Endothelial Dysfunction in Adolescent Hypertension: Diagnostic Challenges and Early Cardiovascular Risk
Abstract
1. Introduction
2. Materials and Methods
Literature Search
3. Clinical Relevance of Endothelial Dysfunction in Youth Hypertension
3.1. Endothelial Dysfunction as Early Indicator of Risk
3.2. “Target Organ” Implications—Heart and Vessels
3.3. White-Coat and Masked Hypertension: ED as a Differentiator
4. Assessing Endothelial Function in Adolescent
4.1. Functional Tests: Brachial Flow-Mediated Dilation and Peripheral Arterial Tonometry
4.2. Non-Invasive Vascular Imaging in Adolescents
5. Pathophysiology and Biomarkers of ED in Adolescent Hypertension
5.1. Nitric Oxide and Oxidative Stress
5.2. Inflammation and Endothelial Activation
5.3. Pubertal Modulation of Endothelial Function
6. Reversibility of Endothelial Dysfunction and Therapeutic Interventions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, B.; Ibrahim, T.I.; Wang, T.; Wu, M.; Qin, J. Current status of elevated blood pressure and hypertension among adolescents in Asia: A systematic review. J. Glob. Health 2025, 15, 04115. [Google Scholar] [CrossRef]
- Khoury, M.; Urbina, E.M. Cardiac and Vascular Target Organ Damage in Pediatric Hypertension. Front. Pediatr. 2018, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Bachetti, T.; D’Anna, S.E.; Galloway, B.; Bianco, A.; D’Agnano, V.; Papa, A.; Motta, A. Mechanisms and Clinical Implications of Endothelial Dysfunction in Arterial Hypertension. J. Cardiovasc. Dev. Dis. 2022, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Järvisalo, M.J.; Jartti, L.; Näntö-Salonen, K.; Irjala, K.; Rönnemaa, T.; Hartiala, J.J.; Celermajer, D.S.; Raitakari, O.T. Increased aortic intima-media thickness: A marker of preclinical atherosclerosis in high-risk children. Circulation 2001, 104, 2943–2947. [Google Scholar] [CrossRef]
- McGill, H.C., Jr.; McMahan, C.A.; Zieske, A.W.; Malcom, G.T.; Tracy, R.E.; Strong, J.P. Effects of nonlipid risk factors on atherosclerosis in youth with a favorable lipoprotein profile. Circulation 2001, 103, 1546–1550. [Google Scholar] [CrossRef]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; De Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef]
- Beckman, J.A.; Goldfine, A.B.; Gordon, M.B.; Garrett, L.A.; Keaney, J.F., Jr.; Creager, M.A. Oral antioxidant therapy improves endothelial function in Type 1 but not Type 2 diabetes mellitus. Am. J. Physiol.—Heart Circ. Physiol. 2003, 285, H2392–H2398. [Google Scholar] [CrossRef]
- Litwin, M.; Niemirska, A.; Sladowska-Kozlowska, J.; Wierzbicka, A.; Janas, R.; Wawer, Z.T.; Wisniewski, A.; Feber, J. Regression of target organ damage in children and adolescents with primary hypertension. Pediatr. Nephrol. 2010, 25, 2489–2499. [Google Scholar] [CrossRef]
- Urbina, E.M.; Khoury, P.R.; McCoy, C.E.; Daniels, S.R.; Kimball, T.R.; Dolan, L.M. Cardiac and vascular consequences of pre-hypertension in youth. J. Clin. Hypertens. 2011, 13, 332–342. [Google Scholar] [CrossRef]
- Tain, Y.-L. Pediatric Hypertension: Current Definition and Knowledge Gaps. Pediatr. Neonatol. 2025; in press. [Google Scholar] [CrossRef]
- Flynn, J.T.; Urbina, E.M.; Brady, T.M.; Baker-Smith, C.; Daniels, S.R.; Hayman, L.L.; Mitsnefes, M.; Tran, A.; Zachariah, J.P. Ambulatory Blood Pressure Monitoring in Children and Adolescents: 2022 Update: A Scientific Statement from the American Heart Association. Hypertension 2022, 79, e114–e124. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, X.; Yin, H.; Sun, Z.; Zügel, M.; Steinacker, J.M.; Schumann, U. Exercise training and endothelial function in patients with type 2 diabetes: A meta-analysis. Cardiovasc. Diabetol. 2018, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Lurbe, E.; Torró, M.I.; Álvarez, J. Ambulatory blood pressure monitoring in children and adolescents: Coming of age? Curr. Hypertens. Rep. 2013, 15, 143–149. [Google Scholar] [CrossRef]
- Lurbe, E.; Invitti, C.; Torro, I.; Maronati, A.; Aguilar, F.; Sartorio, A.; Redon, J.; Parati, G. The impact of the degree of obesity on the discrepancies between office and ambulatory blood pressure values in youth. J. Hypertens. 2006, 24, 1557–1564, Erratum in J. Hypertens. 2007, 25, 258. [Google Scholar] [CrossRef]
- Aggoun, Y.; Farpour-Lambert, N.J.; Marchand, L.M.; Golay, E.; Maggio, A.B.R.; Beghetti, M. Impaired endothelial and smooth muscle functions and arterial stiffness appear before puberty in obese children and are associated with elevated ambulatory blood pressure. Eur. Heart J. 2008, 29, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Walther, G.; Benamo, E.; Perez-Martin, A.; Vinet, A. Effects of Exercise Training on Arterial Function in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Sports Med. 2013, 43, 1191–1199. [Google Scholar] [CrossRef]
- Woo, K.S.; Chook, P.; Yu, C.W.; Sung, R.Y.; Qiao, M.; Leung, S.S.; Lam, C.W.; Metreweli, C.; Celermajer, D.S. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation 2004, 109, 1981–1986. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Rescaldani, M.; Tadic, M.; Sala, C.; Grassi, G.; Mancia, G. White-coat Hypertension, as Defined by Ambulatory Blood Pressure Monitoring, and Subclinical Cardiac Organ Damage: A Meta-analysis. J. Hypertens. 2015, 33, 24–32. [Google Scholar] [CrossRef]
- Allalou, A.; Peng, J.; Robinson, G.A.; Ulloa, A.; Lovinsky-Desir, S.; Zafar, H.; Argemi, G.; Patel, S.R.; Salman, R.; Chung, S.T.; et al. Impact of puberty, sex determinants and chronic inflammation on cardiovascular risk in young people. Front. Cardiovasc. Med. 2023, 10, 1191119. [Google Scholar] [CrossRef]
- Jurko, T.; Mešťaník, M.; Jurková, E.; Zeleňák, K.; Klásková, E.; Jurko, A. The effect of age, hypertension, and overweight on arterial stiffness assessed using carotid wall echo-tracking in childhood and adolescence. Life 2024, 14, 300. [Google Scholar] [CrossRef]
- Wilkinson, I.B.; Franklin, S.S.; Hall, I.R.; Tyrrell, S.; Cockcroft, J.R. Pressure amplification explains why pulse pressure is unrelated to risk in young subjects. Hypertension 2001, 38, 1461–1466. [Google Scholar] [CrossRef]
- Reusz, G.S.; Cseprekal, O.; Temmar, M.; Kis, É.; Cherif, A.B.; Thaleb, A.; Fekete, A.; Szabó, A.J.; Benetos, A.; Salvi, P. Reference values of pulse wave velocity in healthy children and teenagers. Hypertension 2010, 56, 217–224. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the Management of Arterial Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef]
- Jurko, A., Jr.; Jurko, T.; Minarík, M.; Mešťaník, M.; Mešťaníková, A.; Mičieta, V.; Višňovcová, Z.; Tonhajzerová, I. Endothelial Function in Children with White-Coat Hypertension. Heart Vessel. 2018, 33, 657–663. [Google Scholar] [CrossRef]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef] [PubMed]
- Järvisalo, M.J.; Raitakari, O.T. Ultrasound Assessment of Endothelial Function in Children. Vasc. Health Risk Manag. 2005, 1, 227–233. [Google Scholar] [PubMed]
- Black, M.A.; Cable, N.T.; Thijssen, D.H.; Green, D.J. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 2008, 51, 203–210. [Google Scholar] [CrossRef]
- Doğan, K.; Başar, E.Z.; Aytaç, M.B.; Şahin, N.; Bayrak, Y.E.; Bek, K.; Güngör, H.S.; Sönmez, H.E.; Babaoğlu, K. Evaluation of Endothelial Dysfunction in Hypertensive Children and Adolescents. Pediatr. Nephrol. 2024, 39, 1193–1199. [Google Scholar] [CrossRef]
- Mueller, U.M.; Walther, C.; Adam, J.; Fikenzer, K.; Erbs, S.; Mende, M.; Adams, V.; Linke, A.; Schuler, G. Endothelial Function in Children and Adolescents Is Mainly Influenced by Age, Sex and Physical Activity—An Analysis of Reactive Hyperemic Peripheral Artery Tonometry. Circ. J. 2017, 81, 717–725. [Google Scholar] [CrossRef]
- Jurko, T.; Mešťaník, M.; Mešťaníková, A.; Zeleňák, K.; Jurko, A. Early Signs of Microvascular Endothelial Dysfunction in Adolescents with Newly Diagnosed Essential Hypertension. Life 2022, 12, 1048. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Urbina, E.M.; Dennison, B.A.; Jacobson, M.S.; Steinberger, J.; Rocchini, A.P.; Hayman, L.L.; Daniels, S.R. Drug Therapy of High-Risk Lipid Abnormalities in Children and Adolescents: A Scientific Statement from the American Heart Association. Circulation 2007, 115, 1948–1967. [Google Scholar] [CrossRef]
- Watts, K.; Beye, P.; Siafarikas, A.; Davis, E.A.; Jones, T.W.; O’DRiscoll, G.; Green, D.J. Exercise training normalizes vascular dysfunction and improves central adiposity in obese adolescents. J. Am. Coll. Cardiol. 2004, 43, 1823–1827. [Google Scholar] [CrossRef]
- Ateya, A.M.; El Hakim, I.; Shahin, S.M.; El Borolossy, R.; Kreutz, R.; Sabri, N.A. Effects of Ramipril on Biomarkers of Endothelial Dysfunction and Inflammation in Hypertensive Children on Maintenance Hemodialysis: The SEARCH Randomized Placebo-Controlled Trial. Hypertension 2022, 79, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Dias, K.A.; Green, D.J.; Ingul, C.B.; Pavey, T.G.; Coombes, J.S. Exercise and Vascular Function in Child Obesity: A Meta-Analysis. Pediatrics 2015, 136, e648–e659. [Google Scholar] [CrossRef]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2021, 8, 798958. [Google Scholar] [CrossRef]
- de Giorgis, T.; Marcovecchio, M.L.; Giannini, C.; Chiavaroli, V.; Chiarelli, F.; Mohn, A. Blood Pressure from Childhood to Adolescence in Obese Youths in Relation to Insulin Resistance and Asymmetric Dimethylarginine. J. Endocrinol. Investig. 2016, 39, 169–176. [Google Scholar] [CrossRef]
- Gruber, H.-J.; Mayer, C.; Meinitzer, A.; Almer, G.; Horejsi, R.; Möller, R.; Pilz, S.; März, W.; Gasser, R.; Truschnig-Wilders, M.; et al. Asymmetric dimethylarginine (ADMA) is tightly correlated with growth in juveniles without correlations to obesity-related disorders. Exp. Clin. Endocrinol. Diabetes 2008, 116, 520–524. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Sudano, I.; Salvetti, A. Antihypertensive Drugs and Reversing of Endothelial Dysfunction in Hypertension. Curr. Hypertens. Rep. 2000, 2, 64–70. [Google Scholar] [CrossRef]
- Sinha, M.D.; Azukaitis, K.; Sladowska-Kozłowska, J.; Bårdsen, T.; Merkevicius, K.; Karlsen Sletten, I.S.; Obrycki, Ł.; Pac, M.; Fernández-Aranda, F.; Bjelakovic, B.; et al. Prevalence of left ventricular hypertrophy in children and young people with primary hypertension: Meta-analysis and meta-regression. Front. Cardiovasc. Med. 2022, 9, 993513. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhang, Y.; Yu, J.; Zha, M.; Zhu, Y.; Rahimi, K.; Rudan, I. Global Prevalence of Hypertension in Children: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2019, 173, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
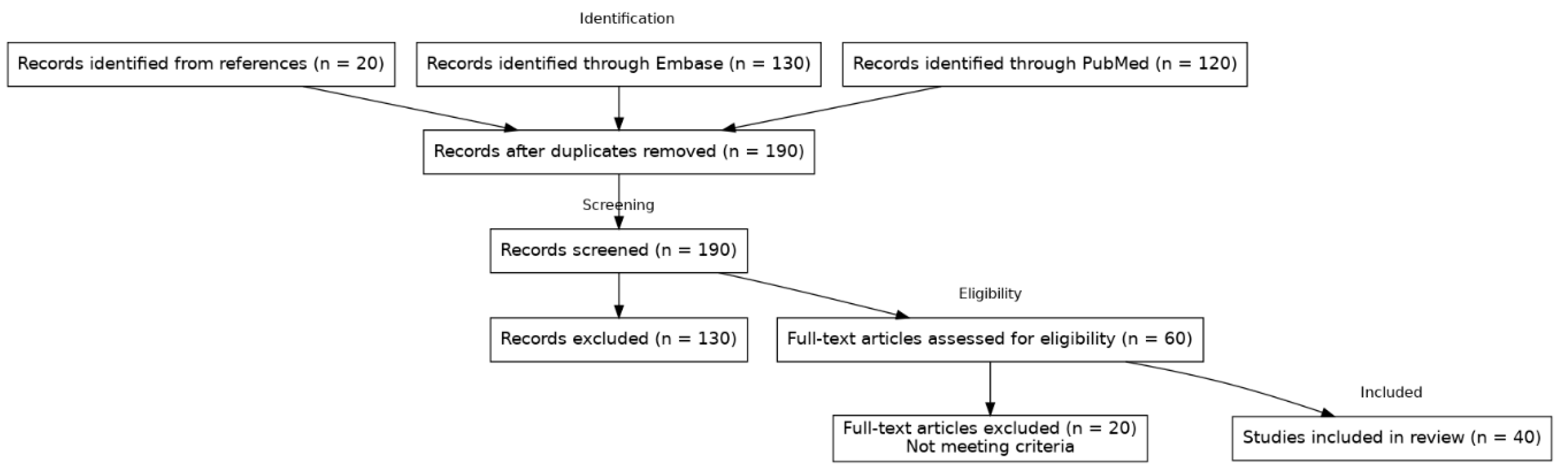
| Study (Year) | Population/Design | Key Findings (Endothelial Function and Outcomes) |
|---|---|---|
| Lurbe et al., 2013 [13] | Referred children and adolescents; ABPM-based classification; cross-sectional prevalence (white-coat hypertension). | Reported prevalence and determinants of white-coat hypertension in youth; highlights need for ABPM to avoid misclassification; WCH associated with higher office BP and adiposity. |
| Jurko Jr. et al., 2018 [24] | ~90 adolescents (cross-sectional)—groups: sustained HTN, white-coat HTN, normotensive controls. Endothelial function by brachial FMD. | FMD was significantly lower in both sustained hypertensive and white-coat hypertensive adolescents compared with controls (p < 0.05 for both). No difference between white-coat vs. sustained HTN FMD, suggesting even WCH had comparable endothelial dysfunction to sustained HTN. Supports that WCH is not entirely benign in youth. |
| Jurko et al., 2022 [30] | 100 adolescents (mean ~15 y)—normotensive (~34), essential HTN (~33), white-coat HTN (~33). Endothelial function measured by PAT (RHI and novel hyperemia indices). | Essential HTN had lower PAT hyperemic AUC index than normotensive and WCH groups (p = 0.02–0.03). Conventional RHI did not differ, but AUC was sensitive to ED in sustained HTN. AUC inversely correlated with mean BP (r = –0.23) and positively with pubertal hormone (DHEA). White-coat HTN had AUC similar to normals, indicating preserved microvascular function in WCH (contrast to macrovascular FMD findings). First study to show microvascular ED via PAT in adolescent HTN. |
| Doğan et al., 2024 [28] | 40 adolescents (~16 y; 20 newly diagnosed essential HTN without treatment, 20 healthy controls). Cross-sectional measurement of carotid IMT, brachial FMD, and capillary density (nailfold capillaroscopy). | Hypertensive youth had higher carotid IMT (mean diff p = 0.04), lower FMD (HTN ~5.8% vs. control ~8.3%, p = 0.02), and reduced functional capillary density (p < 0.001) compared with controls. No difference between dipper vs. non-dipper HTN subgroups. Conclusion: Essential HTN is associated with increased arterial stiffness/thickness and significant endothelial dysfunction in adolescents, emphasizing the need for early management. |
| Watts et al., 2004 [32] | Obese adolescents (n = 19; 14.3 ± 1.5 y), randomized crossover; 8-week supervised circuit training vs. habitual activity; lean controls (n = 20) for baseline comparison; conduit-artery endothelial function by brachial FMD; body composition by DXA | Baseline FMD impaired vs. lean controls (5.3 ± 0.9% vs. 8.9 ± 1.5%); after 8 weeks of training FMD normalized (8.8 ± 0.8%); abdominal and trunk fat decreased; fitness and muscular strength improved (all p < 0.05) |
| Ateya et al., 2022 [33] | 135 children (7–15 y) on maintenance hemodialysis with hypertension. Randomized placebo-controlled trial: Ramipril 2.5 mg QD (n = 68) vs. placebo (n = 67) for 16 weeks. Primary endpoints: plasma ADMA (endothelial dysfunction marker) and hs-CRP; secondary: IL-6, TNF-α, BP. | Ramipril group showed significant improvements in endothelial biomarkers: ADMA decreased by 80%, hs-CRP by 46%, IL-6 by 27%, TNF-α by 52% (all p < 0.001 vs. baseline; significantly greater reductions than placebo). BP fell in both groups, but more in Ramipril (−12/−9 mmHg vs. placebo). Notably, reductions in ADMA and inflammatory markers did not correlate with BP changes, suggesting a direct endothelial benefit of ACE inhibition. Implication: ACE inhibitors restore endothelial function (improve NO availability and lower inflammation) in children, beyond their BP-lowering effect. |
| Dias et al., 2015 [34] | Meta-analysis of randomized exercise interventions in overweight/obese children and adolescents (aggregate N = 219); primary outcome: brachial FMD; secondary: VO2peak. | Exercise training vs control improved FMD (mean difference + 1.54 % absolute, p < 0.05) and increased VO2peak (+3.64 mL·kg−1·min−1, p < 0.05). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micieta, V.; Cehakova, M.; Tonhajzerova, I. Endothelial Dysfunction in Adolescent Hypertension: Diagnostic Challenges and Early Cardiovascular Risk. J. Cardiovasc. Dev. Dis. 2025, 12, 326. https://doi.org/10.3390/jcdd12090326
Micieta V, Cehakova M, Tonhajzerova I. Endothelial Dysfunction in Adolescent Hypertension: Diagnostic Challenges and Early Cardiovascular Risk. Journal of Cardiovascular Development and Disease. 2025; 12(9):326. https://doi.org/10.3390/jcdd12090326
Chicago/Turabian StyleMicieta, Vladimir, Michaela Cehakova, and Ingrid Tonhajzerova. 2025. "Endothelial Dysfunction in Adolescent Hypertension: Diagnostic Challenges and Early Cardiovascular Risk" Journal of Cardiovascular Development and Disease 12, no. 9: 326. https://doi.org/10.3390/jcdd12090326
APA StyleMicieta, V., Cehakova, M., & Tonhajzerova, I. (2025). Endothelial Dysfunction in Adolescent Hypertension: Diagnostic Challenges and Early Cardiovascular Risk. Journal of Cardiovascular Development and Disease, 12(9), 326. https://doi.org/10.3390/jcdd12090326