Simple Summary
The corpus luteum plays a key role in pregnancy maintenance and estrous cycle regulation by secreting progesterone. In this study, we investigate the expression and regulation of lncRNA Hand2os1 in the ovaries. We found Hand2os1 was specifically detected in luteal cells during the proestrus and estrus phases, and strongly expressed in the corpus luteum on day 4 and day 18 of pregnancy. Moreover, Hand2os1 regulates the secretion of progesterone in the mouse corpus luteum by affecting the key rate-limiting enzyme StAR, which suggests it may have an impact on the maintenance of pregnancy.
Abstract
The corpus luteum plays a key role in pregnancy maintenance and estrous cycle regulation by secreting progesterone. Hand2os1 is an lncRNA located upstream of Hand2, with which a bidirectional promoter is shared and is involved in the regulation of cardiac development and embryo implantation in mice. The aim of this study was to investigate the expression and regulation of Hand2os1 in the ovaries. Here, we used RNAscope to detect differential expression of Hand2os1 in the ovaries of cycling and pregnant mice. Hand2os1 was specifically detected in luteal cells during the proestrus and estrus phases, showing its highest expression in the corpus luteum at estrus. Additionally, Hand2os1 was strongly expressed in the corpus luteum on day 4 of pregnancy, but the positive signal progressively disappeared after day 8, was detected again on day 18, and gradually decreased after delivery. Hand2os1 significantly promoted the synthesis of progesterone and the expression of StAR and Cyp11a1. The decreased progesterone levels caused by Hand2os1 interference were rescued by the overexpression of StAR. Our findings suggest that Hand2os1 may regulate the secretion of progesterone in the mouse corpus luteum by affecting the key rate-limiting enzyme StAR, which may have an impact on the maintenance of pregnancy.
1. Introduction
The corpus luteum (CL) is a gland formed after ovulation with a temporary endocrine function. The main role of the CL is to secrete progesterone to maintain pregnancy. The CL can be divided into cyclical and pregnancy CLs. If pregnancy does not occur, the CL will degenerate into a white body under the action of prostaglandin F2α (PGF2α) and prolactin (PRL), and then the female animal will enter the next round of the estrus cycle [1,2,3]. Progesterone (P4), secreted by the CL, plays a crucial role in regulating the estrous cycle and maintaining pregnancy. Luteal cells utilize steroidogenic acute regulatory protein (StAR), P450 cholesterol side chain cleavage enzyme (P450scc; encoded by CYP11A1), and 3β-hydroxysteroid dehydrogenase (3β-HSD) to produce progesterone, and this process is regulated tightly. [4]. Ovulation occurs with a sudden increase in luteinizing hormone (LH) levels, followed by luteinization of the granulosa cells (GC), where StAR expression increases. P450scc then catalyzes the conversion of cholesterol to pregnenolone, which is further metabolized to progesterone via 3β-HSD. [4,5,6].
It has been confirmed that lncRNAs can participate in many aspects of gene function and regulation; they mainly interact with mRNAs, DNA, proteins, and miRNAs, and regulate genes in multiple ways [7,8]. At present, the roles of lncRNAs in the field of reproduction have been widely reported, including spermatogenesis, oocyte and embryo development, follicular development and ovulation, and placenta formation [9,10,11]. Additionally, a study found that the lncRNA Neat1 was highly expressed in the CL, and that severe damage to the CL was observed in nearly half of Neat1 knockout mice studied. These data suggest that Neat1 may play a role in maintaining ovarian function [12]. Moreover, it was confirmed that StAR and progesterone production is disturbed in the H19KO mouse model [13]. However, the expression and biological function of lncRNAs in the CL remain unclear.
Hand2os1 (Uph, also known as HAND2-AS1 in humans) is located upstream of Hand2 and shares a bidirectional promoter with it [14]. Hand2os1 can inhibit the proliferation of cardiomyocytes by inhibiting the expression of Hand2, thereby reconciling the balance of cardiomyocyte lineages [15]. Previous studies have shown that the lncRNA Hand2os1 is involved in the regulation of decidualization of the mouse uteri and that it is regulated by P4 [16]. To delineate the role of Hand2os1 in the CL, we collected ovaries at different stages, constructed in vitro and in vivo models of CL formation and degeneration, and used qRT-PCR and RNAscope to explore further biological functions. Taken together, our findings elucidate the physiological functions of Hand2os1 in the production of P4 and provide evidence for antisense lncRNAs in regulating luteal function.
2. Materials and Methods
2.1. Ethics Statement
The methods used in this study were performed in accordance with the guidelines of the Committee for Ethics on Animal Care and Experiments at Northwest A&F University. All experimental protocols involving animal subjects had received prior approval from the Experimental Animal Manage Committee, and the approval license number was 2017ZX08008005.
2.2. Animals, Treatments, and Sample Collection
Adult mice (Kunming strain), 8–10 weeks old, were purchased from the Chengdu Dashuo Experimental Animal Center (Chengdu, China). Animals were housed under a 12 L:12 D cycle and provided with food and water ad libitum.
Sexually mature mice were selected, and the vaginal smear method and hematoxylin and eosin (HE) staining were used to further identify the estrus cycle phase. Mice with different estrus cycle phases (n = 3 for each phase) were selected and sacrificed using the dislocation method, and ovarian tissue from both sides was collected.
To establish a model of CL formation and degeneration, immature female mice were divided into two groups at 21 days of age. The experimental group (n = 3) was intraperitoneally injected with PMSG (5 IU) and, 48 h later, with hCG (5 IU); the control group (n = 3) was injected with saline [12]. Mice were sacrificed by cervical dislocation at 0, 24, 48, 72, and 96 h after the second injection, and the ovarian tissues from both sides of the body were collected.
Sexually mature female mice were mated with fertile male mice of the same breed to induce pregnancy. Pregnancy was confirmed on days 1 and 4 (day 1 = vaginal congestion day) by recovering embryos from the fallopian tubes and uterus, respectively. Mice were killed at 9 a.m. by cervical dislocation on pregnancy days 0 (D0), D1, D2, D4, D8, D11, D14, and D18 as well as at postpartum days 1 (PD1), PD3, and PD5 (n = 3 each). Ovarian tissue samples were collected from both sides of the body.
2.3. Detecting Target RNA via RNAscope
Ovarian tissues were formalin-fixed, paraffin-embedded, and sliced into 5 μm-thick sections. Target gene expression was detected using a Hand2os1-specific targeting probe (Advanced Cell Diagnostics, Silicon Valley, CA, USA). Follow the instructions for the RNAscope® 2.5 HD detection kit (Advanced Cell Diagnostics). Finally, the tissue sections were counterstained with hematoxylin. Images were acquired using a fluorescence Ni-U microscope (Nikon, Tokyo, Japan).
2.4. Isolation and Culture of Mouse Luteum Cells
Four-day pregnant mice were sacrificed by cervical dislocation at 9 a.m., and bilateral ovarian tissues were collected under aseptic conditions. After washing with PBS 2–3 times, ophthalmic forceps were used to peel off the CL tissue inlaid in the ovary under a stereoscope. The CL tissue was incubated with 0.1% collagenase II (Sigma, St. Louis, MO, USA) for 40 min at 37 °C, and digestion was stopped with GibcoDulbecco’s Modified Eagle Medium: F-12 (DMEM/F12) medium containing 10% serum. Digested tissues were filtered through a 70 µm filter (Cell Strainer; Millipore, MA, USA) and precipitated cells were collected [17]. All the cultured cells were maintained in a humidified incubator at 37 °C with 5% CO2. The luteal degeneration model was constructed using mouse primary luteal cells cultured in medium containing PGF2α (1 µM) for 24 h [18].
2.5. Isolation, Culture, and Luteinization of Mouse Granulosa Cells
Mice in the estrus phase that had been detected by HE staining were killed by cervical dislocation, and bilateral ovarian tissues were collected under aseptic conditions. In DMEM/F-12 medium, a needle was used to pierce the follicle on the ovary to release granulosa cells. The collected solution was filtered through a 70-µm filter (Cell Strainer; Millipore, MA, USA). The cells were then seeded in DMEM/F12 culture medium with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% charcoal-treated fetal bovine serum (FBS; Life Technologies, Carlsbad, CA, USA) at a concentration of 2 × 105 cells/well in 35-mm dishes. The granulosa cells were treated with 100 ng/mL luteinizing hormone (LH) to induce luteinization and harvested after 0, 12, and 24 h [19].
2.6. Transfection of Hand2os1 siRNA and Overexpression Vector
Mouse luteal cells (LCs) were transfected either with a Hand2os1 siRNA or negative control, designed, and synthesized by Gene Pharma Co., Ltd. (Shanghai, China), using TurboFect Transfection Reagent (Thermo Scientific, Shanghai, China), according to the manufacturer’s instructions. Overexpression experiments were performed using the previously constructed vector pcDNA3.1-Hand2os1 [16].
2.7. Cell Proliferation Assay
LCs were plated in 96-well plates at a density of 1 × 104 cells/well and treated with Hand2os1 siRNA and overexpression vector after 24 h of culture. Then, the instructions of the Cell Counting Kit-8 (Beyotime, Shanghai, China) were followed to conduct the experiment, using a microplate reader 680 (Bio-Rad Laboratories Inc., Hercules, CA, USA) to detect the absorbance. All experiments were performed in triplicate.
2.8. P4 Level Detection by ELISA
According to the experimental requirements, the culture supernatants of LCs and GCs were collected, pretreated by centrifugation at 3000× g at 4 °C for 20 min, and then transferred to a clean centrifuge tube. Conditioned media were collected for the analysis of P4 content using a P4 ELISA kit according to the manufacturer’s instructions (Mlbio, Shanghai, China). Absorbance at 450 nm was measured using a microplate reader 680 (Bio-Rad).
2.9. Cell Apoptosis Assay
LCs were plated into 6-well plates and incubated for 24 h. Apoptosis was assessed using an Annexin V-FITC/PI Apoptosis Detection Kit (Keygen Biotech, Nanjing, China). The samples were subjected to flow cytometry using a FACSAria III Cell Sorter (BD Biosciences, San Jose, CA, USA). All data were analyzed using FlowJo X 10.0.7 software (Palo Alto, CA, USA).
2.10. qRT-PCR Analysis
Total RNA was extracted using TRIzol reagent (Invitrogen, Shanghai, China). RT was performed using the 5× All-In-One RT Master Mix with the AccuRT Genomic DNA Removal Kit (Applied Biological Materials, Inc.; Vancouver, BC, Canada). qRT-PCR was performed using the Eva Green qPCR Master Mix Kit (Vazyme Biotech Co., Ltd., Nanjing, China) on a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories Inc.). All reactions were conducted in biological triplicates. The primers and temperatures used are listed in Table 1.

Table 1.
Primer sequence for RT-qPCR.
2.11. Data Analysis and Statistics
Each experiment was repeated at least 3 times in each group. GraphPad Prism 6 software (GraphPad Software, San Diego, CA, USA)was used for statistical analysis of data, and one-way ANOVA or independent samples t-test was used for significant difference analysis. p < 0.05 means significant difference.
3. Results
3.1. Differential Expression of Hand2os1 during the CL Development
To evaluate the physiological functions of Hand2os1 in the CL, RNAscope was first performed to analyze the spatial distribution of this lncRNA in the ovary during the estrus cycle and pregnancy (Figure 1). In all the ovaries studied, Hand2os1 was specifically expressed in LCs; no positive staining for Hand2os1 was observed in any other cell type. During the estrous cycle, highly positive staining for Hand2os1 was detected in the cytoplasm of LCs during the estrus phase compared to that found during the proestrus phase (Figure 1A). In contrast, no positive staining for Hand2os1 was observed in the CL during the diestrus and metestrus phases. During pregnancy, no positive signal for Hand2os1 was detected in the CL on D1, but Hand2os1 expression was strongly detected in the cytoplasm of CL cells on D4 and then disappeared from D8 to D14 (Figure 1B). Notably, a strong Hand2os1 signal peaked again in the nucleus of the LCs during the delivery period (D18). Then, the expression of Hand2os1 gradually decreased from PD1 to PD3. These results indicated that Hand2os1 might be involved in CL formation and regression. Surprisingly, no positive signal for Hand2os1 was detected in the hCG-induced CL formation and regression model (Figure 2).
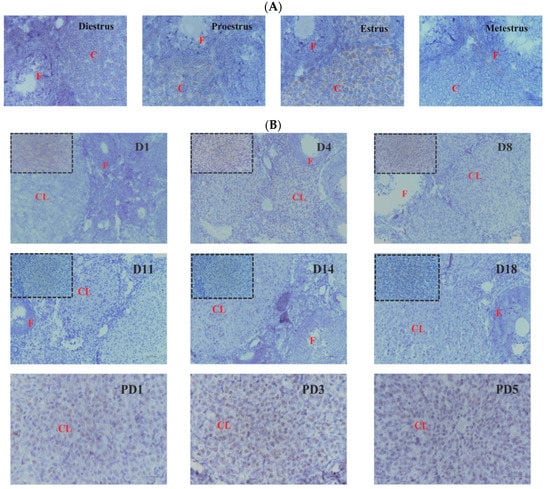
Figure 1.
RNAscope of Hand2os1 expression in the ovary on days 1–18 of pregnancy (D1–D18) and days 1–5 of postpartum (PD1–PD5). Positive expression results in a brown color. (A) Hand2os1 expression and localization in ovaries during the estrus cycle. (B) Hand2os1 expression in ovaries during pregnancy. CL, corpus luteum; F, follicle. Scale bar = 50 µm.
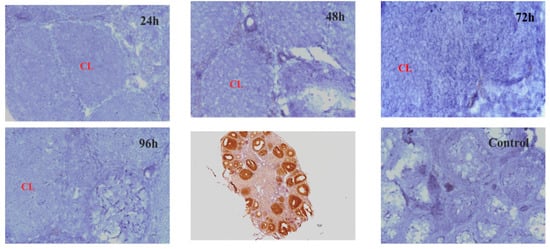
Figure 2.
Hand2os1 expression in the hCG-induced CL formation and regression model for different hours (24 h, 48 h, 72 h, 96 h) was detected by RNAscope. Positive expression results in a brown colour. CL, corpus luteum. Scale bar = 50 µm.
3.2. Effects of Hand2os1 on LC Proliferation and Apoptosis
The proliferation of LCs isolated from the CL on D4 of pregnancy was not affected by silencing or overexpression of Hand2os1 for 24 h (Figure 3A,B). To further analyze the effect of Hand2os1 during LC degeneration, a model of luteal regression was established by the addition of PGF2α (1 µM). Flow cytometry results showed that overexpression of the Hand2os1 gene did not change PGF2α-induced LCs apoptosis (Figure 3C).
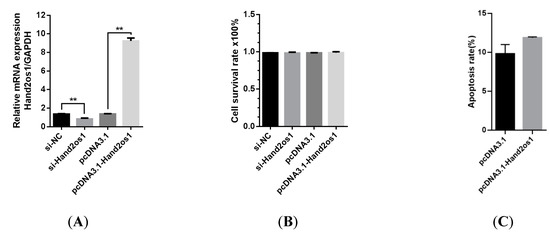
Figure 3.
Effects of Hand2os1 on proliferation and apoptosis in mouse LCs collected on day 4 of pregnancy. (A) The efficiency of Hand2os1 silencing and overexpression were determined using qRT-PCR 24 h after transfection with si-Hand2os1 and pcDNA3.1-Hand2os1, respectively. GAPDH was used as the reference gene for normalization. (B) The proliferation of LCs was determined by CCK-8 assays after silencing or overexpression of Hand2os1 for 24 h. (C) The apoptosis of LCs was analyzed by FCM after transfection with the Hand2os1 overexpression vector, followed by treatment with 1 µM PGF2α for 24 h. The data represent the mean ± SEM from three independent experiments. ** p < 0.01 compared with si-NC group, and different number of asterisks on bars indicate significant differences (p < 0.05).
3.3. The Effect of Hand2os1 on the Formation of the CL
Follicular granulosa cells differentiate into LCs in response to LH stimulation. In this study, primary granulosa cells were cultured and luteinized by a 100 ng/mL LH treatment. As shown in Figure 4, the genes encoding the key steroidogenic enzymes StAR and Cyp11a1 were significantly upregulated with increasing LH treatment time (p < 0.05); however, the expression level of Hand2os1 mRNA was not significantly altered by LH treatment. Furthermore, StAR and Cyp11a1 mRNA levels were unaffected by Hand2os1 overexpression in LH-treated luteinized granulosa cells (Figure 5). ELISA results further confirmed that Hand2os1 overexpression had no effect on progesterone secretion during granulosa cell luteinization (Figure 5).
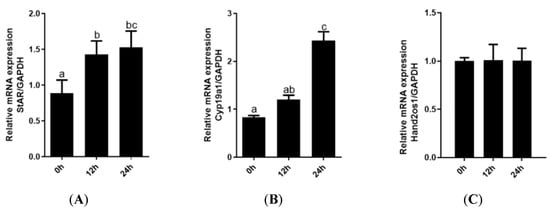
Figure 4.
Expression analyses of StAR, Cyp11a1, and Hand2os1 during luteinization of granulosa cells. The mRNA levels of (A) StAR, (B) Cyp11a1, and (C) Hand2os1 were detected by qRT-PCR after primary granulosa cells were treated with 100 ng/mL LH for 0, 12, and 24 h. GAPDH was used as the reference gene for normalization. The data represent the mean ± SEM from three independent experiments. b (p < 0.05), c (p < 0.01) compared with 0h group (a), the same letter indicates no significant difference and different letters means significant difference (p < 0.05).
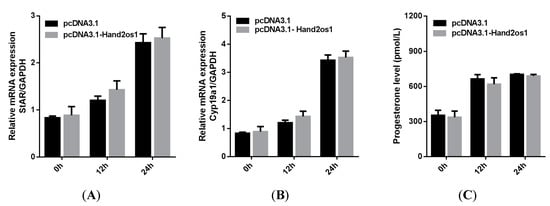
Figure 5.
Effects of Hand2os1 on the expression of steroidogenic enzymes and progesterone production in LH-induced luteinized granulosa cells. The mRNA levels of (A) StAR and (B) Cyp11a1 were detected by qRT-PCR in 1 IU/mL LH-treated granulosa cells after transfection with pcDNA3.1-Hand2os1 for 0, 12, and 24 h. (C) Meanwhile, the concentration of progesterone in the culture supernatants were determined by ELISA. GAPDH was used as the reference gene for normalization. The data represent the mean ± SEM from three independent experiments.
3.4. Effect of Hand2os1 on Progesterone Synthesis in LCs
To further explore the role of Hand2os1 in progesterone synthesis, we either silenced or overexpressed the Hand2os1 gene in cultured primary LCs isolated from the CL of D4 of pregnancy samples. The results showed that silencing of Hand2os1 significantly suppressed progesterone production in cultured primary LCs compared to that in control cells (Figure 6A, p < 0.05). Conversely, Hand2os1 overexpression markedly promoted progesterone production in cultured primary LCs (Figure 6A, p < 0.05). In addition, the mRNA levels of the steroidogenic enzymes StAR and Cyp11a1 were significantly decreased or increased after the silencing or overexpression, respectively, of the Hand2os1 gene in cultured primary LCs (Figure 6B,C, p < 0.05).
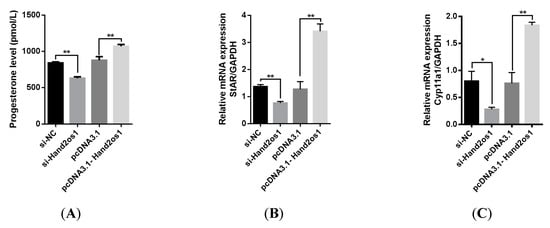
Figure 6.
Effects of Hand2os1 on progesterone production in LCs. LCs were isolated from the CL on D4 of pregnancy following transfection with si-Hand2os1 or pcDNA3.1-Hand2os1 for 24 h. Then, (A) the concentration of progesterone in the culture supernatants was determined by ELISA, and the expression levels of the steroidogenic enzyme (B) StAR and (C) Cyp11a1 genes were analyzed by qRT-PCR. GAPDH was used as the reference gene for normalization. The data represent the mean ± SEM from three independent experiments. * p < 0.05, ** p < 0.01 compared with si-NC group, and different number of asterisks on bars indicate statistically significant differences (p < 0.05).
To verify the mechanism underlying Hand2os1 regulation of progesterone synthesis through steroidogenic enzymes, we constructed a StAR overexpression vector and used it to transfect primary LCs from Hand2os1-silenced mice. As shown in Figure 7A,B, StAR overexpression did not change Hand2os1 expression in mouse primary LCs, but significantly upregulated the mRNA expression of StAR inhibited by Hand2os1. Importantly, overexpression of StAR significantly increased the progesterone production that was inhibited by si-Hand2os1 in mouse primary LCs (Figure 7C, p < 0.05).
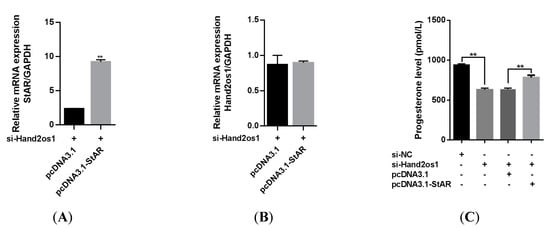
Figure 7.
Effects of StAR overexpression on the progesterone production inhibited by si-Hand2os1 in LCs. qRT−PCR analyses of (A) StAR and (B) Hand2os1 mRNA levels 24 h after transfection with si-Hand2os1 and pcDNA3.1-StAR. (C) The concentration of progesterone in the culture supernatants were determined by ELISA. GAPDH was used as the reference gene for normalization. The data represent the mean ± SEM from three independent experiments. ** p < 0.01 compared with si−NC group, and different number of asterisks on bars indicate statistically significant differences (p < 0.05).
4. Discussion
After ovulation, somatic cells gradually differentiate into LCs. Then, the CL develops into an operational endocrine gland involved in the growth and development of steroid cells [20]. The main function of the CL is to secrete progesterone, which is required to preserve pregnancy in most mammalian species. As important regulatory factors, lncRNAs are involved in the early embryonic development process and in maintaining reproductive ability, including oocyte maturation, zygotic genome activation, and mitochondrial function [21,22,23,24]. However, there are few reports on the effect of lncRNA on the maintenance of the CL in the ovaries during early pregnancy. Our previous study showed that the lncRNA Hand2os1 is involved in mouse embryo implantation [16]. In this study, we found that Hand2os1 was highly expressed in the luteal cells of mice during embryo implantation and delivery. This is consistent with the results of a previous study, in which some lncRNAs were found to play a role in embryo implantation and labor through immune responses [25]. These results suggest that Hand2os1 may be involved in embryo attachment and delivery mechanisms. In addition, highly positive staining for Hand2os1 was detected in the CL during the estrous phase. However, through hormone-induced CL formation and degeneration, no positive staining of Hand2os1 was found in immature mouse ovaries, indicating that Hand2os1 exists only in sexually mature ovaries.
After pregnancy occurs, the CL secretes hormones such as P4 to participate in the regulation of implantation and early maintenance of pregnancy [26]. If pregnancy fails or if the fetus is delivered, the CL regresses normally to start a new reproductive cycle [27]. Therefore, the maintenance and regression of the CL are crucial for pregnancy and the continuity of the sexual cycle in females. The formation and degeneration of the CL involve the proliferation and apoptosis of LCs. PGF2α can reduce the concentration of P4 in the serum and CL. PGF2α may also participate in the induction of CL cell membrane damage and CL cell apoptosis, leading to the degradation of the CL structure, which involves a variety of cytokines and immune functions [28]. Previous studies have shown that lncRNA SRA stimulates mouse granulosa cell growth, changes the cell cycle distribution with an increase in cyclins D1, E, and B, and inhibits cell apoptosis by upregulating Bcl2 and downregulating Bax [29,30]. However, our results showed that neither overexpression nor silencing of Hand2os1 affected the proliferation and apoptosis of LCs. This suggests that Hand2os1 has no significant effect on CL formation and degeneration.
P4 is a key reproductive hormone in the establishment and maintenance of early pregnancy, and its synthesis is regulated by many factors [4]. Some lncRNAs, such as SRA, H19, and Neat1, also play a role in the production of steroid hormones and the expression of key enzymes [12,31,32]. The main function of the CL is to maintain pregnancy by secreting P4. In this study, we found that Hand2os1 affects the level of P4 secreted by LCs during early pregnancy. This result suggests that Hand2os1 may be involved in the secretion of P4 in LCs. StAR and Cyp11a1 are key enzymes in progesterone synthesis [4]. We inhibited Hand2os1 with a specific siRNA and reduced the expression of the key steroid genes StAR and Cyp11a1. In contrast, Hand2os1 overexpression enhanced the expression of StAR and Cyp11a1. In addition, overexpression of StAR rescued the decrease in StAR and P4 caused by Hand2os1 interference, further showing the effect of Hand2os1 on P4 synthesis and suggesting that it may affect the secretion of P4 through the StAR pathway. Interestingly, Hand2os1 does not participate in the regulation of key steroid genes (i.e., StAR and Cyp11a1) during granulosa cell luteinization, implying that its role in different stages of the reproductive process may be quite different. Hand2os1 is mainly involved in the regulation of progesterone secretion in CL during estrus and pregnancy.
5. Conclusions
This study characterized the expression of Hand2os1 in different luteal phases. Our results show that Hand2os1 can participate in the function of progesterone secretion in mouse CL by affecting the expression of the key rate-limiting enzyme StAR. Accordingly, this study provides a valuable resource for identifying functional lncRNAs associated with the CL and pregnancy. However, the specific molecular mechanism by which Hand2os1 is involved in the regulation of luteal function requires further study.
Author Contributions
P.L. and Y.J. (Yaping Jin) designed the experiments and bioinformatics analysis; Y.J. (Yanni Jia) and L.L analyzed the data and drafted the manuscript; Y.J. (Yanni Jia) and L.L. constructed animal models and collected the sample; Y.J. (Yanni Jia) and S.G. conducted the experiments; H.L., X.Z. and R.Z. prepared and checked figures. A.W. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32172934), the Key R&D Program project of the Ningxia Hui Autonomous Region (2021BBF02037) and Shaanxi Agricultural Science and Technology innovation Drive project.
Institutional Review Board Statement
The study was conducted in accordance with the Ethics on Animal Care and Experiments at Northwest A&F University, and approved by the Experimental Animal Manage Committee, and the approval license number was 2017ZX08008005.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Proietto, S.; Cortasa, S.A.; Corso, M.C.; Inserra, P.I.F.; Charif, S.E.; Schmidt, A.R.; Di Giorgio, N.P.; Lux-Lantos, V.; Vitullo, A.D.; Dorfman, V.B.; et al. Prolactin Is a Strong Candidate for the Regulation of Luteal Steroidogenesis in Vizcachas (Lagostomus maximus). Int. J. Endocrinol. 2018, 2018, 1910672. [Google Scholar] [CrossRef] [PubMed]
- Jonczyk, A.W.; Piotrowska-Tomala, K.K.; Skarzynski, D.J. Effects of prostaglandin F(2α) (PGF(2α)) on cell-death pathways in the bovine corpus luteum (CL). BMC Vet. Res. 2019, 15, 416. [Google Scholar] [CrossRef] [PubMed]
- Horihata, K.; Yoshioka, S.; Sano, M.; Yamamoto, Y.; Kimura, K.; Skarzynski, D.J.; Okuda, K. Expressions of lipoprotein receptors and cholesterol efflux regulatory proteins during luteolysis in bovine corpus luteum. Reprod. Fertil. Dev. 2017, 29, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, N.A.; Whirledge, S.; Kallen, A.N. Updates on molecular and environmental determinants of luteal progesterone production. Mol. Cell. Endocrinol. 2020, 515, 110930. [Google Scholar] [CrossRef]
- Lee-Thacker, S.; Jeon, H.; Choi, Y.; Taniuchi, I.; Takarada, T.; Yoneda, Y.; Ko, C.; Jo, M. Core Binding Factors are essential for ovulation, luteinization, and female fertility in mice. Sci. Rep. 2020, 10, 9921. [Google Scholar] [CrossRef]
- Choi, H.; Ryu, K.-Y.; Roh, J. Krüppel-like factor 4 plays a role in the luteal transition in steroidogenesis by downregulating Cyp19A1 expression. Am. J. Physiol. Metab. 2019, 316, E1071–E1080. [Google Scholar] [CrossRef]
- Rinn, J.L.; Kertesz, M.; Wang, J.K.; Squazzo, S.L.; Xu, X.; Brugmann, S.A.; Goodnough, L.H.; Helms, J.A.; Farnham, P.J.; Segal, E.; et al. Functional Demarcation of Active and Silent Chromatin Domains in Human HOX Loci by Noncoding RNAs. Cell 2007, 129, 1311–1323. [Google Scholar] [CrossRef]
- Sun, T.-T.; He, J.; Liang, Q.; Ren, L.-L.; Yan, T.-T.; Yu, T.-C.; Tang, J.-Y.; Bao, Y.-J.; Hu, Y.; Lin, Y.; et al. LncRNA GClnc1 Promotes Gastric Carcinogenesis and May Act as a Modular Scaffold of WDR5 and KAT2A Complexes to Specify the Histone Modification Pattern. Cancer Discov. 2016, 6, 784–801. [Google Scholar] [CrossRef]
- Bouska, M.J.; Bai, H. Long noncoding RNA regulation of spermatogenesis via the spectrin cytoskeleton in Drosophila. G3 2021, 11, jkab080. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qi, B.; Xie, J.; Wu, X.; Ling, Y.; Cao, X.; Kong, F.; Xin, J.; Jiang, X.; Wu, Q.; et al. Filtered reproductive long non-coding RNAs by genome-wide analyses of goat ovary at different estrus periods. BMC Genom. 2018, 19, 866. [Google Scholar] [CrossRef]
- Zhou, F.; Sun, Y.; Chi, Z.; Gao, Q.; Wang, H. Long noncoding RNA SNHG12 promotes the proliferation, migration, and invasion of trophoblast cells by regulating the epithelial-mesenchymal transition and cell cycle. J. Int. Med. Res. 2020, 48, 300060520922339. [Google Scholar] [CrossRef]
- Nakagawa, S.; Shimada, M.; Yanaka, K.; Mito, M.; Arai, T.; Takahashi, E.; Fujita, Y.; Fujimori, T.; Standaert, L.; Marine, J.-C.; et al. The lncRNA Neat1 is required for corpus luteum formation and the establishment of pregnancy in a subpopulation of mice. Development 2014, 141, 4618–4627. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Fan, Y.; Qin, C.; Xia, X.; Johnson, J.; Kallen, A.N. Absence of the long noncoding RNA H19 results in aberrant ovarian STAR and progesterone production. Mol. Cell. Endocrinol. 2019, 490, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.M.; Anderson, D.M.; McAnally, J.R.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature 2016, 539, 433–436. [Google Scholar] [CrossRef]
- Han, X.; Zhang, J.; Liu, Y.; Fan, X.; Ai, S.; Luo, Y.; Li, X.; Jin, H.; Luo, S.; Zheng, H.; et al. The lncRNA Hand2os1/Uph locus orchestrates heart development through regulation of precise expression of Hand. Development 2019, 146, dev176198. [Google Scholar] [CrossRef]
- Jia, Y.; Cai, R.; Yu, T.; Zhang, R.; Liu, S.; Guo, X.; Shang, C.; Wang, A.; Jin, Y.-P.; Lin, P. Progesterone-induced RNA Hand2os1 regulates decidualization in mice uteri. Reproduction 2020, 159, 303–314. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Liu, J.; Luo, H.; Gou, K.; Cui, S. Prostaglandin F2αupregulates Slit/Robo expression in mouse corpus luteum during luteolysis. J. Endocrinol. 2013, 218, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Mejia, R.; Waite, C.; Ascoli, M. Activation of Gq/11 in the Mouse Corpus Luteum Is Required for Parturition. Mol. Endocrinol. 2015, 29, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.Y.; O’Connor, A.; Shitanaka, M.; Shimada, M.; Liu, Z.; Richards, J.S. Beta-catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Mol Endocrinol. 2010, 24, 1529–1542. [Google Scholar] [CrossRef]
- Abedel-Majed, M.A.; Romereim, S.M.; Davis, J.S.; Cupp, A.S. Perturbations in Lineage Specification of Granulosa and Theca Cells May Alter Corpus Luteum Formation and Function. Front. Endocrinol. 2019, 10, 832. [Google Scholar] [CrossRef]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.-J.; Ren, Z.-R.; Yan, J.-B. Identification and functional analysis of long non-coding RNAs in human and mouse early embryos based on single-cell transcriptome data. Oncotarget 2016, 7, 61215–61228. [Google Scholar] [CrossRef]
- Xue, Z.; Huang, K.; Cai, C.; Cai, L.; Jiang, C.-Y.; Feng, Y.; Liu, Z.; Zeng, Q.; Cheng, L.; Sun, Y.E.; et al. Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature 2013, 500, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-S.; Li, T.-P.; Ton, H.; Mao, X.-D.; Chen, Y.-J. Advances of Long Noncoding RNAs-mediated Regulation in Reproduction. Chin. Med. J. 2018, 131, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, L.; Cao, M.; Chen, T.; Huang, Y.; Wang, N.; Zhang, B.; Li, F.; Chen, K.; Yuan, C.; et al. Comparison of lncRNA Expression in the Uterus between Periods of Embryo Implantation and Labor in Mice. Animals 2022, 12, 399. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Souza, A.H.; Carvalho, P.D.; Cunha, A.P.; Giordano, J.O.; Fricke, P.M.; Baez, G.M.; Diskin, M.G. Physiological and practical effects of progesterone on reproduction in dairy cattle. Animal 2014, 8, 70–81. [Google Scholar] [CrossRef]
- Mesen, T.B.; Young, S.L. Progesterone and the luteal phase: A requisite to reproduction. Obstet. Gynecol. Clin. N. Am. 2015, 42, 135–151. [Google Scholar] [CrossRef]
- Rueda, B.R.; Hendry, I.R.; Tilly, J.L.; Hamernik, D.L. Accumulation of Caspase-3 Messenger Ribonucleic Acid and Induction of Caspase Activity in the Ovine Corpus Luteum Following Prostaglandin F2α Treatment In Vivo. Biol. Reprod. 1999, 60, 1087–1092. [Google Scholar] [CrossRef][Green Version]
- Abolghasemi, M.; Mahjoub, S. Long noncoding RNAs as a piece of polycystic ovary syndrome puzzle. Mol. Biol. Rep. 2021, 48, 3845–3851. [Google Scholar] [CrossRef]
- Hao, E.-Y.; Wang, D.-H.; Chang, L.-Y.; Huang, C.-X.; Chen, H.; Yue, Q.-X.; Zhou, R.-Y.; Huang, R.-L. Melatonin regulates chicken granulosa cell proliferation and apoptosis by activating the mTOR signaling pathway via its receptors. Poult. Sci. 2020, 99, 6147–6162. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Zhou, D.; Shuang, T.; Zhao, H.; Chen, B. Up-Regulation of Long Noncoding RNA SRA Promotes Cell Growth, Inhibits Cell Apoptosis, and Induces Secretion of Estradiol and Progesterone in Ovarian Granular Cells of Mice. Med. Sci. Monit. 2018, 24, 2384–2390. [Google Scholar] [CrossRef] [PubMed]
- Kallen, A.N.; Zhou, X.-B.; Xu, J.; Qiao, C.; Ma, J.; Yan, L.; Lu, L.; Liu, C.; Yi, J.-S.; Zhang, H.; et al. The Imprinted H19 LncRNA Antagonizes Let-7 MicroRNAs. Mol. Cell 2013, 52, 101–112. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
