Green Tea Extract in the Extender Improved the Post-Thawed Semen Quality and Decreased Amino Acid Mutation of Kacang Buck Sperm
Abstract
:Simple Summary
Abstract
1. Introduction
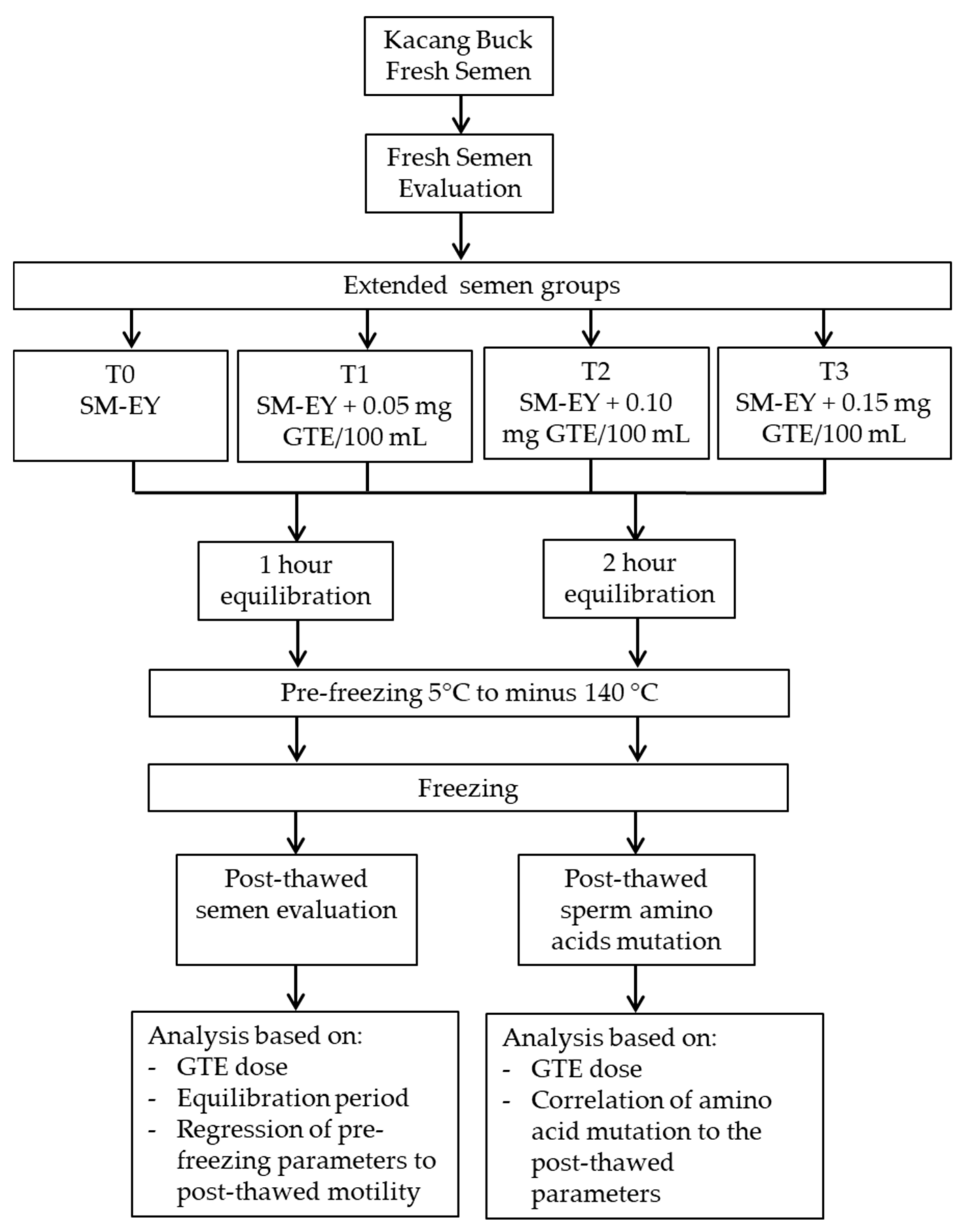
2. Materials and Methods
2.1. GTE Preparation and Extender
2.2. Frozen Semen
2.3. Semen Quality Assessment
2.3.1. Sperm Viability
2.3.2. IPM
2.3.3. Malondialdehyde Levels
2.3.4. Sperm DNA Fragmentation
2.3.5. Capacitation and Acrosome Reaction
2.4. Amino Acid Sequencing
2.5. Data Analysis
3. Results
3.1. Effect of Equilibration Period and GTE Dose on Pre-Freezing and Post-Thawed Sperm Quality
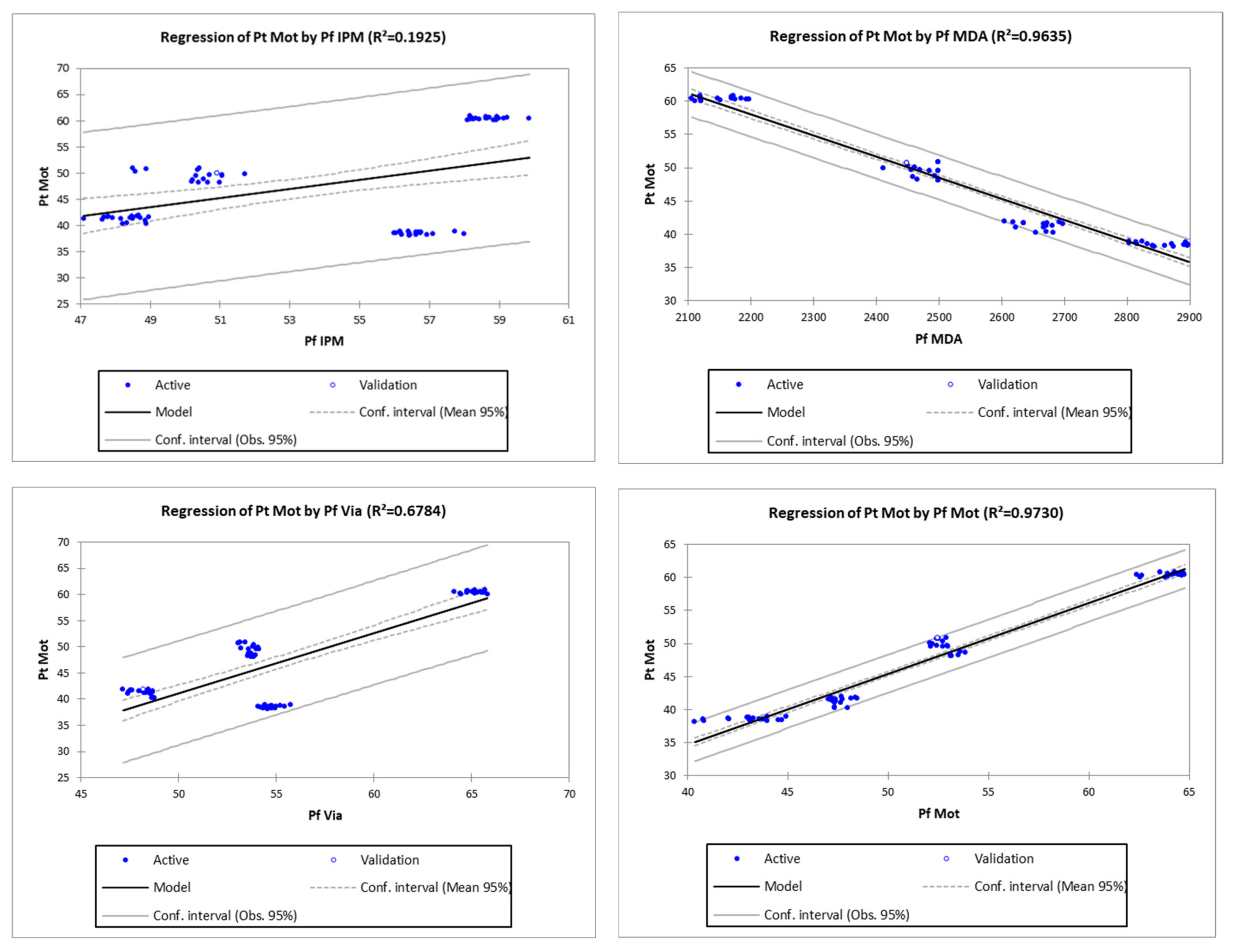
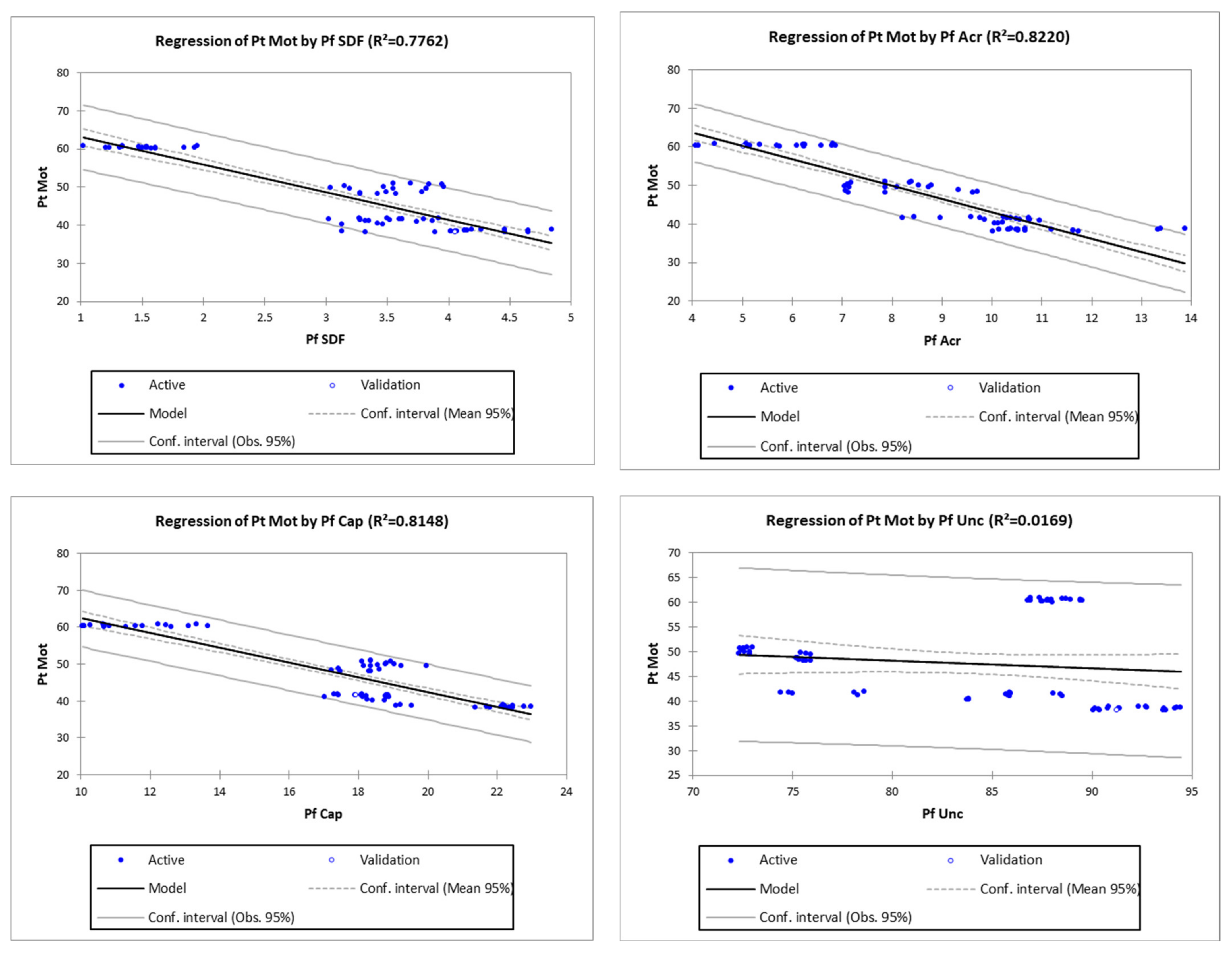
3.2. Regression Analysis for Identification of the Most Potent Pre-Freezing Parameter on Post-Thawed Sperm Motility
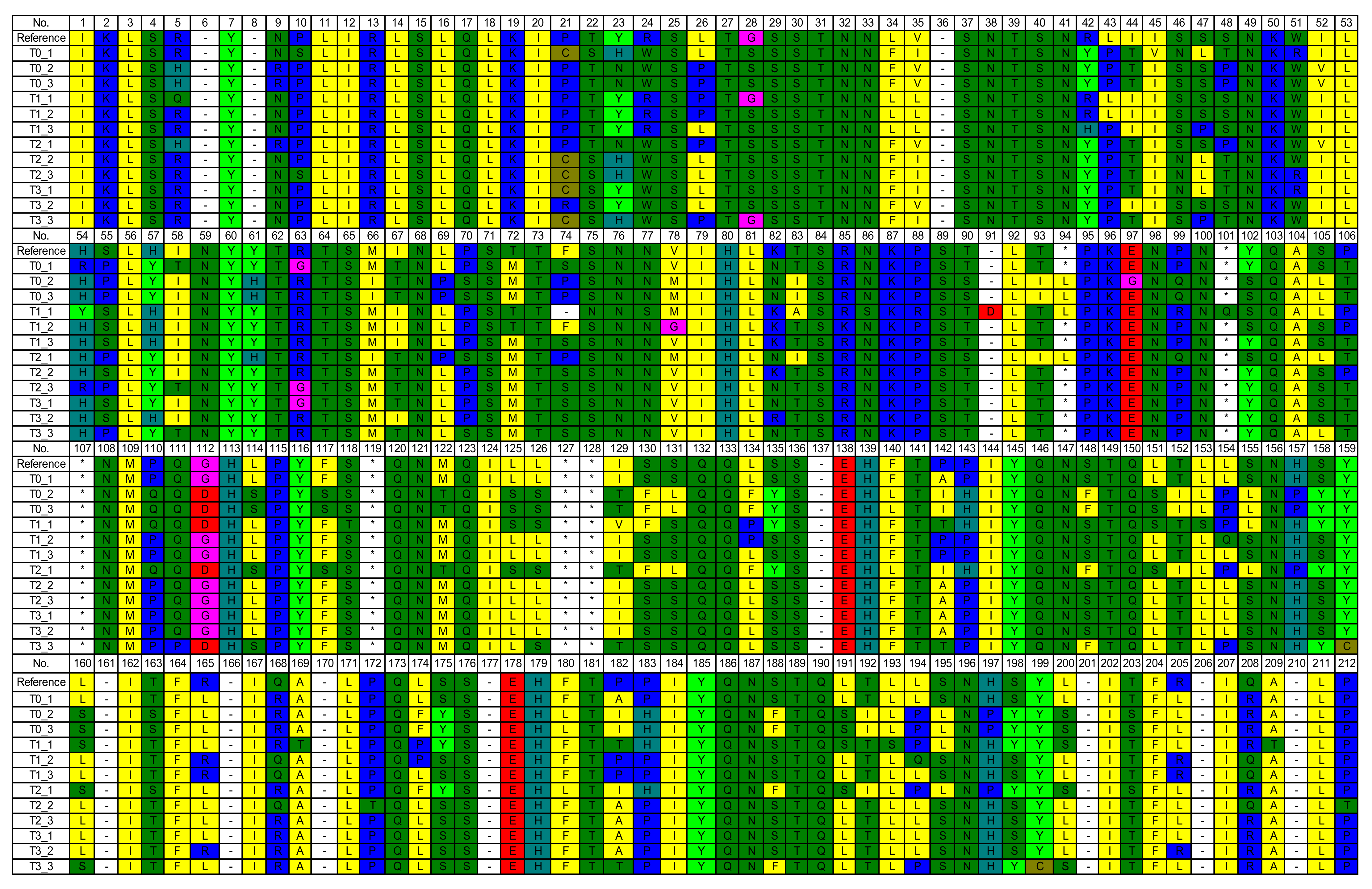
3.3. Mutation of Amino Acids Encoded by NADH Dehydrogenase 1 (ND1) of Mitochondrial Deoxyribonucleic Acid (mtDNA)
3.4. The Correlation Coefficient of Amino Acid Mutation and Post-Thawed Kacang Buck Semen Quality Parameter
4. Discussion
4.1. GTE Dose
4.2. The Equilibration Period
4.3. Mutation of Amino Acids Encoded by NADH Dehydrogenase 1 (ND1) of Mitochondrial Deoxyribonucleic Acid (mtDNA)
4.4. The Correlation Coefficient of Amino Acid Mutation and Post-Thawed Kacang Buck Semen Quality Parameter
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suswono, S. Decree of the Minister of Agriculture of the Republic of Indonesia Number 2840/Kits/LB.430/8/2012. 2012. Available online: http://bibit.ditjenpkh.pertanian.go.id/sites/default/files/Kambing%20Kacang.pdf (accessed on 17 June 2020).
- Lv, C.; Wu, G.; Hong, Q.; Quan, G. Spermatozoa Cryopreservation: State of Art and Future in Small Ruminants. Biopreserv. Biobank. 2018, 17, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.A.J.A.; Batista, A.M.; Arruda, L.C.P.; De Souza, H.M.; Nery, I.H.D.A.V.; Gomes, W.A.; Soares, P.D.C.; Silva, S.V.; Guerra, M.M.P. Concentration of soybean lecithin affects short-term storage success of goat semen related with seminal plasma removal. Anim. Reprod. 2019, 16, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Anand, M.; Yadav, S. Assessment of motion and kinematic characteristics of frozen-thawed Sirohi goat semen using computer-assisted semen analysis. Vet. World 2016, 9, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Susilowati, S.; Triana, I.N.; Sardjito, T.; Suprayogi, T.W.; Wurlina, W.; Mustofa, I. Effect of Simmental bull seminal plasma protein in egg yolk-citrate extender on Kacang buck semen fertility. Cryobiology 2020, 97, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Indonesian National Standard Agency. Frozen Semen–Part 3: Goat and Sheep. 2014. Available online: http://bibit.ditjenpkh.pertanian.go.id/sites/ (accessed on 30 January 2021).
- Mehdipour, M.; Kia, H.D.; Najafi, A.; Dodaran, H.V.; García-Álvarez, O. Effect of green tea (Camellia sinensis) extract and pre-freezing equilibration time on the post-thawing quality of ram semen cryopreserved in a soybean lecithin-based extender. Cryobiology 2016, 73, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Jahan, S.; Riaz, M.; Khan, B.T.; Ijaz, M.U. Epigallocatechin-3-gallate (EGCG) addition as an antioxidant in a cryo-diluent media improves microscopic parameters, and fertility potential, and alleviates oxidative stress parameters of buffalo spermatozoa. Cryobiology 2020, 97, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Doležalová, M.; Stádník, L.; Biniová, Z.; Ducháček, J.; Stupka, R. Equilibration and freezing interactions affecting bull sperm characteristics after thawing. Czech J. Anim. Sci. 2016, 61, 515–525. [Google Scholar] [CrossRef]
- Dwinofanto, H.; Rimayanti, R.; Mustofa, E.; Susilowati, S.; Hernawati, T. The effect of duration of preservation on the quality, MDA level, and DNA damage of post-thawed Bali cattle bull sperm. Iraqi J. Vet. Sci. 2018, 32, 249–252. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; Ling, X.; Zou, P.; Yang, H.; Chen, Q.; Zhou, N.; Sun, L.; Gao, J.; Zhou, Z.; et al. Mitochondrial Biomarkers Reflect Semen Quality: Results from the MARCHS Study in Chongqing, China. PLoS ONE 2016, 11, e0168823. [Google Scholar] [CrossRef]
- Tourmente, M.; Hirose, M.; Ibrahim, S.; Dowling, D.K.; Tompkins, D.M.; Roldan, E.R.S.; Gemmell, N.J. mtDNA polymorphism and metabolic inhibition affect sperm performance in conplastic mice. Reproduction 2017, 154, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Xu, M.; Huang, Z.; Li, L.; Zheng, H.; Yang, S.; Li, S.; Jin, L.; Ling, X.; Xia, Y.; et al. Mitochondrial DNA sequencing and large-scale genotyping identifies MT-ND4 gene mutation m.11696G>A associated with idiopathic oligoasthenospermia. Oncotarget 2017, 8, 52975–52982. [Google Scholar] [CrossRef]
- Mustofa, I.; Susilowati, S.; Wurlina, W.; Hernawati, T.; Oktanella, Y. Green tea extract increases the quality and reduced DNA mutation of post-thawed Kacang buck sperm. Heliyon 2021, 7, e06372. [Google Scholar] [CrossRef] [PubMed]
- Susilowati, S.; Sardjito, T.; Mustofa, I.; Widodo, O.S.; Kurnijasanti, R. Effect of green tea extract in extender of Simmental bull semen on pregnancy rate of recipients. Anim. Biosci. 2021, 34, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Susilowati, S.; Mustofa, I.; Wurlina, W.; Triana, I.N.; Utama, S.; Rimayanti, R. Effect of insulin-like growth factor-1 complex of Simmental bull seminal plasma on post-thawed Kacang buck semen fertility. Vet. World 2021, 14, 2073–2084. [Google Scholar] [CrossRef]
- Üstüner, B.; Nur, Z.; Alçay, S.; Toker, M.B.; Sağirkaya, H.; Soylu, M.K. Effect of freezing rate on goat sperm morphology and DNA integrity. Turk. J. Vet. Anim. Sci. 2015, 39, 110–114. [Google Scholar] [CrossRef]
- Gürler, H.; Malama, E.; Heppelmann, M.; Calisici, O.; Leiding, C.; Kastelic, J.; Bollwein, H. Effects of cryopreservation on sperm viability, synthesis of reactive oxygen species, and DNA damage of bovine sperm. Theriogenology 2016, 86, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Allai, L.; Benmoula, A.; da Silva, M.M.; Nasser, B.; El Amiri, B. Supplementation of ram semen extender to improve seminal quality and fertility rate. Anim. Reprod. Sci. 2018, 192, 6–17. [Google Scholar] [CrossRef]
- Pereira, R.; Sá, R.; Barros, A.; Sousa, M. Major regulatory mechanisms involved in sperm motility. Asian J. Androl. 2017, 19, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Bibov, M.Y.; Kuzmin, A.V.; Alexandrova, A.A.; Chistyakov, V.A.; Dobaeva, N.M.; Kundupyan, O.L. Role of the reactive oxygen species induced DNA damage in human spermatozoa dysfunction. AME Med. J. 2018, 3, 1–12. [Google Scholar] [CrossRef]
- Rahman, M.; Gofur, M.R.; Bari, F.Y.; Juyena, N.S. Effect of Skim Milk and Tris-citrate Extenders to Preserve the Semen of Indigenous Ram of Bangladesh. Asian J. Biol. 2018, 5, 1–11. [Google Scholar] [CrossRef]
- Takeshima, T.; Kuroda, S.; Yumura, Y. Reactive Oxygen Species and Sperm Cells. In Reactive Oxygen Species (ROS) in Living Cells; Filip, C., Albu, E., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- Ribas-Maynou, J.; Benet, J. Single and Double Strand Sperm DNA Damage: Different Reproductive Effects on Male Fertility. Genes 2019, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Bloom, K.; Costanzo, V. Centromere Structure and Function. In Centromeres and Kinetochores; Black, B., Ed.; Progress in Molecular and Subcellular Biology; Springer: Cham, Switzerland, 2017; Volume 56, pp. 515–539. [Google Scholar] [CrossRef]
- Akmal, M.; Aulanni’Am, A.; Widodo, M.A.; Sumitro, S.B.; Purnomo, B.B.; Widodo. The important role of protamine in spermatogenesis and quality of sperm: A mini review. Asian Pac. J. Reprod. 2016, 5, 357–360. [Google Scholar] [CrossRef]
- Martin-Hidalgo, D.; Bragado, M.J.; Batista, A.R.; Oliveira, P.F.; Alves, M.G. Antioxidants and Male Fertility: From Molecular Studies to Clinical Evidence. Antioxidants 2019, 8, 89. [Google Scholar] [CrossRef]
- Ugur, M.R.; Dinh, T.; Hitit, M.; Kaya, A.; Topper, E.; Didion, B.; Memili, E. Amino Acids of Seminal Plasma Associated with Freezability of Bull Sperm. Front. Cell Dev. Biol. 2019, 7, 347. [Google Scholar] [CrossRef]
- Goodla, L.; Morrell, J.M.; Yusnizar, Y.; Stålhammar, H.; Johannisson, A. Quality of bull spermatozoa after preparation by single-layer centrifugation. J. Dairy Sci. 2014, 97, 2204–2212. [Google Scholar] [CrossRef]
- Korochkina, E.; Johannisson, A.; Goodla, L.; Morrell, J.M.; Axner, E. Effect of prostatic fluid on the quality of fresh and frozen-thawed canine epididymal spermatozoa. Theriogenology 2014, 82, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, A.; Agarwal, A. Systematic review of antioxidant types and doses in male infertility: Benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab J. Urol. 2018, 16, 113–124. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Agarwal, A.; Virk, G.; Cho, C.-L. Potential role of green tea catechins in the management of oxidative stress-associated infertility. Reprod. Biomed. Online 2017, 34, 487–498. [Google Scholar] [CrossRef]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef]
- Rahman, S.U.; Huang, Y.; Zhu, L.; Feng, S.; Khan, I.M.; Wu, J.; Li, Y.; Wang, X. Therapeutic Role of Green Tea Polyphenols in Improving Fertility: A Review. Nutrients 2018, 10, 834. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Agarwal, A. A systematic review on sperm DNA fragmentation in male factor infertility: Laboratory assessment. Arab J. Urol. 2018, 16, 65–76. [Google Scholar] [CrossRef]
- Park, S.-H.; Yu, I.-J. Effect of Antioxidant Supplementation in Freezing Extender on Porcine Sperm Viability, Motility and Reactive Oxygen Species. J. Embryo Transf. 2017, 32, 9–15. [Google Scholar] [CrossRef]
- Wagner, H.; Cheng, J.W.; Ko, E.Y. Role of reactive oxygen species in male infertility: An updated review of the literature. Arab J. Urol. 2018, 16, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Martorell, M.; Arbiser, J.; Sureda, A.; Martins, N.; Maurya, P.; Sharifi-Rad, M.; Kumar, P.; Sharifi-Rad, J. Antioxidants: Positive or Negative Actors? Biomolecules 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Leite, T.G.; Filho, V.R.D.V.; de Arruda, R.P.; de Andrade, A.F.C.; Emerick, L.L.; Zaffalon, F.G.; Martins, J.A.M.; de Andrade, V.J. Effects of extender and equilibration time on post-thaw motility and membrane integrity of cryopreserved Gyr bull semen evaluated by CASA and flow cytometry. Anim. Reprod. Sci. 2010, 120, 31–38. [Google Scholar] [CrossRef]
- Amal, A.S.; Arifiantini, R.I.; Setiadi, M.A.; Said, S. Characteristics of the post-thawed Balinese bull semen extended in three different extenders and equilibration periods. J. Indones. Trop. Anim. Agric. 2019, 44, 135–145. [Google Scholar] [CrossRef]
- Murphy, E.; Eivers, B.; O’Meara, C.; Lonergan, P.; Fair, S. Effect of increasing equilibration time of diluted bull semen up to 72 h prior to freezing on sperm quality parameters and calving rate following artificial insemination. Theriogenology 2018, 108, 217–222. [Google Scholar] [CrossRef]
- Ramachandran, N.; Yadav, S.; Sikarwar, A.; Saraswat, S.; Ranjan, R.; Jindal, S. Effect of equilibration periods on post-thaw semen quality of Jamunapari bucks. Indian J. Small Rumin. 2015, 21, 234–237. [Google Scholar] [CrossRef]
- Ahmad, M.; Nasrullah, R.; Ahmad, N. Effect of cooling rate and equilibration period on pre-freeze and post-thaw survival of buck sperm. Cryobiology 2015, 70, 233–238. [Google Scholar] [CrossRef]
- Purdy, P.H.; Mocé, E.; Stobart, R.; Murdoch, W.J.; Moss, G.E.; Larson, B.; Ramsey, S.; Graham, J.K.; Blackburn, H.D. The fertility of ram sperm held for 24 h at 5 °C prior to cryopreservation. Anim. Reprod. Sci. 2010, 118, 231–235. [Google Scholar] [CrossRef]
- Câmara, D.R.; Pinto, L.C.; Pinto, M.M.C.M.; Kastelic, J.P.; Nunes, J.F.; Barbosa, J.M.P.; Guerra, M.M.P. Influence of catalase and pre-freezing equilibration on post-thaw semen quality and conception rate in ewes laparoscopically inseminated. Anim. Reprod. 2016, 13, 21–27. [Google Scholar] [CrossRef]
- Henkel, R.; Sandhu, I.S.; Agarwal, A. The excessive use of antioxidant therapy: A possible cause of male infertility? Andrologia 2019, 51, e13162. [Google Scholar] [CrossRef]
- Vaught, R.C.; Dowling, D.K. Maternal inheritance of mitochondria: Implications for male fertility? Reproduction 2018, 155, R159–R168. [Google Scholar] [CrossRef]
- Durairajanayagam, D.; Singh, D.; Agarwal, A.; Henkel, R. Causes and consequences of sperm mitochondrial dysfunction. Andrologia 2021, 53, e13666. [Google Scholar] [CrossRef]
- Dzudzor, B.; Bimah, B.; Amarh, V.; Ocloo, A. Sperm parameters and mitochondrial DNA sequence variants among patients at a fertility clinic in Ghana. PLoS ONE 2021, 16, e0252923. [Google Scholar] [CrossRef]
- Elrahman, M.M.A.; El Makawy, A.I.; Hassanane, M.S.; Alam, S.S.; Hassan, N.H.A.; Amer, M.K. Assessment of correlation between asthenozoospermia and mitochondrial DNA mutations in Egyptian infertile men. J. Genet. Eng. Biotechnol. 2021, 19, 11. [Google Scholar] [CrossRef]
- Thomas, H.I.S.; Chen, Y.-S.; Hung, C.-H.; Urs, D.B.S.; Liao, T.-L.; Lai, Y.-C.; Komrskova, K.; Postlerová, P.; Lin, Y.-F.; Kao, S.-H. Genetic Association in the Maintenance of the Mitochondrial Microenvironment and Sperm Capacity. Oxid. Med. Cell. Longev. 2021, 2021, 5561395. [Google Scholar] [CrossRef]
- Talebi, E.; Karimian, M.; Nikzad, H. Association of sperm mitochondrial DNA deletions with male infertility in an Iranian population. Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2017, 29, 615–623. [Google Scholar] [CrossRef]
- Wu, H.; Huffman, A.M.; Whitcomb, B.W.; Josyula, S.; Labrie, S.; Tougias, E.; Rahil, T.; Sites, C.K.; Pilsner, J.R. Sperm mitochondrial DNA measures and semen parameters among men undergoing fertility treatment. Reprod. Biomed. Online 2019, 38, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Faja, F.; Carlini, T.; Coltrinari, G.; Finocchi, F.; Nespoli, M.; Pallotti, F.; Lenzi, A.; Lombardo, F.; Paoli, D. Human sperm motility: A molecular study of mitochondrial DNA, mitochondrial transcription factor A gene and DNA fragmentation. Mol. Biol. Rep. 2019, 46, 4113–4121. [Google Scholar] [CrossRef] [PubMed]
- Podolak, A.; Liss, J.; Kiewisz, J.; Pukszta, S.; Cybulska, C.; Rychlowski, M.; Lukaszuk, A.; Jakiel, G.; Lukaszuk, K. Mitochondrial DNA Copy Number in Cleavage Stage Human Embryos—Impact on Infertility Outcome. Curr. Issues Mol. Biol. 2022, 44, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Al Zoubi, M.S.; Al-Talafha, A.M.; Al Sharu, E.; Al-Trad, B.; Alzu’Bi, A.; AbuAlarjah, M.I.; Shehab, Q.; Alsmadi, M.; Al-Batayneh, K.M. Correlation of Sperm Mitochondrial DNA 7345 bp and 7599 bp Deletions with As-thenozoospemia in Jordanian Population. J. Reprod. Infertil. 2021, 22, 165–172. [Google Scholar] [CrossRef]
- Elsanousi, S.A.; Javed, M.; Sufyan, H.; Amer, S.A. Forensic Relationship between ATP6 Gene and Sperm Motility in a Saudi Population. Am. J. Biochem. Biotechnol. 2020, 16, 15–24. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, W.; Zou, P.; Jiang, F.; Zeng, Y.; Chen, Q.; Sun, L.; Yang, H.; Zhou, N.; Wang, X.; et al. Mitochondrial functionality modifies human sperm acrosin activity, acrosome reaction capability and chromatin integrity. Hum. Reprod. 2019, 34, 3–11. [Google Scholar] [CrossRef]
- Khalil, W.A.; El-Harairy, M.A.; Zeidan, A.E.; Hassan, M.A. Impact of selenium nano-particles in semen extender on bull sperm quality after cryopreservation. Theriogenology 2019, 126, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Piran, F.; Khoshkhoo, Z.; Hosseini, S.E.; Azizi, M.H. Controlling the Antioxidant Activity of Green Tea Extract through Encapsulation in Chitosan-Citrate Nanogel. J. Food Qual. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; Mongioì, L.M.; La Vignera, S.; Calogero, A.E. Molecular Biology of Spermatogenesis: Novel Targets of Apparently Idiopathic Male Infertility. Int. J. Mol. Sci. 2020, 21, 1728. [Google Scholar] [CrossRef]
| Parameters | Values |
|---|---|
| Volume (mL) | 2.35 ± 0.21 |
| Concentration (million/mL) | 3680.17 ± 165.96 |
| Viability (%) | 91.50 ± 0.67 |
| Progressive motility (%) | 88.17 ± 0.72 |
| Intact plasma membrane (%) | 85.42 ± 0.79 |
| Parameters | Group | 1 h Equilibration | 2 h Equilibration | ||
|---|---|---|---|---|---|
| Pre-Freezing | Post-Thawed | Pre-Freezing | Post-Thawed | ||
| IPM | T0 | 56.68 ± 0.33 Ab | 41.72 ± 0.38 Bc | 38.50 ± 0.89 Cd | 35.11 ± 0.41 Dc |
| (%) | T1 | 50.32 ± 0.94 Ac | 46.52 ± 0.41 Bb | 46.98 ± 0.51 Bb | 41.39 ± 0.70 Cb |
| T2 | 58.73 ± 0.41 Aa | 58.58 ± 0.57 Aa | 53.77 ± 0.39 Ba | 50.65 ± 0.55 Ca | |
| T3 | 48.17 ± 0.32 Ad | 46.60 ± 0.48 Ab | 43.24 ± 0.88 Bc | 39.99 ± 0.36 Cb | |
| MDA | T0 | 2895.00 ± 183.72 Da | 3258.20 ± 48.55 Ba | 3018.60 ± 29.39 Ca | 3378.48 ± 20.44 Aa |
| levels | T1 | 2498.76 ± 26.83 Dc | 2858.76 ± 33.94 Bc | 2618.76 ± 31.29 Cc | 3102.37 ± 46.69 Ab |
| (nmol/mL) | T2 | 2119.43 ± 7.96 Cd | 2383.44 ± 26.29 Bd | 2143.50 ± 40.16 Cd | 2455.44 ± 16.97 Ac |
| T3 | 2671.58 ± 32.45 Cb | 2983.58 ± 78.67 Bb | 2683.58 ± 22.57 Cb | 3342.42 ± 16.97 Aa | |
| Sperm | T0 | 54.74 ± 0.44 Ab | 39.20 ± 0.39 Cd | 49.56 ± 0.36 Bb | 35.07 ± 0.31 Dc |
| viability | T1 | 53.69 ± 0.37 Ab | 50.40 ± 0.27 Bb | 42.35 ± 0.28 Cc | 38.15 ± 0.10 Db |
| (%) | T2 | 65.10 ± 0.39 Aa | 61.67 ± 0.29 Ba | 57.47 ± 0.66 Ca | 50.61 ± 0.44 Da |
| T3 | 48.23 ± 0.41 Ac | 42.00 ± 0.42 Bc | 41.09 ± 0.13 Bc | 35.63 ± 0.43 Cc | |
| Sperm | T0 | 42.97 ± 1.26 Ad | 38.50 ± 0.19 Bd | 37.33 ± 0.44 Bd | 34.44 ± 0.26 Cc |
| motility | T1 | 52.73 ± 0.39 Ab | 49.50 ± 1.23 Bb | 41.59 ± 0.28 Cb | 37.61 ± 0.60 Db |
| (%) | T2 | 63.94 ± 0.72 Aa | 60.50 ± 0.26 Ba | 56.21 ± 0.43 Ca | 49.71 ± 0.30 Da |
| T3 | 47.37 ± 0.39 Ac | 41.25 ± 0.43 Bc | 40.41 ± 0.50 Bc | 34.99 ± 0.37 Cc | |
| SDF | T0 | 4.05 ± 0.11 Ca | 6.24 ± 0.10 Ba | 4.19 ± 0.12 Ca | 6.90 ± 0.15 Aa |
| (%) | T1 | 3.55 ± 0.19 Db | 5.56 ± 0.08 Ab | 4.22 ± 0.14 Ca | 4.82 ± 0.09 Bc |
| T2 | 1.47 ± 0.11 Cc | 4.31 ± 0.09 Ac | 1.94 ± 0.05 Bc | 4.32 ± 0.06 Ad | |
| T3 | 3.42 ± 0.13 Bb | 6.16 ± 0.05 Aa | 3.62 ± 0.08 Bb | 6.37 ± 0.10 Ab | |
| Group | Incapacitated | Capacitated | Acrosome Reaction | |||
|---|---|---|---|---|---|---|
| 1 h | 2 h | 1 h | 2 h | 1 h | 2 h | |
| T0 | 62.60 ± 0.55 Ad | 60.20 ± 2.17 Ac | 24.40 ± 1.34 Ba | 26.40 ± 0.89 Ba | 12.60 ± 1.34 Ca | 13.40 ± 1.34 Ca |
| T1 | 69.40 ± 0.89 Ac | 66.20 ± 2.68 Ab | 21.00 ± 0.71 Bb | 24.00 ± 2.24 Ba | 9.60 ± 0.89 Ca | 9.80 ± 0.45 Cb |
| T2 | 80.60 ± 0.55 Aa | 78.20 ± 1.79 Aa | 13.00 ± 1.41 Bc | 15.20 ± 1.30 Bc | 6.40 ± 0.89 Cb | 6.60 ± 0.55 Cc |
| T3 | 74.20 ± 3.83 Ab | 69.00 ± 4.80 Ab | 20.40 ± 0.89 Bb | 20.80 ± 0.45 Bb | 11.40 ± 0.89 Ca | 12.20 ± 1.30 Ca |
| No | Parameters | Equation | p-Value | R | R2 (%) |
|---|---|---|---|---|---|
| 1 | Pf IPM | Pt Mot = 1.13776 + 0.86461 × Pf IPM | 0.0001 | 0.44 | 19.25 |
| 2 | Pf MDA | Pt Mot = 127.81664 − 0.03171 × Pf MDA | <0.0001 | 0.98 | 96.35 |
| 3 | Pf Via | Pt Mot = −16.32235 + 1.15038 × Pf Via | <0.0001 | 0.82 | 67.84 |
| 4 | Pf Mot | Pt Mot = −8.07232 + 1.07062 × Pf Mot | <0.0001 | 0.99 | 97.30 |
| 5 | Pf SDF | Pt Mot = 70.34792 − 7.21669 × Pf SDF | <0.0001 | 0.88 | 77.62 |
| 6 | Pf Acr | Pt Mot = 77.51510 − 3.43959 × Pf Acr | <0.0001 | 0.91 | 82.20 |
| 7 | Pf Cap | Pt Mot = 82.35528 − 1.99723 × Pf Cap | <0.0001 | 0.90 | 81.48 |
| 8 | Pf Unc | Pt Mot = 60.48636 − 0.15341 × Pf Unc | 0.2797 | 0.13 | 1.69 |
| AA | Ref | T0 | T1 | T2 | T3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| G | 2 | 2.00 | 100.00 | 1.00 | 50.00 | 0.50 | 25.00 | 1.67 | 83.33 |
| A | 3 | 1.00 | 33.33 | 1.33 | 44.44 | 0.00 | 0.00 | 1.00 | 33.33 |
| V | 2 | 2.00 | 100.00 | 1.33 | 66.67 | 1.00 | 50.00 | 2.00 | 100.00 |
| L | 28 | 5.60 | 20.00 | 4.67 | 16.67 | 2.00 | 7.14 | 5.00 | 17.86 |
| I | 17 | 3.00 | 17.65 | 1.00 | 5.88 | 0.00 | 0.00 | 0.67 | 3.92 |
| S | 30 | 9.00 | 30.00 | 4.00 | 13.33 | 1.75 | 5.83 | 5.67 | 18.89 |
| T | 19 | 6.00 | 31.58 | 4.67 | 24.56 | 1.75 | 9.21 | 3.33 | 17.54 |
| D | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| E | 3 | 0.20 | 6.67 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| N | 19 | 2.00 | 10.53 | 2.00 | 10.53 | 0.50 | 2.63 | 2.33 | 12.28 |
| Q | 14 | 0.00 | 0.00 | 0.33 | 2.38 | 0.50 | 3.57 | 1.00 | 7.14 |
| K | 6 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| R | 8 | 2.80 | 35.00 | 3.33 | 41.67 | 2.75 | 34.38 | 3.00 | 37.50 |
| H | 8 | 2.00 | 25.00 | 1.00 | 12.50 | 0.25 | 3.13 | 0.67 | 8.33 |
| C | 0 | 0.00 | 0.00 | 1.00 | n/a | 0.00 | 0.00 | 0.67 | n/a |
| M | 3 | 1.00 | 33.33 | 0.67 | 22.22 | 0.00 | 0.00 | 0.33 | 11.11 |
| P | 15 | 3.00 | 20.00 | 2.33 | 15.56 | 2.75 | 18.33 | 3.33 | 22.22 |
| F | 6 | 2.00 | 33.33 | 0.67 | 11.11 | 0.25 | 4.17 | 0.00 | 0.00 |
| Y | 10 | 1.00 | 10.00 | 2.33 | 23.33 | 0.50 | 5.00 | 2.00 | 20.00 |
| W | 1 | 0.00 | 0.00 | 1.00 | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sum | 194 | 42.60 | 21.96 | 32.67 | 16.84 | 14.50 | 7.47 | 32.67 | 16.84 |
| Aa | Pt IPM | Pt MDA | Pt Via | Pt Mot | Pt SDF | Pt Inc | Pt Cap | Pt Acr |
|---|---|---|---|---|---|---|---|---|
| G | −0.98 * | 0.95 * | −0.98 * | −0.98 * | 0.94 * | −0.75 * | 0.85 * | 0.92 * |
| A | −0.68 * | 0.77 * | −0.73 * | −0.72 * | 0.68 * | −0.71 * | 0.83 * | 0.69 * |
| V | −0.93 * | 0.9 * | −0.97 * | −0.97 * | 0.93 * | −0.63 * | 0.79 * | 0.9 * |
| L | −0.94 * | 0.97 * | −0.96 * | −0.96 * | 0.91 * | −0.84 * | 0.95 * | 0.91 * |
| I | −0.86 * | 0.86 * | −0.76 * | −0.75 * | 0.71 * | −0.92 * | 0.83 * | 0.74 * |
| S | −0.98 * | 0.96 * | −0.93 * | −0.93 * | 0.88 * | −0.85 * | 0.88 * | 0.89 * |
| T | −0.85 * | 0.9 * | −0.78 * | −0.77 * | 0.72 * | −0.97 * | 0.92 * | 0.76 * |
| D | −0.10 | 0.13 | −0.11 | −0.11 | 0.07 | −0.14 | 0.12 | 0.07 |
| E | −0.74 * | 0.7 * | −0.6 * | −0.6 * | 0.56 * | −0.77 * | 0.64 * | 0.6 * |
| N | −0.80 * | 0.85 * | −0.89 * | −0.88 * | 0.85 * | −0.66 * | 0.84 * | 0.82 * |
| Q | −0.27 ** | 0.3 ** | −0.09 | −0.08 | 0.06 | −0.63 * | 0.37 * | 0.13 |
| K | −0.10 | 0.13 | −0.11 | −0.11 | 0.07 | −0.14 | 0.12 | 0.07 |
| R | −0.03 | 0.15 | −0.11 | −0.1 | 0.09 | −0.2 | 0.31 ** | 0.1 |
| H | −0.86 * | 0.87 * | −0.76 * | −0.75 * | 0.71 * | −0.95 * | 0.86 * | 0.74 * |
| C | −0.1 | 0.13 | −0.11 | −0.11 | 0.07 | −0.14 | 0.12 | 0.07 |
| M | −0.84 * | 0.88 * | −0.76 * | −0.75 * | 0.7 * | −0.97 * | 0.91 * | 0.75 * |
| P | −0.45 * | 0.35 * | −0.53 * | −0.53 * | 0.52 * | −0.02 | 0.17 | 0.46 * |
| F | −0.66 * | 0.66 * | −0.5 * | −0.5 * | 0.46 * | −0.83 * | 0.65 * | 0.52 * |
| Y | −0.27 ** | 0.37 * | −0.4 * | −0.39 * | 0.38 * | −0.26 ** | 0.45 * | 0.37 * |
| W | −0.14 | 0.01 | −0.13 | −0.14 | 0.15 | − 0.20 | 0.18 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susilowati, S.; Mustofa, I.; Wurlina, W.; Hernawati, T.; Oktanella, Y.; Soeharsono, S.; Purwanto, D.A. Green Tea Extract in the Extender Improved the Post-Thawed Semen Quality and Decreased Amino Acid Mutation of Kacang Buck Sperm. Vet. Sci. 2022, 9, 403. https://doi.org/10.3390/vetsci9080403
Susilowati S, Mustofa I, Wurlina W, Hernawati T, Oktanella Y, Soeharsono S, Purwanto DA. Green Tea Extract in the Extender Improved the Post-Thawed Semen Quality and Decreased Amino Acid Mutation of Kacang Buck Sperm. Veterinary Sciences. 2022; 9(8):403. https://doi.org/10.3390/vetsci9080403
Chicago/Turabian StyleSusilowati, Suherni, Imam Mustofa, Wurlina Wurlina, Tatik Hernawati, Yudit Oktanella, Soeharsono Soeharsono, and Djoko Agus Purwanto. 2022. "Green Tea Extract in the Extender Improved the Post-Thawed Semen Quality and Decreased Amino Acid Mutation of Kacang Buck Sperm" Veterinary Sciences 9, no. 8: 403. https://doi.org/10.3390/vetsci9080403
APA StyleSusilowati, S., Mustofa, I., Wurlina, W., Hernawati, T., Oktanella, Y., Soeharsono, S., & Purwanto, D. A. (2022). Green Tea Extract in the Extender Improved the Post-Thawed Semen Quality and Decreased Amino Acid Mutation of Kacang Buck Sperm. Veterinary Sciences, 9(8), 403. https://doi.org/10.3390/vetsci9080403






