The Role of Artificial Intelligence in Cardiac Amyloidosis: A Focus on Diagnosis and Clinical Application
Abstract
1. Introduction
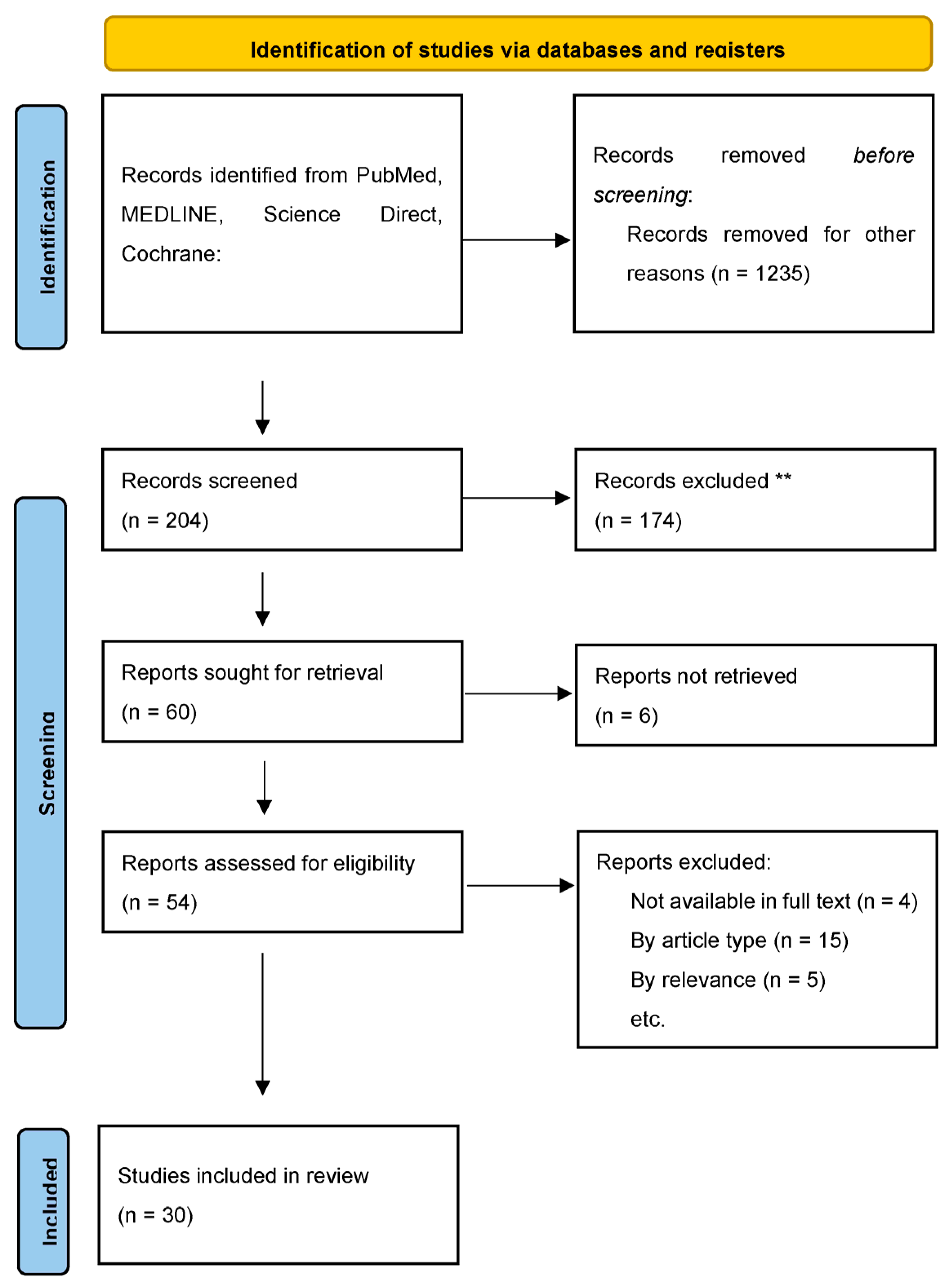
2. Materials and Methods
3. Discussion
3.1. A Brief Introduction to Machine Learning
3.2. A Brief Introduction to Neural Networks and Deep Learning
3.3. Using AI to Detect CA from Electrocardiogram
3.4. AI Detected CA Using Echocardiography
3.5. Can AI Help Novice Operators Obtain Echocardiographic Images?
3.6. Scintigraphy Focused AI in Detecting CA
3.7. Use of CMR to Aide in the Diagnosis of CA
3.8. Differentiating CA from Other Cardiac Disease States
3.9. Use of AI in Genetics to Predict CA
3.10. A Hopeful Future of Diagnosing CA
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| AL | Light chain amyloid |
| ATTR | Transthyretin amyloid |
| CA | Cardiac amyloidosis |
| CMR | Cardiac magnetic resonance |
| ML | Machine learning |
| DL | Deep learning |
| NN | Neural networks |
| LV | Left ventricular |
| CP | Constrictive pericarditis |
| DPD | 3,3-diphosphono-1,2-propanodicarboxylic acid |
| HMDP | hydroxymethylene diphosphonate |
| PYP | pyrophosphate |
| SPECT | single-photon emission computed tomography |
| CNN | convolutional neural networks |
| AUROC | area under the receiver operating characteristic curve |
| HCM | hypertrophic cardiomyopathy |
| LGLS | LV global longitudinal strain |
| TAPSE | tricuspid annular plane systolic excursion |
| ESRD | end-stage renal disease |
| POCUS | point-of-care ultrasound |
| FDA | US Food and Drug Administration |
| TTE | transthoracic echocardiographic |
| WBS | whole body bone scintigraphy |
| 99mTc | technetium-99m |
| LGE | late gadolinium enhancement |
| ECV | extracellular volume |
| SVM | support vector machine |
| PAH | Pulmonary arterial hypertension |
| MVP | Mitral valve prolapse |
| GBM | Gradient Boosted Machine |
| LVH | Left ventricular hypertrophy |
| HTN | Hypertension |
| LC | Light chains |
References
- Goto, S.; Mahara, K.; Beussink-Nelson, L.; Ikura, H.; Katsumata, Y.; Endo, J.; Gaggin, H.K.; Shah, S.J.; Itabashi, Y.; MacRae, C.A.; et al. Artificial Intelligence-Enabled Fully Automated Detection of Cardiac Amyloidosis Using Electrocardiograms and Echocardiograms. Nat. Commun. 2021, 12, 2726. [Google Scholar] [CrossRef] [PubMed]
- Alwan, L.; Benz, D.C.; Cuddy, S.A.M.; Dobner, S.; Shiri, I.; Caobelli, F.; Bernhard, B.; Stämpfli, S.F.; O’Sullivan, C.J.; Reyes, M.; et al. Current and Evolving Multimodality Cardiac Imaging in Managing Transthyretin Amyloid Cardiomyopathy. JACC Cardiovasc. Imaging 2024, 17, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jimenez, F.; Attia, Z.; Arruda-Olson, A.M.; Carter, R.; Chareonthaitawee, P.; Jouni, H.; Kapa, S.; Lerman, A.; Luong, C.; Medina-Inojosa, J.R.; et al. Artificial Intelligence in Cardiology: Present and Future. Mayo Clin. Proc. 2020, 95, 1015–1039. [Google Scholar] [CrossRef]
- Infante, T.; Francone, M.; De Rimini, M.L.; Cavaliere, C.; Canonico, R.; Catalano, C.; Napoli, C.; Salvatore, M.; Cademartiri, F.; Punzo, B. Machine Learning and Network Medicine: A Novel Approach for Precision Medicine and Personalized Therapy in Cardiomyopathies. J. Cardiovasc. Med. 2020, 22, 429–440. [Google Scholar] [CrossRef]
- Ahammed, M.R.; Ananya, F.N. Cardiac Amyloidosis: A Comprehensive Review of Pathophysiology, Diagnostic Approach, Applications of Artificial Intelligence, and Management Strategies. Cureus 2024, 16, e63673. [Google Scholar] [CrossRef]
- Vrudhula, A.; Stern, L.; Cheng, P.C.; Ricchiuto, P.; Daluwatte, C.; Witteles, R.; Patel, J.; Ouyang, D.; Kwan, A.C.; Cheng, S.C. Impact of Case and Control Selection on Training Artificial Intelligence Screening of Cardiac Amyloidosis. JACC Adv. 2024, 3, 100998. [Google Scholar] [CrossRef] [PubMed]
- Tison, G.H.; Zhang, J.; Delling, F.N.; Deo, R.C.; Attia, Z.; Carter, R.; Chareonthaitawee, P.; Jouni, H.; Kapa, S.; Lerman, A.; et al. Automated and Interpretable Patient ECG Profiles for Disease Detection, Tracking, and Discovery. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005289. [Google Scholar] [CrossRef]
- Grogan, M.; Lopez-Jimenez, F.; Cohen-Shelly, M.; Dispenzieri, A.; Attia, Z.I.; Abou Ezzedine, O.F.; Lin, G.; Kapa, S.; Borgeson, D.D.; Friedman, P.A. Artificial Intelligence–Enhanced Electrocardiogram for the Early Detection of Cardiac Amyloidosis. Mayo Clin. Proc. 2021, 96, 2768–2778. [Google Scholar] [CrossRef]
- Haimovich, J.S.; Diamant, N.; Khurshid, S.; Di Achille, P.; Reeder, C.; Friedman, S.; Singh, P.; Spurlock, W.; Ellinor, P.T.; Philippakis, A.; et al. Artificial Intelligence–Enabled Classification of Hypertrophic Heart Diseases Using Electrocardiograms. Cardiovasc. Digit. Health J. 2023, 4, 48–59. [Google Scholar] [CrossRef]
- Goto, S.; Solanki, D.; John, J.E.; Yagi, R.; Homilius, M.; Ichihara, G.; Katsumata, Y.; Gaggin, H.K.; Itabashi, Y.; MacRae, C.A.; et al. Multinational Federated Learning Approach to Train ECG and Echocardiogram Models for Hypertrophic Cardiomyopathy Detection. Circulation 2022, 146, 755–769. [Google Scholar] [CrossRef]
- Harmon, D.M.; Mangold, K.; Baez Suarez, A.; Scott, C.G.; Murphree, D.H.; Malik, A.; Attia, Z.I.; Lopez-Jimenez, F.; Friedman, P.A.; Dispenzieri, A.; et al. Postdevelopment performance and validation of the artificial intelligence-enhanced electrocardiogram for detection of cardiac amyloidosis. JACC Adv. 2023, 2, 100612. [Google Scholar] [CrossRef] [PubMed]
- Arana-Achaga, X.; Goena-Vives, C.; Villanueva-Benito, I.; Solla-Ruiz, I.; Rengel Jiménez, A.; Gaspar, T.I.; Urreta-Barallobre, I.; Barge-Caballero, G.; Seijas-Marcos, S.; Cabrera, E.; et al. Development and Validation of a Prediction Model and Score for Transthyretin Cardiac Amyloidosis Diagnosis. JACC Cardiovasc. Imaging 2023, 16, 1567–1580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gajjala, S.; Agrawal, P.; Tison, G.H.; Hallock, L.A.; Beussink-Nelson, L.; Lassen, M.H.; Fan, E.; Aras, M.A.; Jordan, C.; et al. Fully Automated Echocardiogram Interpretation in Clinical Practice. Circulation 2018, 138, 1623–1635. [Google Scholar] [CrossRef]
- Chao, C.-J.; Jeong, J.; Arsanjani, R.; Kim, K.; Tsai, Y.-L.; Yu, W.-C.; Farina, J.M.; Mahmoud, A.K.; Ayoub, C.; Grogan, M.; et al. Echocardiography-Based Deep Learning Model to Differentiate Constrictive Pericarditis and Restrictive Cardiomyopathy. JACC Cardiovasc. Imaging 2024, 17, 349–360. [Google Scholar] [CrossRef]
- Duffy, G.; Cheng, P.P.; Yuan, N.; He, B.; Kwan, A.C.; Shun-Shin, M.J.; Alexander, K.M.; Ebinger, J.; Lungren, M.P.; Rader, F.; et al. High-Throughput Precision Phenotyping of Left Ventricular Hypertrophy with Cardiovascular Deep Learning. JAMA Cardiol. 2022, 7, 386. [Google Scholar] [CrossRef]
- Li, J.; Chao, C.-J.; Jeong, J.J.; Farina, J.M.; Seri, A.R.; Barry, T.; Newman, H.; Campany, M.; Abdou, M.; O’Shea, M.; et al. Developing an Echocardiography-Based, Automatic Deep Learning Framework for the Differentiation of Increased Left Ventricular Wall Thickness Etiologies. J. Imaging 2023, 9, 48. [Google Scholar] [CrossRef]
- Narang, A.; Bae, R.; Hong, H.; Thomas, Y.; Surette, S.; Cadieu, C.; Chaudhry, A.; Martin, R.P.; McCarthy, P.M.; Rubenson, D.S.; et al. Utility of a Deep-Learning Algorithm to Guide Novices to Acquire Echocardiograms for Limited Diagnostic Use. JAMA Cardiol. 2021, 6, 624. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Vaid, A.; Holste, G.; Coppi, A.; McNamara, R.L.; Baloescu, C.; Krumholz, H.M.; Wang, Z.; Apakama, D.J.; Nadkarni, G.N.; et al. Artificial Intelligence-Guided Detection of Under-Recognised Cardiomyopathies on Point-of-Care Cardiac Ultrasonography: A Multicentre Study. Lancet Digit. Health 2025, 7, e113–e123. [Google Scholar] [CrossRef]
- Ouyang, D.; He, B.; Ghorbani, A.; Yuan, N.; Ebinger, J.; Langlotz, C.P.; Heidenreich, P.A.; Harrington, R.A.; Liang, D.H.; Ashley, E.A.; et al. Video-Based AI for Beat-To-Beat Assessment of Cardiac Function. Nature 2020, 580, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Soh, C.; Wright, L.; Baumann, A.; Seidel, B.; Yu, C.; Nolan, M.; Mylius, T.; Marwick, T.H.; AGILE-Echo Investigators. An Artificial Intelligence-Guided Echocardiography in Primary Care Patients from Rural and Remote Communities: The AGILE-Echo Study. Heart Lung Circ. 2024, 33, S161. [Google Scholar] [CrossRef]
- Delbarre, M.-A.; Girardon, F.; Roquette, L.; Blanc-Durand, P.; Hubaut, M.-A.; Hachulla, É.; Semah, F.; Huglo, D.; Garcelon, N.; Marchal, E.; et al. Deep Learning on Bone Scintigraphy to Detect Abnormal Cardiac Uptake at Risk of Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2023, 16, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Spielvogel, C.P.; Haberl, D.; Mascherbauer, K.; Ning, J.; Kluge, K.; Traub-Weidinger, T.; Davies, R.H.; Pierce, I.; Patel, K.; Nakuz, T.; et al. Diagnosis and Prognosis of Abnormal Cardiac Scintigraphy Uptake Suggestive of Cardiac Amyloidosis Using Artificial Intelligence: A Retrospective, International, Multicentre, Cross-Tracer Development and Validation Study. Lancet Digit. Health 2024, 6, e251–e260. [Google Scholar] [CrossRef] [PubMed]
- Halme, H.-L.; Ihalainen, T.; Suomalainen, O.; Loimaala, A.; Mätzke, S.; Uusitalo, V.; Sipilä, O.; Hippeläinen, E. Convolutional Neural Networks for Detection of Transthyretin Amyloidosis in 2D Scintigraphy Images. EJNMMI Res. 2022, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Maggialetti, N.; Torrente, A.; Lorusso, G.; Villanova, I.; Ficco, M.; Gravina, M.; Ferrari, C.; Giordano, L.; Granata, V.; Rubini, D.; et al. Role of Cardiovascular Magnetic Resonance in Cardiac Amyloidosis: A Narrative Review. J. Pers. Med. 2024, 14, 407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martini, N.; Aimo, A.; Barison, A.; Della Latta, D.; Vergaro, G.; Aquaro, G.D.; Ripoli, A.; Emdin, M.; Chiappino, D.; Chiappino, S. Deep Learning to Diagnose Cardiac Amyloidosis from Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 84. [Google Scholar] [CrossRef]
- Agibetov, A.; Törnquist, K.; Perez-Rodriguez, D.; Smistad, E.; Oliveira, G.S.; Rudd, J.; Rueckert, D.; Jørstad, O.K.; Østvik, A.; Blaschke, T.F.; et al. Convolutional Neural Networks for Fully Automated Diagnosis of Cardiac Amyloidosis by Cardiac Magnetic Resonance Imaging. J. Pers. Med. 2021, 11, 1268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eckstein, J.; Moghadasi, N.; Körperich, H.; Weise Valdés, E.; Sciacca, V.; Paluszkiewicz, L.; Burchert, W.; Piran, M. A Machine Learning Challenge: Detection of Cardiac Amyloidosis Based on Bi-Atrial and Right Ventricular Strain and Cardiac Function. Diagnostics 2022, 12, 2693. [Google Scholar] [CrossRef]
- Garofalo, M.; Piccoli, L.; Romeo, M.; Barzago, M.M.; Ravasio, S.; Foglierini, M.; Matkovic, M.; Sgrignani, J.; De Gasparo, R.; Prunotto, M.; et al. Machine Learning Analyses of Antibody Somatic Mutations Predict Immunoglobulin Light Chain Toxicity. Nat. Commun. 2021, 12, 3532. [Google Scholar] [CrossRef]
- Khera, R. Transforming Cardiovascular Care with Artificial Intelligence: From Discovery to Practice. J. Am. Coll. Cardiol. 2024, 84, 97–114. [Google Scholar] [CrossRef]
- Olawade, D.B.; Adertinto, N.; Olatunji, G.; Kokori, E.; David-Olawade, A.C.; Hadi, M. Advancements and Applications of Artificial Intelligence in Cardiology: Current Trends and Future Prospects. J. Med. Surg. Public Health 2024, 3, 100109. [Google Scholar] [CrossRef]
| Study | Year | Design | Sample Size | Population/Data | Objective | Key Findings | Limitations | Comparison to Other Disease States |
|---|---|---|---|---|---|---|---|---|
| Vrudhula et al. [6] | 2022 | Deep learning models | 341,989 | Cedars-Sinai Medical Center | Training AI models using different selection of cases and controls in screening for CA | AUC of 0.714–0.733 for detecting CA, similar performance using different curated cases for training | Model was not externally validated | N/A |
| Tison et al. [7] | 2017 | Machine learning algorithms | 36,186 | University of California, San Franciso (UCSF) ECG Database | Detect and track four disease states including PAH, HCM, CA, and MVP | AUC of 0.86 for predicting CA | Single center. Only optimized to analyze ECGs in normal sinus rhythm. | N/A |
| Goto et al. [1] | 2021 | CNN based model | 10,933 | Brigham and Woman’s Hospital System | Automated strategy to augment CA detection | AUC of 0.85–0.91 for detecting CA before clinical diagnosis | Probability of undetected cases in the control group, false labels | N/A |
| Grogan et al. [8] | 2021 | DNN algorithm | 2541 | Mayo Clinic | Detect CA from a standard 12-lead ECG | AUC of 0.91 for predicting CA more than six months before clinical diagnosis | Single center, uncertainty regarding cardiac involvement in some patients | N/A |
| Haimovich et al. [9] | 2019 | Deep learning models LVH-Net and LVH-Net Leads I and II | 50,709 | Mass General Brigham Healthcare System | Deep learning models to classify LVH etiology using 12-lead and single-lead ECGs | AUC of 0.95 for diagnosing CA | Patients with multiple LVH etiologies may be misclassified. Dataset included mostly older white individuals making it difficult to generalize to patients who are younger or those with different racial backgrounds | AUC for HCM 0.92; AUC for AS 0.90; AUC for HTN 0.76; AUC for other LVH 0.69 |
| Goto et al. [10] | 2021 | Federated learning, a machine learning technique | 56,129 | Three US, one Japanese academic medical centers | ML models to detect and discriminate HCM from other causes of LVH using ECGs | AUC of 0.88 for discriminating CA | Features of the training model remain obscure resulting in some ambiguity | AUC for HCM 0.90–0.96; AUC for HTN 0.84; AUC for AS 0.83 |
| Harmon et al. [11] | 2022 | Follow up validation study of AI-ECG DNN algorithm | 7040 | Mayo Clinic | Evaluate post development performance of the AI-enhanced ECG to detect CA with respect to different subgroups | AUC of 0.84, acceptable performance across various subgroups | Lower performance noted in LBBB, LVH, and ethnically diverse populations like Hispanics | N/A |
| Study | Year | Design | Sample Size | Population/Data | Objective | Key Findings | Limitations | Comparison to Other Disease States |
|---|---|---|---|---|---|---|---|---|
| Goto et al. [1] | 2021 | CNN based model | 4565 | Three US, one Japanese academic medical centers | Automated strategy to augment CA detection | AUC of 0.89–1 for detecting CA before clinical diagnosis | Probability of undetected cases in the control group, false labels | AUC for HCM 0.87–0.96; AUC for HTN 0.89–0.96; AUC for ESRD 0.90–0.96 |
| Zhang et al. [13] | 2018 | CNN based models | 14,035 echocardiograms | UCSF | CNN to develop an analytic pipeline for the automated analysis of echocardiograms | AUC of 0.87 for detecting CA | Problems with segmentation arose due to complexity of tasks | AUC for HCM 0.93; AUC for PAH 0.85 |
| Chao et al. [14] | 2021 | ResNet50 a deep learning model | 381 | Mayo Clinic | Deep learning approach based on echocardiography to differentiate CP from CA as a representative of restrictive cardiomyopathy | AUC 0.97 in differentiating CP and CA | Small sample size and single center. Designed to differentiate CP from CA but not necessarily other forms of RCM. | N/A |
| Duffy et al. [15] | 2020 | Deep learning algorithm | 23,745 | Stanford and Ceders-Sinai medical centers | Deep learning algorithm measuring LV dimensions and identifying HCM and CA | AUC of 0.83 for detecting CA | Certain populations with known prevalence of CA like Black individuals were underrepresented | AUC for HCM 0.98; AUC for AS 0.89 |
| Li et al. [16] | 2019 | DL model with a fusion architecture | 586 | Mayo Clinic | Using DL model with fusion architecture to facilitate the evaluation and diagnosis of LV wall thickness | AUC of 0.90 for detecting CA | Single center, retrospective, potential referral bias | AUC for HCM 0.93; AUC for HTN/Other 0.92 |
| Narang et al. [17] | 2019 | Deep learning algorithm | 240 | Northwestern Memorial Hospital and Minneapolis Heart Institute | Test whether novice users could obtain 10-view echocardiographic studies using DL-based software | Novice users identified LV size, LV function, pericardial effusion 98.8% of the time and RV size 92.5% using DL algorithm | Small sample size, no control group for novice scanners | N/A |
| Oikonomou et al. [18] | 2024 | Video-based deep learning algorithms | 38,751 | Yale–New Haven and Mount Sinai Health System | Develop video-based deep learning algorithms to diagnose HCM and ATTR on POCUS | AUC of 0.98 for detecting ATTR | Retrospective study. POCUS was not used to train the AI models | AUC for HCM 0.95 |
| Ouyang et al. [19] | 2018 | EchoNet-Dynamic a video-based deep learning algorithm | 10,030 | Stanford Health Care | DL approach to segment the LV, estimate EF and assess for cardiomyopathy | AUC of 0.97 for predicting EF < 50% | Model was trained using videos obtained by trained sonographers | N/A |
| Goto et al. [10] | 2021 | Federated learning, a machine learning technique | 6825 | Three US, one Japanese academic medical centers | ML models to detect and discriminate HCM from other causes of LVH using echocardiograms | AUC of 0.85 for discriminating CA | Features of the training model remain obscure resulting in some ambiguity | AUC for HCM 0.90–0.96; AUC for HTN 0.93; AUC for AS 0.94 |
| Study | Year | Design | Sample Size | Population/Data | Objective | Key Findings | Limitations |
|---|---|---|---|---|---|---|---|
| Delbarre et al. [21] | 2022 | Deep learning algorithm | 4681 | Two Health Systems in France | Develop deep learning algorithm to detect significant cardiac uptake on scintigraphy to identify patients at risk of CA | AUC of 0.99 for detecting cardiac uptake Perugini grade ≥ 2 | Model is solely based on Perugini score; therefore, unable to distinguish between amyloidosis-related and nonamyloidosis-related cardiac uptake |
| Spielvogel et al. [22] | 2023 | Deep learning algorithm | 16,241 | Nine International Centers | Develop deep learning algorithm to detect high-grade cardiac uptake on scintigraphy | AUC of 0.925–1 for detecting CA-suggestive uptake on scintigraphy | Study was based on visual image assessment of Perugini grade ≥ 2, suggesting CA |
| Halme et al. [23] | 2021 | CNN | 1334 | Four Finnish Centers | Train CNN to detect ATTR from scintigraphy | AUC of 0.88–0.94 for detecting ATTR patients | Small sample size of ATTR positive patients, simple architectures of CNN models, lack of clinical validation |
| Study | Year | Design | Sample Size | Population/Data | Objective | Key Findings | Limitations |
|---|---|---|---|---|---|---|---|
| Martini et al. [25] | 2019 | Deep learning and Machine learning algorithms | 206 | Italy | Assess diagnostic performance of CMR-based DL and ML algorithms | AUC of 0.98 for DL detection of CA; AUC of 0.95 for ML detection of CA | Small sample size, high prevalence of CA from a CA specialized center, no external validation |
| Agibetov et al. [26] | 2018 | CNNs | 502 | Austria | CNNs to recognize imaging patterns associated with CA | AUC of 0.96 for detecting CA, 94% sensitivity, 90% specificity | Single center, most patients had advanced HF, and my not identify early disease |
| Eckstein et al. [27] | 2021 | Machine learning algorithms | 107 | Germany | ML algorithms using multi-chamber strain and cardiac function | AUC of 0.96 for detecting CA | Small sample size, unmatched retrospective cohort, unsupervised algorithmic model |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wardak, R.; Snipelisky, D. The Role of Artificial Intelligence in Cardiac Amyloidosis: A Focus on Diagnosis and Clinical Application. J. Cardiovasc. Dev. Dis. 2025, 12, 221. https://doi.org/10.3390/jcdd12060221
Wardak R, Snipelisky D. The Role of Artificial Intelligence in Cardiac Amyloidosis: A Focus on Diagnosis and Clinical Application. Journal of Cardiovascular Development and Disease. 2025; 12(6):221. https://doi.org/10.3390/jcdd12060221
Chicago/Turabian StyleWardak, Roshan, and David Snipelisky. 2025. "The Role of Artificial Intelligence in Cardiac Amyloidosis: A Focus on Diagnosis and Clinical Application" Journal of Cardiovascular Development and Disease 12, no. 6: 221. https://doi.org/10.3390/jcdd12060221
APA StyleWardak, R., & Snipelisky, D. (2025). The Role of Artificial Intelligence in Cardiac Amyloidosis: A Focus on Diagnosis and Clinical Application. Journal of Cardiovascular Development and Disease, 12(6), 221. https://doi.org/10.3390/jcdd12060221






