Transcatheter Aortic Valve Implantation in Nonagenarians: A Comparative Analysis of Baseline Characteristics and 1-Year Outcomes
Abstract
1. Introduction
2. Methods
3. Patient Evaluation, Selection and Data Collection
4. Procedural Details
5. Outcome Measures and Follow-Up
6. Statistical Analysis
7. Results
8. Discussion
9. Conclusions
10. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vincent, G.K. The Next Four Decades: The Older Population in the United States: 2010 to 2050; US Department of Commerce, Economics and Statistics Administration: Washington, DC, USA, 2010.
- Attinger-Toller, A.; Ferrari, E.; Tueller, D.; Templin, C.; Muller, O.; Nietlispach, F.; Toggweiler, S.; Noble, S.; Roffi, M.; Jeger, R. Age-related outcomes after transcatheter aortic valve replacement: Insights from the SwissTAVI registry. Cardiovasc. Interv. 2021, 14, 952–960. [Google Scholar]
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis. the Tromsø study. Heart 2013, 99, 396–400. [Google Scholar] [CrossRef]
- Assmann, A.; Minol, J.-P.; Mehdiani, A.; Akhyari, P.; Boeken, U.; Lichtenberg, A. Cardiac surgery in nonagenarians: Not only feasible, but also reasonable? Interact. Cardiovasc. Thorac. Surg. 2013, 17, 340–343. [Google Scholar] [CrossRef]
- Members, W.C.; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin III, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar]
- Akin, I.; Kische, S.; Paranskaya, L.; Schneider, H.; Rehders, T.C.; Turan, G.R.; Divchev, D.; Kundt, G.; Bozdag-Turan, I.; Ortak, J. Morbidity and mortality of nonagenarians undergoing CoreValve implantation. BMC Cardiovasc. Disord. 2012, 12, 80. [Google Scholar] [CrossRef]
- Havakuk, O.; Finkelstein, A.; Steinvil, A.; Halkin, A.; Arbel, Y.; Abramowitz, Y.; Assa, E.B.; Konigstein, M.; Keren, G.; Banai, S. Comparison of outcomes in patients ≤85 versus >85 years of age undergoing transcatheter aortic-valve implantation. Am. J. Cardiol. 2014, 113, 138–141. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; Van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.-A. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. J. Am. Coll. Cardiol. 2012, 60, 1438–1454. [Google Scholar] [CrossRef]
- Kharbanda, R.K.; Perkins, A.D.; Kennedy, J.; Banning, A.P.; Baumbach, A.; Blackman, D.J.; Dodd, M.; Evans, R.; Hildick-Smith, D.; Jamal, Z. Routine cerebral embolic protection in transcatheter aortic valve implantation: Rationale and design of the randomised British Heart Foundation PROTECT-TAVI trial. EuroIntervention 2023, 18, 1428. [Google Scholar] [CrossRef]
- Vendrik, J.; van Mourik, M.S.; van Kesteren, F.; Henstra, M.J.; Piek, J.J.; Henriques, J.P.; Wykrzykowska, J.J.; de Winter, R.J.; Vis, M.M.; Koch, K.T. Comparison of outcomes of transfemoral aortic valve implantation in patients <90 with those >90 years of age. Am. J. Cardiol. 2018, 121, 1581–1586. [Google Scholar]
- Yamamoto, M.; Mouillet, G.; Meguro, K.; Gilard, M.; Laskar, M.; Eltchaninoff, H.; Fajadet, J.; Iung, B.; Donzeau-Gouge, P.; Leprince, P. Clinical results of transcatheter aortic valve implantation in octogenarians and nonagenarians: Insights from the FRANCE-2 registry. Ann. Thorac. Surg. 2014, 97, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Arsalan, M.; Szerlip, M.; Vemulapalli, S.; Holper, E.M.; Arnold, S.V.; Li, Z.; DiMaio, M.J.; Rumsfeld, J.S.; Brown, D.L.; Mack, M.J. Should transcatheter aortic valve replacement be performed in nonagenarians? Insights from the STS/ACC TVT Registry. J. Am. Coll. Cardiol. 2016, 67, 1387–1395. [Google Scholar] [CrossRef]
- Yokoyama, H.; Tobaru, T.; Muto, Y.; Hagiya, K.; Higuchi, R.; Saji, M.; Takamisawa, I.; Shimizu, J.; Takanashi, S.; Takayama, M. Long-term outcomes in Japanese nonagenarians undergoing transcatheter aortic valve implantation: A multi-center analysis. Clin. Cardiol. 2019, 42, 605–611. [Google Scholar] [CrossRef]
- Stehli, J.; Koh, J.Q.S.; Duffy, S.J.; Zamani, J.; Yeong, C.C.; Paratz, E.; Martin, C.; Htun, N.M.; Stub, D.; Dick, R. Comparison of outcomes of transcatheter aortic valve implantation in patients aged >90 years versus <90 years. Am. J. Cardiol. 2019, 124, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, Y.; Fu, M.; Ma, Y.; Wang, D.; Zhang, J.; Liu, W.; Zhao, Y.; Zhou, Y. Clinical outcomes of transcatheter aortic valve replacement in nonagenarians: A systematic review and meta-analysis. J. Interv. Cardiol. 2019, 2019, 5819232. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N. STS-ACC TVT registry of transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.; Linder, M.; Seiffert, M.; Witberg, G.; Pilgrim, T.; Tomii, D.; Barkan, Y.T.; Van Mieghem, N.M.; Adrichem, R.; Codner, P. The impact of cerebral embolic protection devices on characteristics and outcomes of stroke complicating TAVR. Cardiovasc. Interv. 2024, 17, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Vlastra, W.; Chandrasekhar, J.; Vendrik, J.; Gutierrez-Ibanes, E.; Tchétché, D.; de Brito, F.S.; Barbanti, M.; Kornowski, R.; Latib, A.; D’Onofrio, A. Transfemoral TAVR in nonagenarians: From the CENTER collaboration. JACC Cardiovasc. Interv. 2019, 12, 911–920. [Google Scholar] [CrossRef]
- Thourani, V.H.; Jensen, H.A.; Babaliaros, V.; Kodali, S.K.; Rajeswaran, J.; Ehrlinger, J.; Blackstone, E.H.; Suri, R.M.; Don, C.W.; Aldea, G. Outcomes in nonagenarians undergoing transcatheter aortic valve replacement in the PARTNER-I trial. Ann. Thorac. Surg. 2015, 100, 785–793. [Google Scholar] [CrossRef]

| General Population (n = 620) | Age < 90 (n = 545) | Age ≥ 90 (n = 75) | p-Value | |
|---|---|---|---|---|
| Age (years) | 79.0 (73.3–84.0) | 78.0 (72.0–83.0) | 92.0 (90.0–97.0) | n/a |
| Gender | 0.622 1 | |||
| Male | 281 (45.3%) | 249 (45.7%) | 32 (42.7%) | |
| Female | 339 (54.7%) | 296 (54.3%) | 43 (57.3%) | |
| BMI (kg/m2) | 24.2 (23.0–28.0) | 24.2 (23.0–28.0) | 23.3 (23.0–25.8) | 0.021 2 |
| NYHA | 0.266 1 | |||
| 2 | 159 (25.6%) | 143 (28.2%) | 16 (21.3%) | |
| 3 | 352 (56.8%) | 312 (57.2%) | 40 (53.3%) | |
| 4 | 95 (15.3%) | 79 (14.5%) | 16 (21.3%) | |
| Pulmonary edema | 14 (2.3%) | 11 (2.0%) | 3 (4.0%) | |
| STS score | 7.40 (5.91–10.10) | 6.70 (5.60–10.38) | 7.48 (5.98–10.00) | 0.421 2 |
| COPD | 0.637 1 | |||
| None | 324 (52.3%) | 285 (52.3%) | 39 (52.0%) | |
| Mild | 35 (5.6%) | 33 (6.1%) | 2 (2.7%) | |
| Medium | 177 (28.5%) | 153 (28.1%) | 24 (32.0%) | |
| Severe | 84 (13.5%) | 74 (13.6%) | 10 (13.3%) | |
| CVA | 36 (5.8%) | 34 (6.2%) | 2 (2.7%) | 0.295 3 |
| PAD | 48 (7.7%) | 43 (7.9%) | 5 (6.7%) | 0.888 4 |
| DM | 179 (28.9%) | 166 (30.5%) | 13 (17.3%) | 0.027 4 |
| HT | 512 (82.6%) | 453 (83.1%) | 59 (78.7%) | 0.429 4 |
| HL | 308 (49.7%) | 280 (51.4%) | 28 (37.3%) | 0.023 1 |
| AF | 149 (24.1%) | 130 (23.9%) | 19 (25.7%) | 0.849 4 |
| CKD | 23 (3.7%) | 22 (4.0%) | 1 (1.3%) | 0.342 3 |
| CAD | 0.097 1 | |||
| Normal | 198 (32.0%) | 172 (31.6%) | 26 (35.1%) | |
| Non-obstructive | 268 (43.4%) | 244 (44.9%) | 24 (32.4%) | |
| Obstructive | 152 (24.6%) | 128 (23.5%) | 24 (32.4%) | |
| ACS history | >0.999 5 | |||
| None | 603 (97.3%) | 530 (97.2%) | 73 (97.3%) | |
| NSTEMI | 8 (1.3%) | 7 (1.3%) | 1 (1.3%) | |
| STEMI | 8 (1.3%) | 7 (1.3%) | 1 (1.3%) | |
| USAP | 1 (0.2%) | 1 (0.2%) | 0 (0.0%) | |
| PCI history | 80 (12.9%) | 69 (12.7%) | 11 (14.9%) | 0.734 4 |
| CABG history | 135 (21.8%) | 133 (24.4%) | 2 (2.7%) | <0.001 4 |
| BAV | 74 (12.2%) | 71 (13.3%) | 3 (4.2%) | 0.042 4 |
| History of previous valve surgery | 26 (4.2%) | 24 (4.4%) | 2 (2.7%) | 0.758 3 |
| Anticoagulant/Antiplatelet * | ||||
| ASA | 460 (75.8%) | 495 (75.9%) | 55 (75.3%) | >0.999 4 |
| Clopidogrel | 26 (4.3%) | 20 (3.8%) | 6 (8.2%) | 0.113 3 |
| Warfarine | 122 (20.1%) | 108 (20.2%) | 14 (19.2%) | 0.957 4 |
| NOAC | 22 (3.6%) | 19 (3.6%) | 3 (4.1%) | 0.740 3 |
| Basal GFR | 64.3 (48.0–81.0) | 65.0 (48.0–82.0) | 58.0 (46.0–71.0) | 0.018 2 |
| HbA1c | 5.95 (5.50–6.52) | 5.95 (5.53–6.58) | 5.88 (5.31–6.39) | 0.135 2 |
| Pre-op HBG | 11.7 ± 1.9 | 11.7 ± 1.9 | 11.4 ± 1.6 | 0.135 6 |
| General Population (n = 620) | Age < 90 (n = 545) | Age ≥ 90 (n = 75) | p-Value | |
|---|---|---|---|---|
| Basal EF | 55.0 (45.0–65.0) | 55.0 (45.0–65.0) | 60.0 (50.0–65.0) | 0.048 1 |
| Basal LVEDD | 4.60 (4.30–5.10) | 4.70 (4.30–5.10) | 4.40 (4.10–4.80) | <0.001 1 |
| Basal LVESD | 2.90 (2.50–3.50) | 3.00 (2.60–3.60) | 2.70 (2.30–3.10) | <0.001 1 |
| Basal septal wall thickness | 1.40 (1.20–1.50) | 1.40 (1.20–1.50) | 1.50 (1.30–1.60) | 0.012 1 |
| Basal posterior wall thickness | 1.30 (1.20–1.40) | 1.30 (1.20–1.40) | 1.30 (1.20–1.40) | 0.159 1 |
| Basal LA dimension | 4.60 (4.30–5.00) | 4.60 (4.30–5.00) | 4.60 (4.30–5.00) | 0.720 1 |
| AS group | 0.314 2 | |||
| Normal | 412 (67.0%) | 368 (68.0%) | 44 (59.5%) | |
| LFLG | 41 (6.7%) | 35 (6.5%) | 6 (8.1%) | |
| Paradoxal LFLG | 9 (1.5%) | 7 (1.3%) | 2 (2.7%) | |
| VSAS | 153 (24.9%) | 131 (24.2%) | 22 (29.7%) | |
| Basal AVA | 0.69 (0.54–0.80) | 0.70 (0.54–0.80) | 0.67 (0.53–0.80) | 0.304 1 |
| Basal aortic mean gradient | 47.0 (41.0–58.0) | 46.0 (41.0–58.0) | 49.0 (41.0–60.3) | 0.490 1 |
| Bazal PASP | 40.0 (35.0–55.0) | 40.0 (32.3–55.0) | 45.0 (35.0–60.0) | 0.200 1 |
| Bazal MR | 0.556 3 | |||
| None | 10 (1.6%) | 9 (1.7%) | 1 (1.4%) | |
| Trivial | 134 (21.8%) | 121 (22.4%) | 13 (17.6%) | |
| 1 | 261 (42.5%) | 232 (43.0%) | 29 (39.2%) | |
| 2 | 132 (21.5%) | 114 (21.1%) | 18 (24.3%) | |
| 3 | 70 (11.4%) | 59 (10.9%) | 11 (14.9%) | |
| 4 | 7 (1.1%) | 5 (0.9%) | 2 (2.7%) |
| General Population (n = 620) | Age < 90 (n = 545) | Age ≥ 90 (n = 75) | p-Value | |
|---|---|---|---|---|
| Transfemoral closure | 0.896 1 | |||
| Prostar | 206 (35.2%) | 180 (35.0%) | 26 (36.6%) | |
| Cut-down | 12 (2.0%) | 11 (2.1%) | 1 (1.4%) | |
| Proglide | 368 (62.8%) | 324 (62.9%) | 44 (62.0%) | |
| Pre-dilatation | 435 (71.2%) | 384 (71.4%) | 51 (69.9%) | 0.897 2 |
| Post-dilatation | 19 (3.1%) | 19 (3.5%) | 0 (0.0%) | 0.150 3 |
| Valve size | 0.026 4 | |||
| 20 | 3 (0.5%) | 2 (0.4%) | 1 (1.4%) | |
| 23 | 263 (42.6%) | 225 (41.4%) | 38 (51.4%) | |
| 25 | 16 (2.6%) | 15 (2.8%) | 1 (1.4%) | |
| 26 | 252 (40.8%) | 220 (40.4%) | 32 (43.2%) | |
| 27 | 7 (1.1%) | 7 (1.3%) | 0 (0.0%) | |
| 29 | 77 (12.5%) | 75 (13.8%) A | 2 (2.7%) A | |
| Valve type | 0.293 4 | |||
| Sapien XT | 530 (85.8%) | 470 (86.4%) | 60 (81.1%) | |
| Edwards Sapien 3 | 50 (8.1%) | 42 (7.7%) | 8 (10.8%) | |
| Myval | 29 (4.7%) | 25 (4.6%) | 4 (5.4%) | |
| Accurate neo | 7 (1.1%) | 6 (1.1%) | 1 (1.4%) | |
| Evolut R | 2 (0.3%) | 1 (0.2%) | 1 (1.4%) | |
| Valve in valve | 9 (1.5%) | 7 (1.3%) | 2 (2.7%) | 0.298 3 |
| General Population (n = 620) | Age <90 (n = 545) | Age ≥ 90 (n = 75) | p-Value | |
|---|---|---|---|---|
| Stroke | 17 (2.7%) | 12 (2.2%) | 5 (6.7%) | 0.044 1 |
| Pericardial Tamponade | 0.715 2 | |||
| None | 607 (97.9%) | 534 (98.0%) | 73 (97.3%) | |
| Yes | 11 (1.8%) | 9 (1.7%) | 2 (2.7%) | |
| Percutaneous Surgical drainage needed | 2 (0.3%) | 2 (0.4%) | 0 (0.0%) | |
| Post-op Arrhythmias | 0.774 2 | |||
| None | 536 (86.7%) | 471 (86.6%) | 65 (87.8%) | |
| Complete AV block | 44 (7.1%) | 37 (6.8%) | 7 (9.5%) | |
| AF | 21 (3.4%) | 20 (3.7%) | 1 (1.4%) | |
| LBBB | 14 (2.3%) | 13 (2.4%) | 1 (1.4%) | |
| VT | 3 (0.5%) | 3 (0.6%) | 0 (0.0%) | |
| Other Complications | 0.477 2 | |||
| None | 593 (95.6%) | 522 (95.8%) | 71 (94.7%) | |
| Closure device fail | 14 (2.3%) | 11 (2.0%) | 3 (4.0%) | |
| Valve embolization | 4 (0.6%) | 4 (0.7%) | 0 (0.0%) | |
| AKI | 4 (0.6%) | 4 (0.7%) | 0 (0.0%) | |
| Coronary obstruction | 2 (0.3%) | 2 (0.4%) | 0 (0.0%) | |
| Valve in valve | 2 (0.3%) | 1 (0.2%) | 1 (1.3%) | |
| Annular rupture | 1 (0.2%) | 1 (0.2%) | 0 (0.0%) | |
| Peripheral Complication | 0.542 2 | |||
| None | 576 (92.9%) | 507 (93.0%) | 69 (92.0%) | |
| Hematoma | 9 (1.5%) | 7 (1.3%) | 2 (2.7%) | |
| Pseudoaneurysm | 10 (1.6%) | 9 (1.7%) | 1 (1.3%) | |
| Dissection | 21 (3.4%) | 19 (3.5%) | 2 (2.7%) | |
| Major bleeding | 4 (0.6%) | 3 (0.6%) | 1 (1.3%) | |
| Post-op Pacemaker | 0.833 2 | |||
| None | 572 (92.6%) | 504 (92.6%) | 68 (91.9%) | |
| Permanent | 45 (7.3%) | 39 (7.2%) | 6 (8.1%) | |
| Temporary | 1 (0.2%) | 1 (0.2%) | 0 (0.0%) | |
| Procedural Success | 595 (96.1%) | 522 (96.0%) | 73 (97.3%) | 0.756 1 |
| Hospital stay (day) | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) | 0.329 |
| In-hospital mortality | 26 (4.2%) | 20 (3.7%) | 6 (8.0%) | 0.114 1 |
| General Population (n = 620) | Age < 90 (n = 545) | Age ≥ 90 (n = 75) | p-Value | |
|---|---|---|---|---|
| 1-month NYHA | 0.017 1 | |||
| 1 | 178 (30.5%) | 167 (32.4%) | 11 (15.9%) | |
| 2 | 372 (63.7%) | 320 (62.1%) | 52 (75.4%) | |
| 3 | 34 (5.8%) | 28 (5.4%) | 6 (8.7%) | |
| 1-month mortality | 36 (5.8%) | 30 (5.5%) | 6 (8.0%) | 0.425 2 |
| 6-month mortality | 50 (8.1%) | 43 (7.9%) | 7 (9.3%) | 0.838 3 |
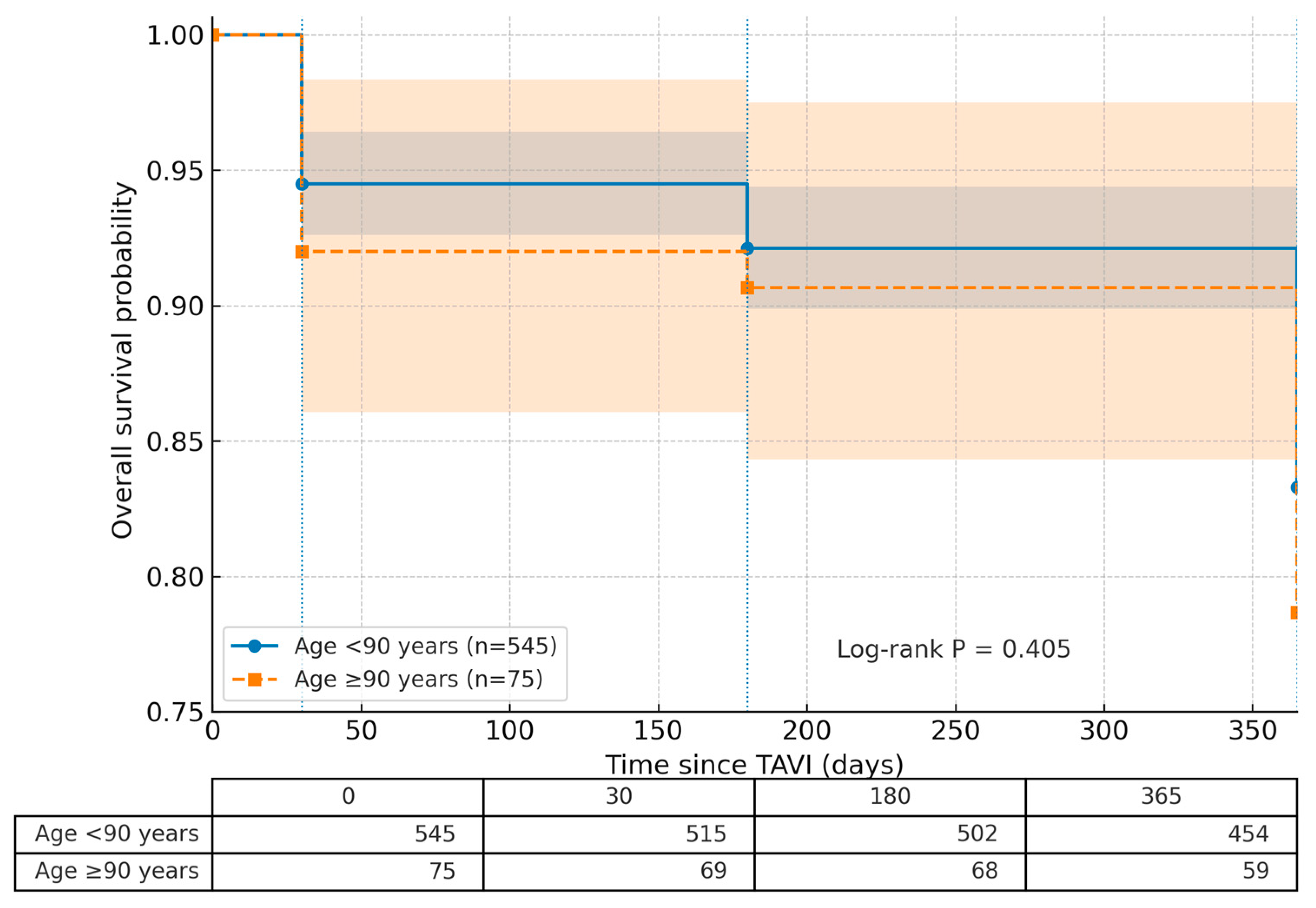
| 1-year mortality | 107 (17.3%) | 91 (16.7%) | 16 (21.3%) | 0.405 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güney, M.C.; Bozkurt, E. Transcatheter Aortic Valve Implantation in Nonagenarians: A Comparative Analysis of Baseline Characteristics and 1-Year Outcomes. J. Cardiovasc. Dev. Dis. 2025, 12, 327. https://doi.org/10.3390/jcdd12090327
Güney MC, Bozkurt E. Transcatheter Aortic Valve Implantation in Nonagenarians: A Comparative Analysis of Baseline Characteristics and 1-Year Outcomes. Journal of Cardiovascular Development and Disease. 2025; 12(9):327. https://doi.org/10.3390/jcdd12090327
Chicago/Turabian StyleGüney, Murat Can, and Engin Bozkurt. 2025. "Transcatheter Aortic Valve Implantation in Nonagenarians: A Comparative Analysis of Baseline Characteristics and 1-Year Outcomes" Journal of Cardiovascular Development and Disease 12, no. 9: 327. https://doi.org/10.3390/jcdd12090327
APA StyleGüney, M. C., & Bozkurt, E. (2025). Transcatheter Aortic Valve Implantation in Nonagenarians: A Comparative Analysis of Baseline Characteristics and 1-Year Outcomes. Journal of Cardiovascular Development and Disease, 12(9), 327. https://doi.org/10.3390/jcdd12090327





