao, Y. The Combined Use of Arbuscular Mycorrhizal Fungi, Biochar and Nitrogen Fertilizer Is Most Beneficial to Cultivate Cichorium intybus L. in Cd-Contaminated Soil. Ecotoxicol. Environ. Saf. 2021, 217, 112154. [Google Scholar] [CrossRef]
- Zhuo, F.; Zhang, X.F.; Lei, L.L.; Yan, T.X.; Lu, R.R.; Hu, Z.H.; Jing, Y.X. The Effect of Arbuscular Mycorrhizal Fungi and Biochar on the Growth and Cd/Pb Accumulation in Zea mays. Int. J. Phytoremediation 2020, 22, 1009–1018. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal Responses to Biochar in Soil-Concepts and Mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Chan, W.F.; Li, H.; Wu, F.Y.; Wu, S.C.; Wong, M.H. Arsenic Uptake in Upland Rice Inoculated with a Combination or Single Arbuscular Mycorrhizal Fungi. J. Hazard. Mater. 2013, 262, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Mulyadi; Jiang, L. The Combined Application of Biochar and Arbuscular Mycorrhizal Fungi (AMF) Enhanced the Physical and Chemical Properties of Soil and Rice Productivity in Indonesia. Sustainability 2023, 15, 9782. [Google Scholar] [CrossRef]
- Wen, Z.; Chen, Y.; Liu, Z.; Meng, J. Biochar and Arbuscular Mycorrhizal Fungi Stimulate Rice Root Growth Strategy and Soil Nutrient Availability. Eur. J. Soil Biol. 2022, 113, 103448. [Google Scholar] [CrossRef]
- Irfan, M.; Hayat, S.; Ahmad, A.; Alyemeni, M.N. Soil Cadmium Enrichment: Allocation and Plant Physiological Manifestations. Saudi J. Biol. Sci. 2013, 20, 1–10. [Google Scholar] [CrossRef]
- Bushra, S.; Faizan, S.; Badar, A.; Pandey, E.; Kumari, R. AMF-Mediated mitigation of Cd-Induced toxicity in Cicer arietinum and their influence on growth, Photosynthesis efficiency, Cell viability, Antioxidants and yield attributes. S. Afr. J. Bot. 2024, 164, 9–22. [Google Scholar] [CrossRef]
- Vejvodová, K.; Száková, J.; García-Sánchez, M.; Praus, L.; Romera, I.G.; Tlustoš, P. Effect of Dry Olive Residue–Based Biochar and Arbuscular Mycorrhizal Fungi Inoculation on the Nutrient Status and Trace Element Contents in Wheat Grown in the As-, Cd-, Pb-, and Zn-Contaminated Soils. J. Soil Sci. Plant Nutr. 2020, 20, 1067–1079. [Google Scholar] [CrossRef]
- Liu, M.; Che, Y.; Wang, L.; Zhao, Z.; Zhang, Y.; Wei, L.; Xiao, Y. Rice Straw Biochar and Phosphorus Inputs Have More Positive Effects on the Yield and Nutrient Uptake of Lolium Multiflorum than Arbuscular Mycorrhizal Fungi in Acidic Cd-Contaminated Soils. Chemosphere 2019, 235, 32–39. [Google Scholar] [CrossRef]
- Zhang, H.; Zhen, H.; Huang, C.; Wang, K.; Qiao, Y. The Effects of Biochar and AM Fungi (Funneliformis mosseae) on Bioavailability Cd in a Highly Contaminated Acid Soil with Different Soil Phosphorus Supplies. Environ. Sci. Pollut. Res. 2020, 27, 44440–44451. [Google Scholar] [CrossRef]
- Rafique, M.; Ortas, I.; Rizwan, M.; Sultan, T.; Chaudhary, H.J.; Işik, M.; Aydin, O. Effects of Rhizophagus Clarus and Biochar on Growth, Photosynthesis, Nutrients, and Cadmium (Cd) Concentration of Maize (Zea mays) Grown in Cd-Spiked Soil. Environ. Sci. Pollut. Res. 2019, 26, 20689–20700. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, M.; Chen, L.; Ji, L.; Zhao, Z.; Wang, L.; Wei, L.; Zhang, Y. Growth and Elemental Uptake of Trifolium Repens in Response to Biochar Addition, Arbuscular Mycorrhizal Fungi and Phosphorus Fertilizer Applications in Low-Cd-Polluted Soils. Environ. Pollut. 2020, 260, 113761. [Google Scholar] [CrossRef]
- Sun, H.; Zheng, Y.; Lin, Y.; Chen, C.; Yang, F. Effects of Biochar, Phosphorus Addition and AMF Inoculation on Switchgrass Growth and Soil Properties under Cd Stress. Acta Prataculturae Sin. 2021, 30, 71. [Google Scholar]
- Murad, Z.; Ahmad, I.; Waleed, M.; Hashim, S.; Bibi, S. Effect of Biochar on Immobilization of Cadmium and Soil Chemical Properties. Gesunde Pflanz. 2022, 74, 151–158. [Google Scholar] [CrossRef]
- Meng, Z.; Huang, S.; Lin, Z.; Mu, W.; Ge, H.; Huang, D. Cadmium Long-Term Immobilization by Biochar and Potential Risks in Soils with Different PH under Combined Aging. Sci. Total Environ. 2022, 825, 154018. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, N.; Zhang, L.J.; Zhao, H.M.; Li, Y.W.; Cai, Q.Y.; Wong, M.H.; Mo, C.H. Do Arbuscular Mycorrhizal Fungi Affect Cadmium Uptake Kinetics, Subcellular Distribution and Chemical Forms in Rice? Sci. Total Environ. 2016, 571, 1183–1190. [Google Scholar] [CrossRef]
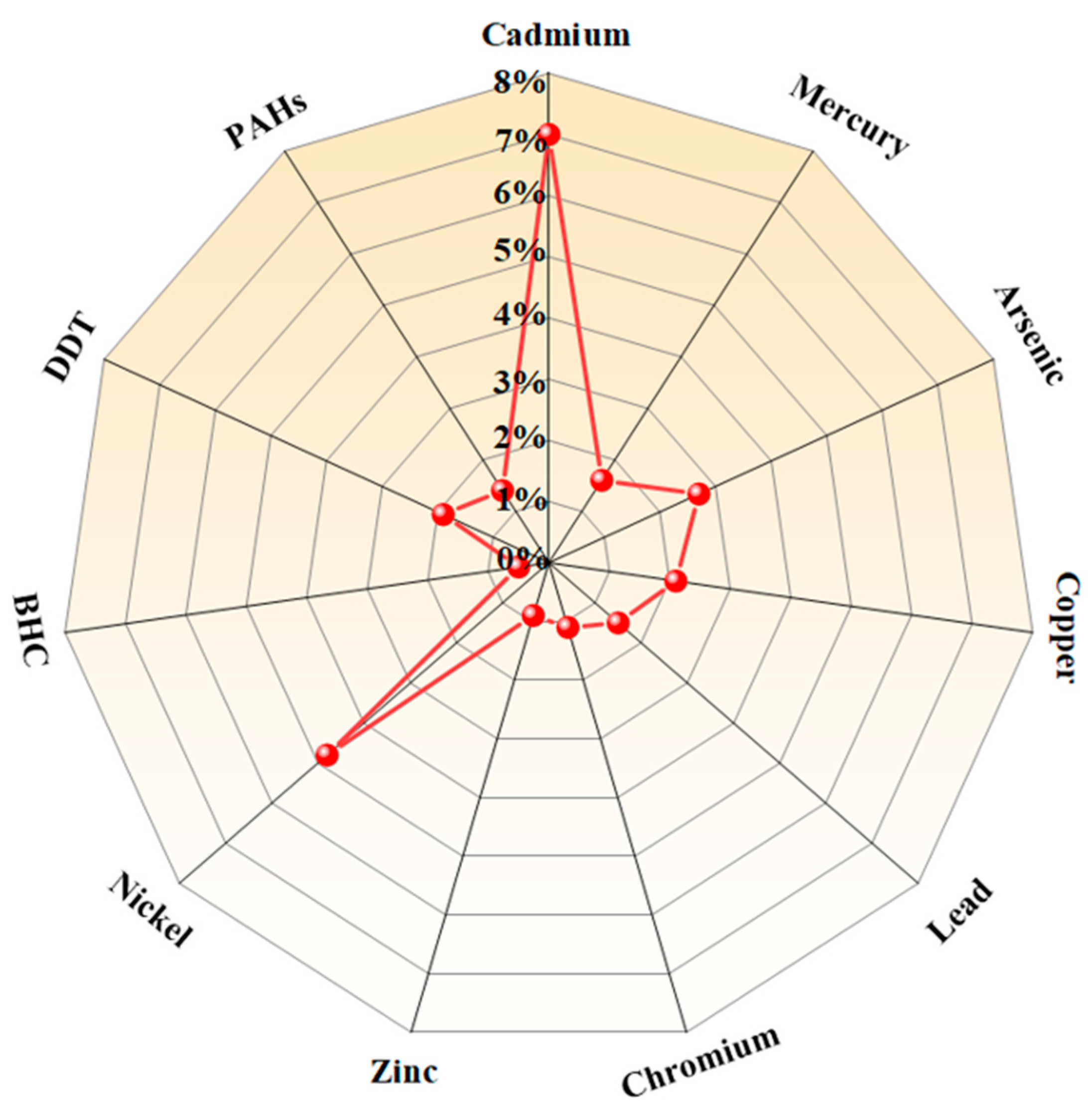
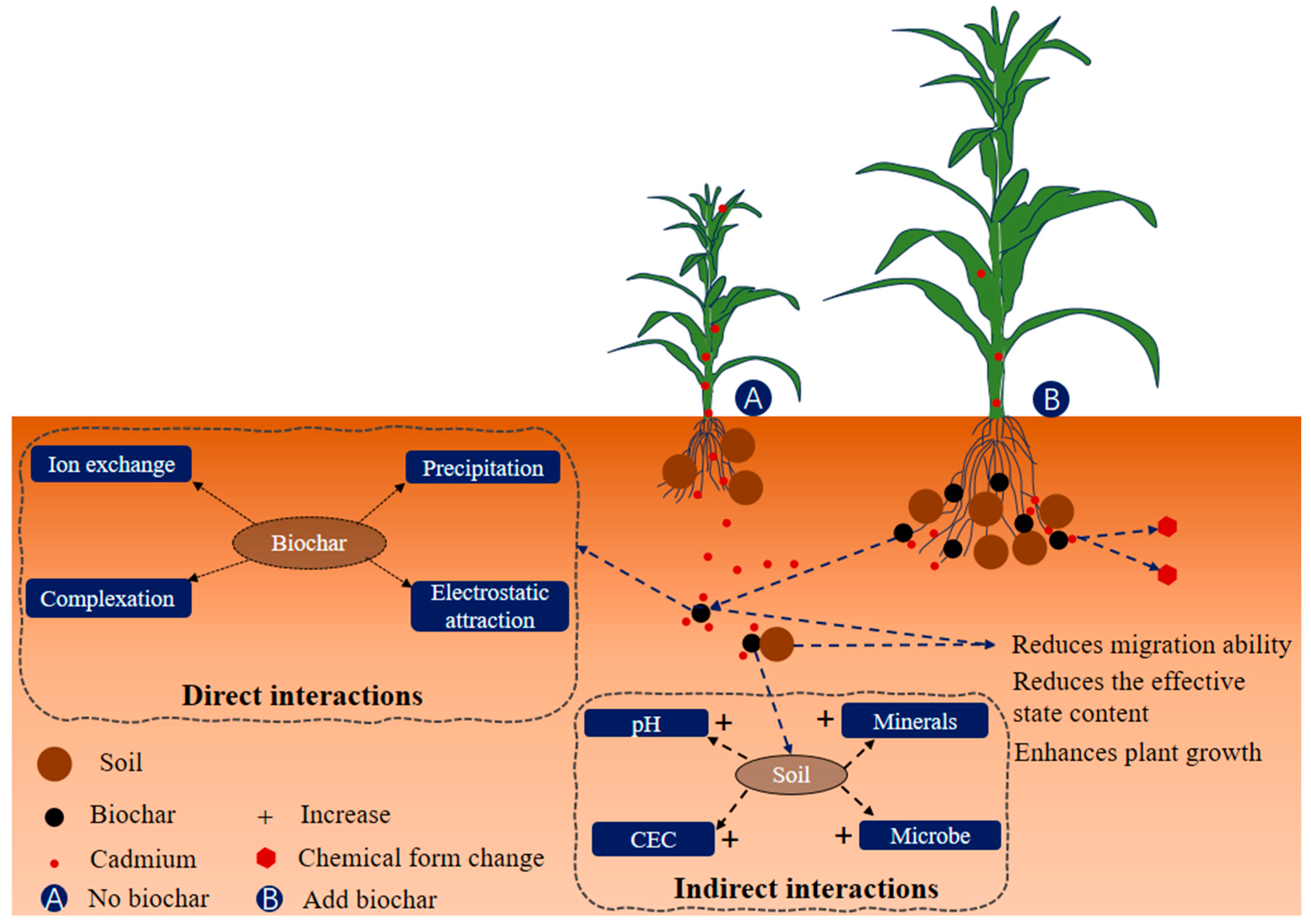
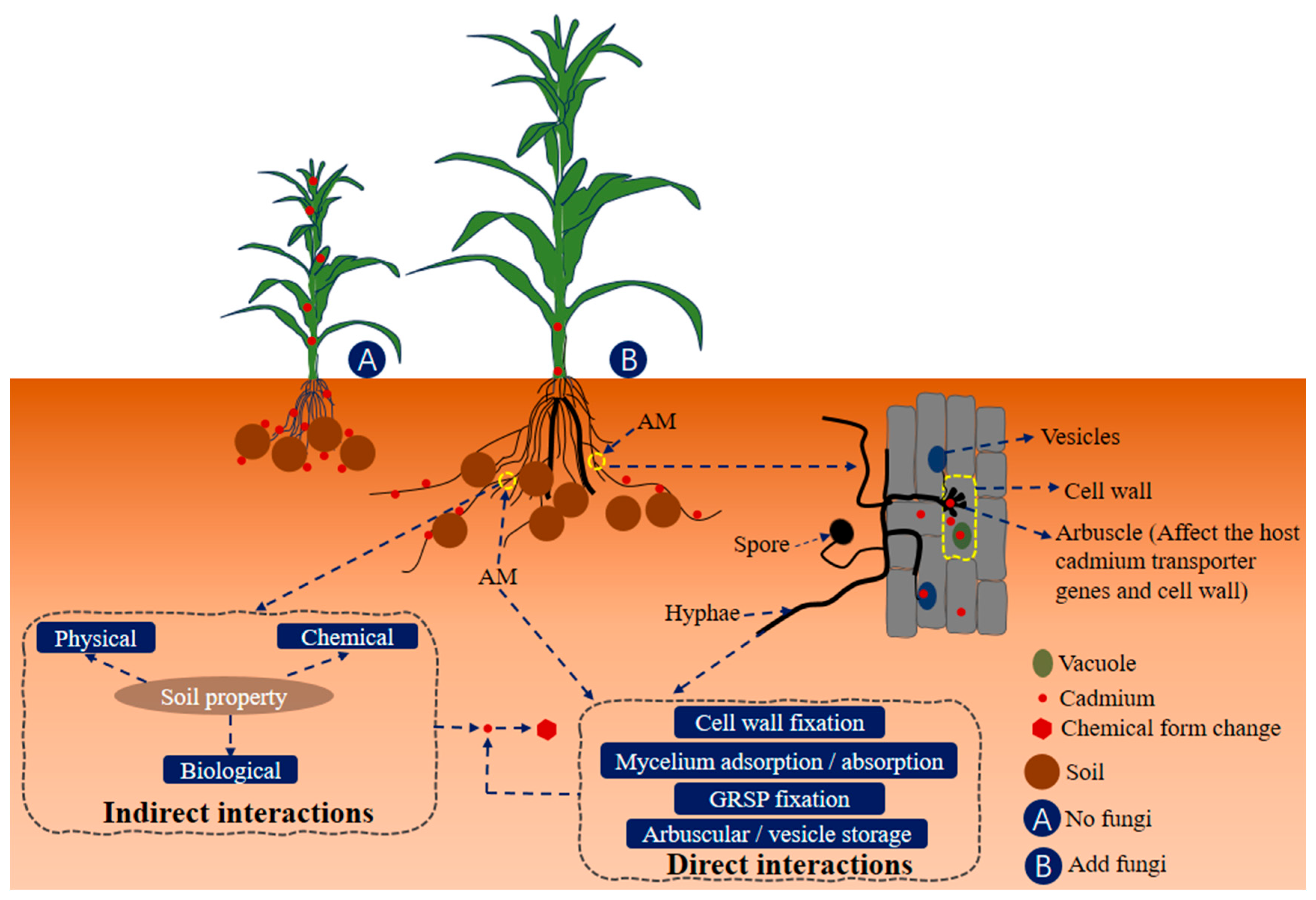
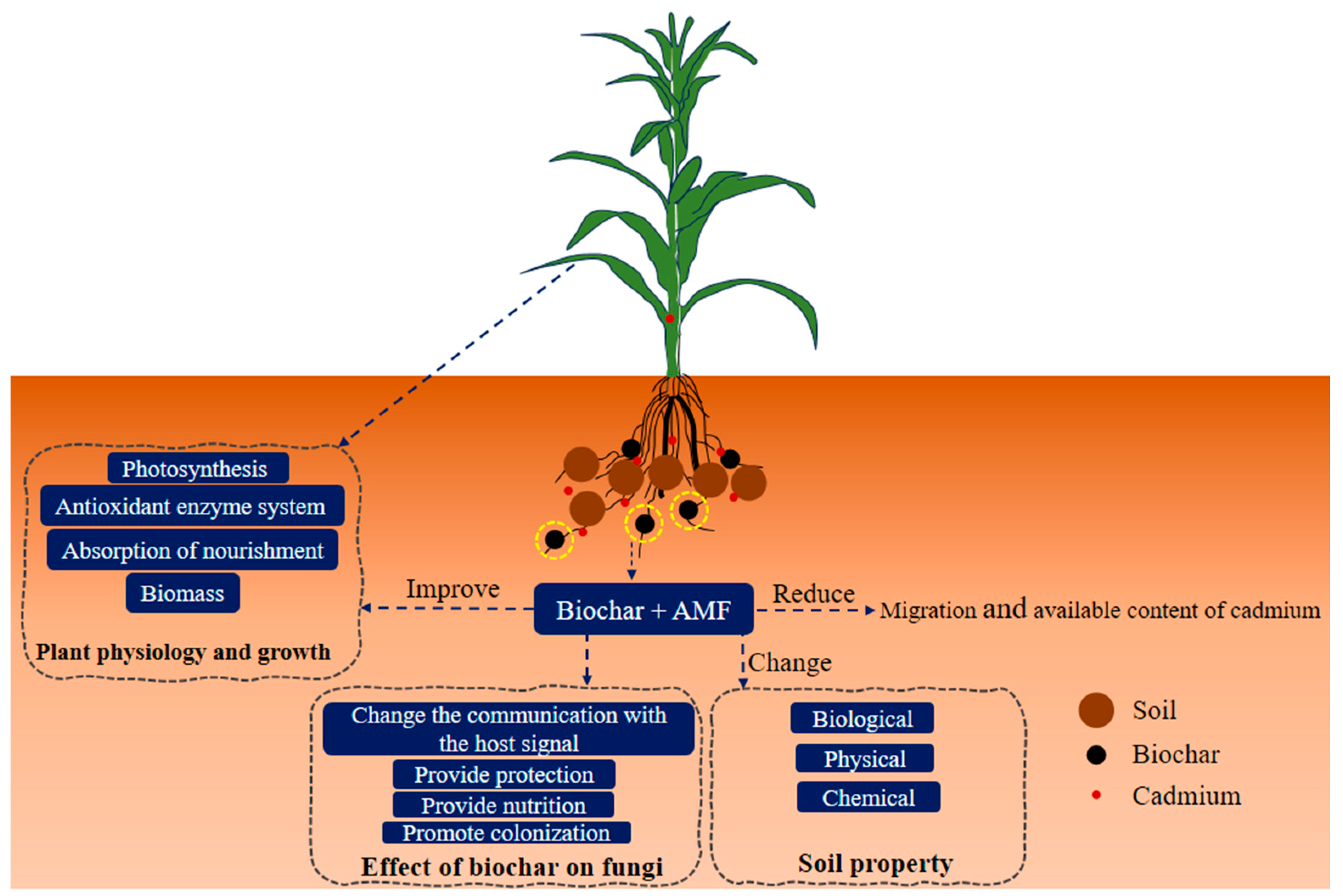
| Influences | Remark | Reference | |
|---|---|---|---|
| Positive | Reduced bioavailability | Biochar has a high surface area and can adsorb cadmium, reducing its bioavailability. When combined with AMF, the mycorrhizal network can facilitate the transport of biochar particles and improve their distribution in the soil, enhancing the immobilization of cadmium. | [100] |
| Enhanced sorption | The porous structure of biochar provides numerous binding sites for cadmium ions, immobilizing them in the soil. The presence of AMF could increase the efficiency of this process by promoting a more extensive root system and facilitating the interaction between biochar and the rhizosphere. | [101] | |
| pH modification | Some biochars have alkaline properties that can influence soil pH. An increase in pH can lead to the precipitation of less soluble forms of cadmium, reducing mobility. The mycorrhizal association may further contribute to pH modification in the rhizosphere, enhancing the immobilization of cadmium. | [85,102] | |
| Mycoextraction | Certain AMF species can accumulate cadmium within their mycelium through mycoextraction. This sequestration reduces cadmium availability in the soil, preventing plant uptake and minimizing the transfer risk to the food chain. | [103,104] | |
| Microbial community enhancement | Both biochar and AMF can positively influence soil microbial communities. Beneficial microbes can contribute to processes immobilizing cadmium, such as microbial-mediated precipitation and complexation reactions. Biochar may provide a supportive habitat for these microbes, and AMF can further enhance their activity. | [40] | |
| Improved soil structure | Combining biochar and AMF may contribute to soil aggregation, improving soil structure. Enhanced soil structure can limit the leaching of cadmium, keeping it bound within the soil matrix and reducing the risk of groundwater contamination. | [100] | |
| Negative | Variable effects | The effectiveness of biochar and AMF in cadmium immobilization can vary depending on factors such as biochar type, AMF species, soil properties, and environmental conditions. In some cases, the synergistic effects may be less pronounced, leading to less effective cadmium immobilization. | [32] |
| Biochar–AMF characteristics | The success of cadmium immobilization in biochar–AMF may depend on the properties of the biochar used. Some biochars may have limited cadmium adsorption capacity, and their effectiveness could be influenced by feedstock and pyrolysis conditions. | [32] | |
| Site-specific considerations | The efficacy of the combination may be influenced by site-specific conditions. Soil characteristics, existing cadmium concentrations, and other contaminants can affect the overall success of the biochar–AMF strategy for cadmium immobilization. | [105] | |
| Long-term stability | The long-term stability of biochar and the sustainability of the mycorrhizal association may influence the persistence of the positive effects on cadmium immobilization. Changes in environmental conditions over time could impact the success of the strategy. | [106] |
| Soil pH | Soil AP (mg/kg) | Soil Organic Matter (g/kg) | Soil TN (g/kg) | Soil AN (mg/kg) | Soil Cadmium Concentration (mg/kg) | Biochar Types | B pH | B TN(g/kg) | BTP(g/kg) | Biochar Application Rate | Plant Species | AMF Species | AMF Application Rate | Synergistic Effect | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4.38 | 21.07 | 1.55 | 71.2 | 20 | rice straw | 10.2 | 8.7 | 2.4 | 3% | Medicago sativa | Mixing of four different fungi | 3.30% | Y | [105] | |
| 7.5 | 15.69 | 13.24 | 0.27 | 50.23 | 4 | wheat straw | 10.2 | 0.27 | 0.26 | 2% | Mulberry | Glomus intraradices | 2% | Y | [110] |
| 5.91 | 52 | 0.0165 | 1.25 | 5 | tobacco straw | 10.3 | 17.7 | 4.8 | 2% | maize | Glomus versiforme | 5% | Y | [112] | |
| 5.91 | 52 | 0.0165 | 1.25 | 5 | tobacco straw | 10.3 | 17.7 | 4.8 | 2% | maize | Funneliformis mosseae | 5% | Y | - | |
| 5.91 | 52 | 0.0165 | 1.25 | 5 | tobacco straw | 10.3 | 17.7 | 4.8 | 2% | maize | Rhizophagus intraradices | 5% | Y | - | |
| 7.6 | 17.13 | 12.62 | 0.54 | 46.66 | 3 | wheat straw | 10.4 | 0.59 | 2% | maize | Glomus intraradices | 2% | Y | [32] | |
| 7.6 | 17.13 | 12.62 | 0.54 | 46.66 | 6 | wheat straw | 10.4 | 0.59 | 2% | maize | Glomus intraradices | 2% | Y | - | |
| 7.6 | 17.13 | 12.62 | 0.54 | 46.66 | 3 | wheat straw | 10.4 | 5.9 | 0.89 | 2% | maize | Glomus intraradices | 2% | Y | [90] |
| 7.6 | 17.13 | 12.62 | 0.54 | 46.66 | 6 | wheat straw | 10.4 | 5.9 | 0.89 | 2% | maize | Glomus intraradices | 2% | Y | - |
| 6.11 | 7.14 | 20.86 | 1.47 | 38.46 | 1 | rice straw | 10.2 | 8.7 | 2.4 | 5% | Trifolium | Mixing two different fungi | 6.50% | N | [123] |
| 6.11 | 37.14 | 20.86 | 1.47 | 38.46 | 1 | rice straw | 10.2 | 8.7 | 2.4 | 5% | Trifolium | Mixing two different fungi | 6.50% | N | - |
| 6.11 | 107.14 | 20.86 | 1.47 | 38.46 | 1 | rice straw | 10.2 | 8.7 | 2.4 | 5% | Trifolium | Mixing two different fungi | 6.50% | N | - |
| 4.38 | 36.61 | 21.07 | 1.55 | 71.2 | 10 | rice straw | 10.2 | 8.7 | 2.4 | 5% | Lolium multiflorum | Mixing of four different fungi | 6% | N | [120] |
| 4.38 | 86.61 | 21.07 | 1.55 | 71.2 | 10 | rice straw | 10.2 | 8.7 | 2.4 | 5% | Lolium multiflorum | Mixing of four different fungi | 6% | N | - |
| 4.38 | 136.61 | 21.07 | 1.55 | 71.2 | 10 | rice straw | 10.2 | 8.7 | 2.4 | 5% | Lolium multiflorum | Mixing of four different fungi | 6% | N | - |
| 8.27 | 15 | 0.0131 | 50.56 | 20 | Pig manure and bamboo powder mixture | 4.50% | Alamo | Rhizophagus irregularis | 1.10% | N | [124] | ||||
| 8.27 | 75 | 0.0131 | 50.56 | 20 | Pig manure and bamboo powder mixture | 4.50% | Alamo | Rhizophagus irregularis | 1.10% | N | - | ||||
| 7.84 | 1.45 | 8 | 5 | reed | 8.98 | 0.0051 | 0.0023 | 1% | maize | Rhizophagus clarus | 1.70% | N | [122] | ||
| 7.84 | 1.45 | 8 | 10 | reed | 8.98 | 0.0051 | 0.0023 | 1% | maize | Rhizophagus clarus | 1.70% | N | - | ||
| 5.71 | 26 | 43.05 | 3.4 | 200 | 3 | maize | 9.74 | 8.53 | 1.21 | 3% | Cichorium intybus | Mixing of four different fungi | 10% | Y | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Lee, X.; Twagirayezu, G.; Cheng, H.; Lu, H.; Huang, S.; Deng, L.; Ji, B. A Critical Review of the Effectiveness of Biochar Coupled with Arbuscular Mycorrhizal Fungi in Soil Cadmium Immobilization. J. Fungi 2024, 10, 182. https://doi.org/10.3390/jof10030182
Fang X, Lee X, Twagirayezu G, Cheng H, Lu H, Huang S, Deng L, Ji B. A Critical Review of the Effectiveness of Biochar Coupled with Arbuscular Mycorrhizal Fungi in Soil Cadmium Immobilization. Journal of Fungi. 2024; 10(3):182. https://doi.org/10.3390/jof10030182
Chicago/Turabian StyleFang, Xin, Xinqing Lee, Gratien Twagirayezu, Hongguang Cheng, Hongyu Lu, Shenglan Huang, Linbo Deng, and Bo Ji. 2024. "A Critical Review of the Effectiveness of Biochar Coupled with Arbuscular Mycorrhizal Fungi in Soil Cadmium Immobilization" Journal of Fungi 10, no. 3: 182. https://doi.org/10.3390/jof10030182
APA StyleFang, X., Lee, X., Twagirayezu, G., Cheng, H., Lu, H., Huang, S., Deng, L., & Ji, B. (2024). A Critical Review of the Effectiveness of Biochar Coupled with Arbuscular Mycorrhizal Fungi in Soil Cadmium Immobilization. Journal of Fungi, 10(3), 182. https://doi.org/10.3390/jof10030182







