Avian Neuropeptide Y: Beyond Feed Intake Regulation
Abstract
1. Introduction
2. Structure of NPY
3. NPY Receptors
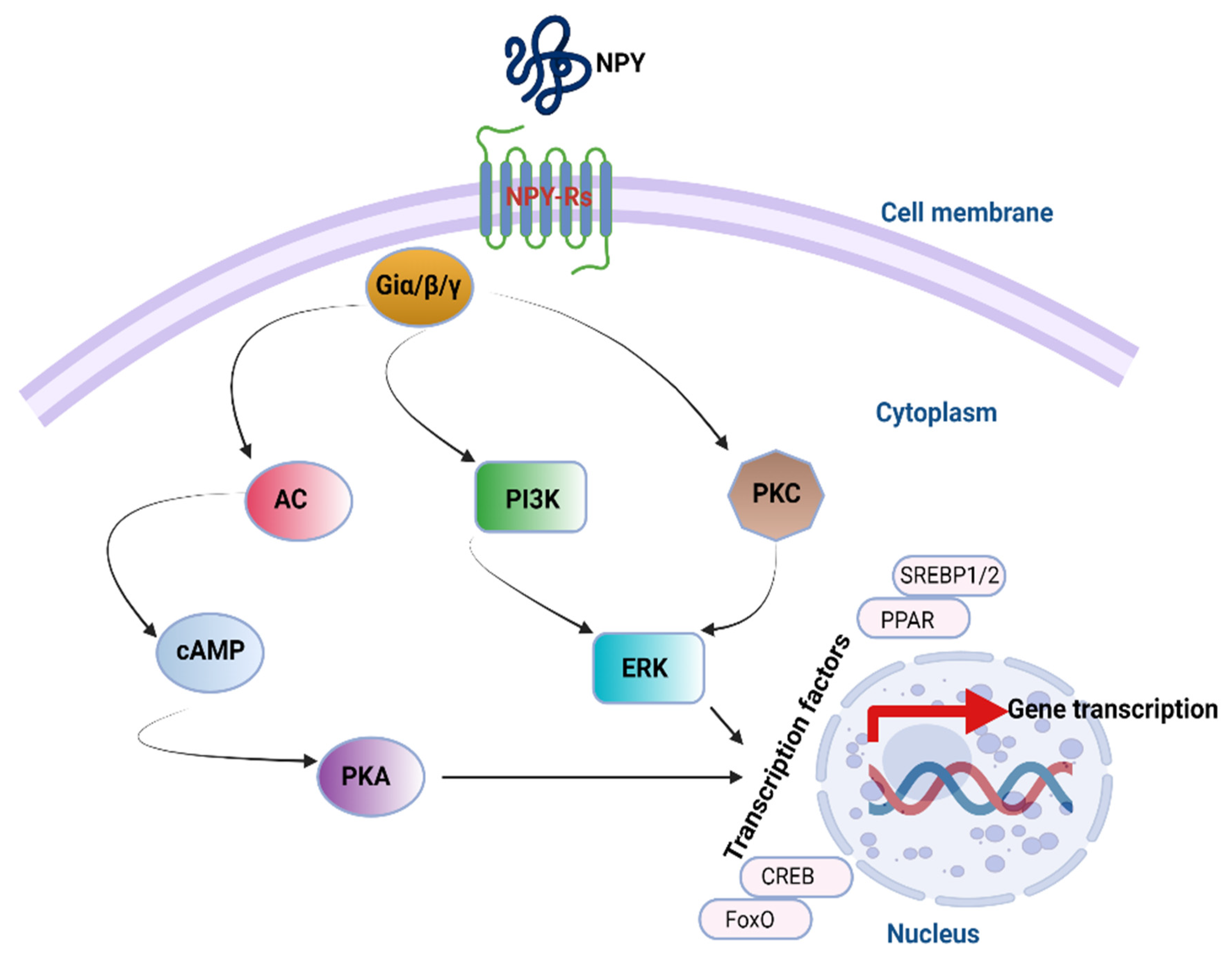
4. NPY Downstream Signaling Cascades
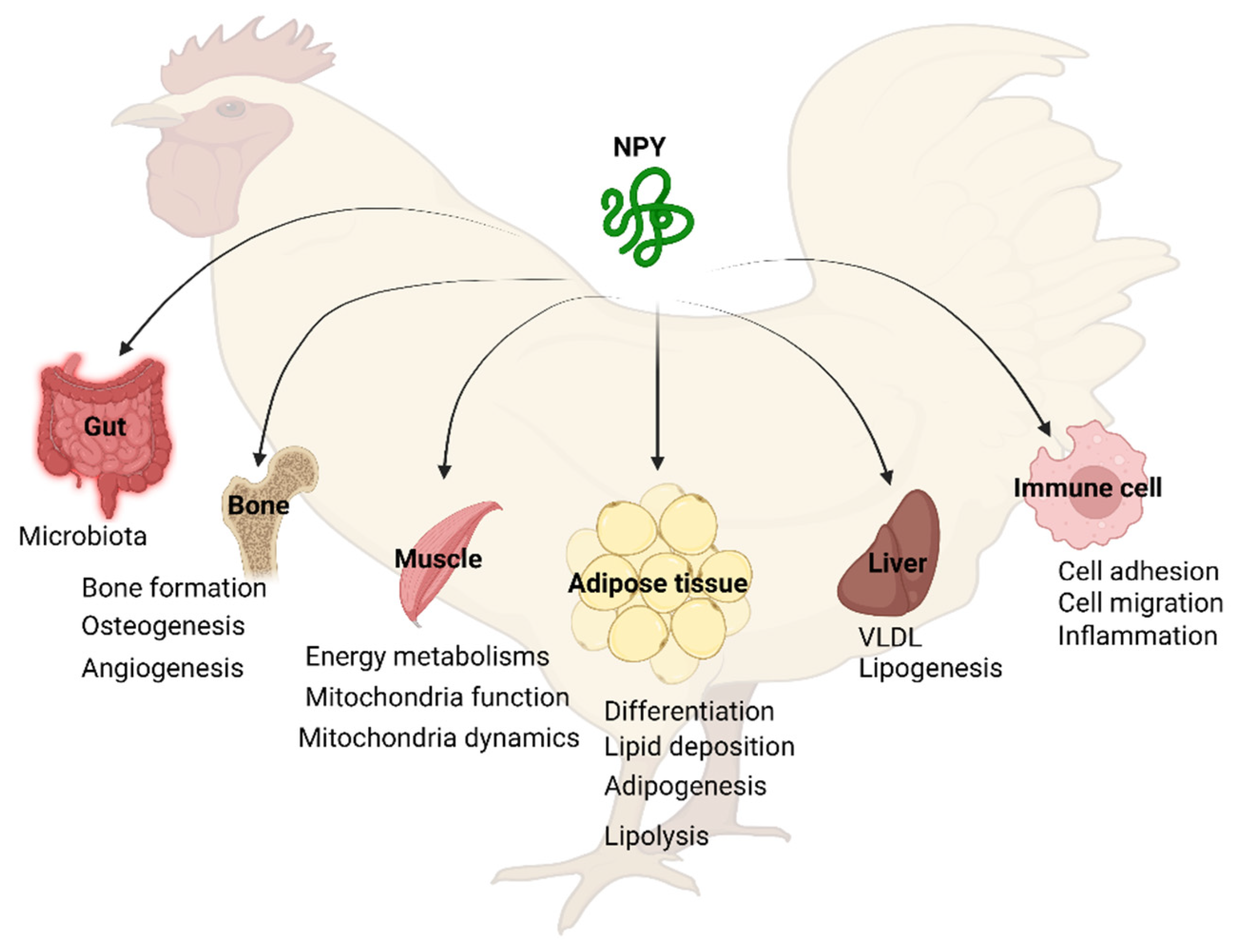
5. Tissue Distribution of NPY System
6. Physiological Functions of NPY
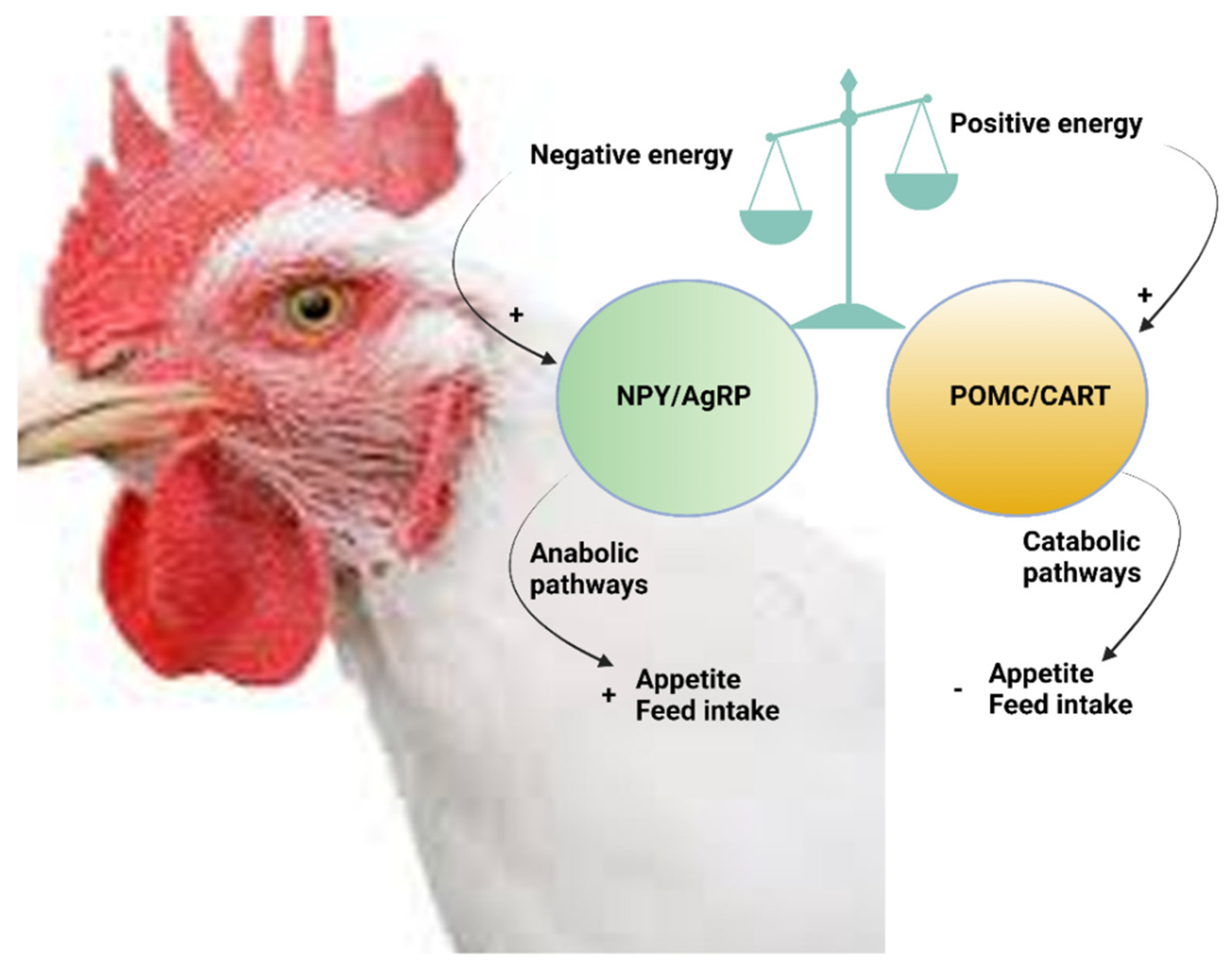
6.1. Central Functions of NPY
6.2. Peripheral Functions of NPY
6.2.1. NPY in Adipose Tissue
6.2.2. NPY in the Liver
6.2.3. NPY in the Muscle
6.2.4. NPY in the Bone
6.2.5. NPY in Macrophage and Immune System
6.2.6. NPY in the Gut
7. Regulation of Avian NPY Expression
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tatemoto, K.; Carlquist, M.; Mutt, V. Neuropeptide Y—A novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 1982, 296, 659–660. [Google Scholar] [CrossRef] [PubMed]
- McConn, B.R.; Gilbert, E.R.; Cline, M.A. Appetite-associated responses to central neuropeptide Y injection in quail. Neuropeptides 2018, 69, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Hökfelt, T.; Broberger, C.; Zhang, X.; Diez, M.; Kopp, J.; Xu, Z.Q.; Landry, M.; Bao, L.; Schalling, M.; Koistinaho, J.; et al. Neuropeptide Y: Some viewpoints on a multifaceted peptide in the normal and diseased nervous system. Brain Res. Rev. 1998, 26, 154–166. [Google Scholar] [CrossRef]
- Lin, S.; Boey, D.; Herzog, H. NPY and Y receptors: Lessons from transgenic and knockout models. Neuropeptides 2004, 38, 189–200. [Google Scholar] [CrossRef]
- Levine, A.S.; Morley, J.E. Neuropeptide Y: A potent inducer of consummatory behavior in rats. Peptides 1984, 5, 1025–1029. [Google Scholar] [CrossRef]
- Morley, J.E.; Levine, A.S.; Gosnell, B.A.; Kneip, J.; Grace, M. Effect of neuropeptide Y on ingestive behaviors in the rat. Am. J. Physiol. Reg. Integr. Comp. Physiol. 1987, 252, R599–R609. [Google Scholar] [CrossRef]
- Miner, J.L.; Della-Fera, M.A.; Paterson, J.A.; Baile, C.A. Lateral cerebroventricular injection of neuropeptide Y stimulates feeding in sheep. Am. J. Physiol. Reg. Integr. Comp. Physiol. 1989, 257, R383–R387. [Google Scholar] [CrossRef]
- Parrott, R.F.; Heavens, R.P.; Baldwin, B.A. Stimulation of feeding in the satiated pig by intracerebroventricular injection of neuropeptide Y. Physiol. Behav. 1986, 36, 523–525. [Google Scholar] [CrossRef]
- Kuenzel, W.J.; Douglass, L.W.; Davison, B.A. Robust feeding following central administration of neuropeptide Y or peptide YY in chicks, Gallus domesticus. Peptides 1987, 8, 823–828. [Google Scholar] [CrossRef]
- Newmyer, B.A.; Nandar, W.; Webster, R.I.; Gilbert, E.; Siegel, P.B.; Cline, M.A. Neuropeptide Y is associated with changes in appetite-associated hypothalamic nuclei but not food intake in a hypophagic avian model. Behav. Brain Res. 2013, 236, 327–331. [Google Scholar] [CrossRef]
- Sainsbury, A.; Zhang, L. Role of the arcuate nucleus of the hypothalamus in regulation of body weight during energy deficit. Mol. Cell. Endocrinol. 2010, 316, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Dryden, S.; Pickavance, L.; Frankish, H.M.; Williams, G. Increased neuropeptide Y secretion in the hypothalamic paraventricular nucleus of obese (fa/fa) Zucker rats. Brain Res. 1995, 690, 185–188. [Google Scholar] [CrossRef]
- Schutz, B.; Schafer, M.K.H.; Eiden, L.E.; Weihe, E. VIP and NPY Expression during Differentiation of Cholinergic and Noradrenergic Sympathetic Neuronsa. Ann. N. Y. Acad. Sci. 1998, 865, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Guan, H.; Arany, E.; Hill, D.J.; Cao, X. Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J. 2008, 22, 2452–2464. [Google Scholar] [CrossRef] [PubMed]
- Baldock, P.A.; Allison, S.J.; Lundberg, P.; Lee, N.J.; Slack, K.; Lin, E.-J.D.; Enriquez, R.F.; McDonald, M.M.; Zhang, L.; During, M.J.; et al. Novel Role of Y1 Receptors in the Coordinated Regulation of Bone and Energy Homeostasis. J. Biol. Chem. 2007, 282, 19092–19102. [Google Scholar] [CrossRef]
- Baldock, P.A.; Lee, N.J.; Driessler, F.; Lin, S.; Allison, S.; Stehrer, B.; Lin, E.-J.D.; Zhang, L.; Enriquez, R.F.; Wong, I.P.L.; et al. Neuropeptide Y Knockout Mice Reveal a Central Role of NPY in the Coordination of Bone Mass to Body Weight. PLoS ONE 2009, 4, e8415. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Baldock, P.A. Central and peripheral mechanisms of the NPY system in the regulation of bone and adipose tissue. Bone 2012, 50, 430–436. [Google Scholar] [CrossRef]
- Edelsbrunner, M.E.; Herzog, H.; Holzer, P. Evidence from knockout mice that peptide YY and neuropeptide Y enforce murine locomotion, exploration and ingestive behaviour in a circadian cycle- and gender-dependent manner. Behav. Brain Res. 2009, 203, 97–107. [Google Scholar] [CrossRef]
- Reichmann, F.; Holzer, P. Neuropeptide Y: A stressful review. Neuropeptides 2016, 55, 99–109. [Google Scholar] [CrossRef]
- Gøtzsche, C.R.; Woldbye, D.P.D. The role of NPY in learning and memory. Neuropeptides 2016, 55, 79–89. [Google Scholar] [CrossRef]
- Colmers, W.F.; El Bahh, B. Neuropeptide Y and Epilepsy. Epilepsy Curr. 2003, 3, 53–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gribkoff, V.K.; Pieschl, R.L.; Wisialowski, T.A.; van den Pol, A.N.; Yocca, F.D. Phase shifting of circadian rhythms and depression of neuronal activity in the rat suprachiasmatic nucleus by neuropeptide Y: Mediation by different receptor subtypes. J. Neurosci. 1998, 18, 3014–3022. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.M.J.; Green, P.; Tapoulal, N.; Lewandowski, A.J.; Leeson, P.; Herring, N. The Role of Neuropeptide Y in Cardiovascular Health and Disease. Front. Physiol. 2018, 9, 1281. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.C. Receptors for neuropeptide Y: Multiple subtypes and multiple second messengers. Trends Pharmacol. Sci. 1991, 12, 389–394. [Google Scholar] [CrossRef]
- Blomqvist, A.G.; Söderberg, C.; Lundell, I.; Milner, R.J.; Larhammar, D. Strong evolutionary conservation of neuropeptide Y: Sequences of chicken, goldfish, and Torpedo marmorata DNA clones. Proc. Natl. Acad. Sci. USA 1992, 89, 2350–2354. [Google Scholar] [CrossRef]
- He, C.; Zhang, J.; Gao, S.; Meng, F.; Bu, G.; Li, J.; Wang, Y. Molecular characterization of three NPY receptors (Y2, Y5 and Y7) in chickens: Gene structure, tissue expression, promoter identification, and functional analysis. Gen. Comp. Endocrinol. 2016, 236, 24–34. [Google Scholar] [CrossRef]
- Bromée, T.; Sjödin, P.; Fredriksson, R.; Boswell, T.; Larsson, T.A.; Salaneck, E.; Zoorob, R.; Mohell, N.; Larhammar, D. Neuropeptide Y-family receptors Y6 and Y7 in chicken. FEBS J. 2006, 273, 2048–2063. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, J.; He, C.; Meng, F.; Bu, G.; Zhu, G.; Li, J.; Wang, Y. Molecular characterization of neuropeptide Y (NPY) receptors (Y1, Y4 and Y6) and investigation of the tissue expression of their ligands (NPY, PYY and PP) in chickens. Gen. Comp. Endocrinol. 2017, 240, 46–60. [Google Scholar] [CrossRef]
- Denbow, D.M.; Cline, M.A. Chapter 21—Food Intake Regulation. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 469–485. [Google Scholar] [CrossRef]
- Kuenzel, W.J.; Beck, M.M.; Teruyama, R. Neural sites and pathways regulating food intake in birds: A comparative analysis to mammalian systems. J. Exper. Zoo. 1999, 283, 348–364. [Google Scholar] [CrossRef]
- Larhammar, D.; Salaneck, E. Molecular evolution of NPY receptor subtypes. Neuropeptides 2004, 38, 141–151. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Novotný, J.; Martin, J.; Heinrich, G. Molecular structure of mammalian neuropeptide Y: Analysis by molecular cloning and computer-aided comparison with crystal structure of avian homologue. Proc. Natl. Acad. Sci. USA 1987, 84, 2532–2536. [Google Scholar] [CrossRef] [PubMed]
- Beck-Sickinger, A.G.; Jung, G. Structure–activity relationships of neuropeptide Y analogues with respect to Y1 and Y2 receptors. Biopolym. Orig. Res. Biomol. 1995, 37, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Kim, J.; Ko, S.-B.; Choi, Y.K.; Jeong, H.; Woo, H.; Kang, H.; Bang, I.; Kim, S.A.; Yoon, T.-Y.; et al. Structural basis of neuropeptide Y signaling through Y1 receptor. Nat. Commun. 2022, 13, 853. [Google Scholar] [CrossRef]
- Brun, C.; Philip-Couderc, P.; Raggenbass, M.; Roatti, A.; Baertschi, A.J. Intracellular targeting of truncated secretory peptides in the mammalian heart and brain. FASEB J. 2006, 20, 732–734. [Google Scholar] [CrossRef][Green Version]
- Merten, N.; Beck-Sickinger, A.G. Molecular ligand-receptor interaction of the NPY/PP peptide family. In NPY Family of Peptides in Neurobiology, Cardiovascular and Metabolic Disorders: From Genes to Therapeutics; Springer: Berlin/Heidelberg, Germany, 2006; pp. 35–62. [Google Scholar]
- Sundström, G.; Xu, B.; Larsson, T.A.; Heldin, J.; Bergqvist, C.A.; Fredriksson, R.; Conlon, J.M.; Lundell, I.; Denver, R.J.; Larhammar, D. Characterization of the neuropeptide Y system in the frog Silurana tropicalis (Pipidae): Three peptides and six receptor subtypes. Gen. Comp. Endocrinol. 2012, 177, 322–331. [Google Scholar] [CrossRef]
- Sundström, G.; Larsson, T.; Xu, B.; Heldin, J.; Larhammar, D. Interactions of zebrafish peptide YYb with the neuropeptide Y-family receptors Y4, Y7, Y8a, and Y8b. Front. Neurosci. 2013, 7, 29. [Google Scholar] [CrossRef]
- Wraith, A.; Törnsten, A.; Chardon, P.; Harbitz, I.; Chowdhary, B.P.; Andersson, L.; Lundin, L.G.; Larhammar, D. Evolution of the neuropeptide Y receptor family: Gene and chromosome duplications deduced from the cloning and mapping of the five receptor subtype genes in pig. Genome Res. 2000, 10, 302–310. [Google Scholar] [CrossRef]
- Krause, J.; Eva, C.; Seeburg, P.H.; Sprengel, R. Neuropeptide Y1 subtype pharmacology of a recombinantly expressed neuropeptide receptor. Mol. Pharmacol. 1992, 41, 817. [Google Scholar]
- Larhammar, D.; Blomqvist, A.G.; Yee, F.; Jazin, E.; Yoo, H.; Wahlested, C. Cloning and functional expression of a human neuropeptide Y/peptide YY receptor of the Y1 type. J. Biol. Chem. 1992, 267, 10935–10938. [Google Scholar] [CrossRef]
- Lundell, I.; Blomqvist, A.G.; Berglund, M.M.; Schober, D.A.; Johnson, D.; Statnick, M.A.; Gadski, R.A.; Gehlert, D.R.; Larhammar, D. Cloning of a human receptor of the NPY receptor family with high affinity for pancreatic polypeptide and peptide YY. J. Biol. Chem. 1995, 270, 29123–29128. [Google Scholar] [CrossRef] [PubMed]
- Burkhoff, A.M.; Linemeyer, D.L.; Salon, J.A. Distribution of a novel hypothalamic neuropeptide Y receptor gene and its absence in rat. Mol. Brain Res. 1998, 53, 311–316. [Google Scholar] [CrossRef]
- Starbäck, P.; Wraith, A.; Eriksson, H.; Larhammar, D. Neuropeptide Y Receptor Gene y6: Multiple Deaths or Resurrections? Biochem. Biophys. Res. Commun. 2000, 277, 264–269. [Google Scholar] [CrossRef]
- Dhamad, A.; Zampiga, M.; Greene, E.S.; Sirri, F.; Dridi, S. Neuropeptide Y and its receptors are expressed in chicken skeletal muscle and regulate mitochondrial function. Gen. Comp. Endocrinol. 2021, 310, 113798. [Google Scholar] [CrossRef]
- Michel, M.C.; Beck-Sickinger, A.; Cox, H.; Doods, H.N.; Herzog, H.; Larhammar, D.; Quirion, R.; Schwartz, T.; Westfall, T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev. 1998, 50, 143–150. [Google Scholar]
- Salaneck, E.; Holmberg, S.K.S.; Berglund, M.M.; Boswell, T.; Larhammar, D. Chicken neuropeptide Y receptor Y2: Structural and pharmacological differences to mammalian Y2. FEBS Lett. 2000, 484, 229–234. [Google Scholar] [CrossRef]
- Larsson, T.A.; Larson, E.T.; Fredriksson, R.; Conlon, J.M.; Larhammar, D. Characterization of NPY receptor subtypes Y2 and Y7 in rainbow trout Oncorhynchus mykiss. Peptides 2006, 27, 1320–1327. [Google Scholar] [CrossRef]
- Yi, M.; Li, H.; Wu, Z.; Yan, J.; Liu, Q.; Ou, C.; Chen, M. A Promising Therapeutic Target for Metabolic Diseases: Neuropeptide Y Receptors in Humans. Cell. Physiol. Biochem. 2018, 45, 88–107. [Google Scholar] [CrossRef]
- Gerald, C.; Walker, M.W.; Criscione, L.; Gustafson, E.L.; Batzl-Hartmann, C.; Smith, K.E.; Vaysse, P.; Durkin, M.M.; Laz, T.M.; Linemeyer, D.L.; et al. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature 1996, 382, 168–171. [Google Scholar] [CrossRef]
- Marsh, D.J.; Hollopeter, G.; Kafer, K.E.; Palmiter, R.D. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nat. Med. 1998, 4, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Stanley, B.G.; Magdalin, W.; Seirafi, A.; Nguyen, M.M.; Leibowitz, S.F. Evidence for neuropeptide Y mediation of eating produced by food deprivation and for a variant of the Y1 receptor mediating this peptide’s effect. Peptides 1992, 13, 581–587. [Google Scholar] [CrossRef]
- Lecklin, A.; Lundell, I.; Paananen, L.; Wikberg, J.E.S.; Männistö, P.T.; Larhammar, D. Receptor subtypes Y1 and Y5 mediate neuropeptide Y induced feeding in the guinea-pig. Br. J. Pharmacol. 2002, 135, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Molosh, A.I.; Sajdyk, T.J.; Truitt, W.A.; Zhu, W.; Oxford, G.S.; Shekhar, A. NPY Y1 receptors differentially modulate GABAA and NMDA receptors via divergent signal-transduction pathways to reduce excitability of amygdala neurons. Neuropsychopharmacology 2013, 38, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Hansel, D.E.; Eipper, B.A.; Ronnett, G.V. Neuropeptide Y functions as a neuroproliferative factor. Nature 2001, 410, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Pellieux, C.; Sauthier, T.; Domenighetti, A.; Marsh, D.J.; Palmiter, R.D.; Brunner, H.-R.; Pedrazzini, T. Neuropeptide Y (NPY) potentiates phenylephrine-induced mitogen-activated protein kinase activation in primary cardiomyocytes via NPY Y5 receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Saneyasu, T.; Fujita, S.; Kitashiro, A.; Fukuzo, S.; Honda, K.; Kamisoyama, H. Hypothalamic Akt-mediated signaling regulates food intake in chicks. Neurosci. Lett. 2018, 670, 48–52. [Google Scholar] [CrossRef]
- Pelletier, G.; Guy, J.; Allen, Y.S.; Polak, J.M. Electron microscope immunocytochemical localization of neuropeptide Y (NPY) in the rat brain. Neuropeptides 1984, 4, 319–324. [Google Scholar] [CrossRef]
- Heilig, M.; Widerlöv, E. Neuropeptide Y: An overview of central distribution, functional aspects, and possible involvement in neuropsychiatric illnesses. Acta Psychiatr. Scand. 1990, 82, 95–114. [Google Scholar] [CrossRef]
- Danger, J.M.; Tonon, M.C.; Jenks, B.G.; Saint-Pierre, S.; Martel, J.C.; Fasolo, A.; Breton, B.; Quirion, R.; Pelletier, G.; Vaudry, H. Neuropeptide Y: Localization in the central nervous system and neuroendocrine functions. Fundam. Clin. Pharmacol. 1990, 4, 307–340. [Google Scholar] [CrossRef]
- Adrian, T.E.; Allen, J.M.; Bloom, S.R.; Ghatei, M.A.; Rossor, M.N.; Roberts, G.W.; Crow, T.J.; Tatemoto, K.; Polak, J.M. Neuropeptide Y distribution in human brain. Nature 1983, 306, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Allen, Y.S.; Adrian, T.E.; Allen, J.M.; Tatemoto, K.; Crow, T.J.; Bloom, S.R.; Polak, J.M. Neuropeptide Y Distribution in the Rat Brain. Science 1983, 221, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Gray, T.S.; Morley, J.E. Neuropeptide Y: Anatomical distribution and possible function in mammalian nervous system. Life Sci. 1986, 38, 389–401. [Google Scholar] [CrossRef]
- Kuenzel, W.J.; McMurtry, J. Neuropeptide Y: Brain localization and central effects on plasma insulin levels in chicks. Physiol. Behav. 1988, 44, 669–678. [Google Scholar] [CrossRef]
- Wang, X.; Day, J.R.; Vasilatos-Younken, R. The distribution of neuropeptide Y gene expression in the chicken brain. Mol. Cell. Endocrinol. 2001, 174, 129–136. [Google Scholar] [CrossRef]
- Ericsson, A.; Schalling, M.; McIntyre, K.R.; Lundberg, J.M.; Larhammar, D.; Seroogy, K.; Hökfelt, T.; Persson, H. Detection of neuropeptide Y and its mRNA in megakaryocytes: Enhanced levels in certain autoimmune mice. Proc. Natl. Acad. Sci. USA 1987, 84, 5585–5589. [Google Scholar] [CrossRef]
- Zhang, W.; Sumners, L.H.; Siegel, P.B.; Cline, M.A.; Gilbert, E.R. Quantity of glucose transporter and appetite-associated factor mRNA in various tissues after insulin injection in chickens selected for low or high body weight. Physiol. Genom. 2013, 45, 1084–1094. [Google Scholar] [CrossRef]
- Gerald, C.; Walker, M.W.; Vaysse, P.J.J.; He, C.; Branchek, T.A.; Weinshank, R.L. Expression Cloning and Pharmacological Characterization of a Human Hippocampal Neuropeptide Y/Peptide YY Y2 Receptor Subtype. J. Biol. Chem. 1995, 270, 26758–26761. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, S.; Liu, D.; Cline, M.A.; Gilbert, E.R. Neuropeptide Y promotes adipogenesis in chicken adipose cells in vitro. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 181, 62–70. [Google Scholar] [CrossRef]
- Richards, M.P.; Proszkowiec-Weglarz, M. Mechanisms regulating feed intake, energy expenditure, and body weight in poultry. Poult. Sci. 2007, 86, 1478–1490. [Google Scholar] [CrossRef]
- Everaert, N.; Decuypere, E.; Buyse, J. 3: Feed intake and regulation. In Poultry and Pig Nutrition; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; pp. 59–75. [Google Scholar] [CrossRef]
- Wynne, K.; Stanley, S.; McGowan, B.; Bloom, S. Appetite control. J. Endocrinol. 2005, 184, 291–318. [Google Scholar] [CrossRef] [PubMed]
- Ando, R.; Kawakami, S.-i.; Bungo, T.; Ohgushi, A.; Takagi, T.; Denbow, D.M.; Furuse, M. Feeding responses to several neuropeptide Y receptor agonists in the neonatal chick. Eur. J. Pharmacol. 2001, 427, 53–59. [Google Scholar] [CrossRef]
- Cline, M.A.; Smith, M.L. Central α-melanocyte stimulating hormone attenuates behavioral effects of neuropeptide Y in chicks. Physiol. Behav. 2007, 91, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.-i.; Bungo, T.; Ando, R.; Ohgushi, A.; Shimojo, M.; Masuda, Y.; Furuse, M. Central administration of α-melanocyte stimulating hormone inhibits fasting-and neuropeptide Y-induced feeding in neonatal chicks. Eur. J. Pharmacol. 2000, 398, 361–364. [Google Scholar] [CrossRef]
- Tachibana, T.; Sato, M.; Oikawa, D.; Takahashi, H.; Boswell, T.; Furuse, M. Intracerebroventricular injection of neuropeptide Y modifies carbohydrate and lipid metabolism in chicks. Reg. Pept. 2006, 136, 1–8. [Google Scholar] [CrossRef]
- Saito, E.-S.; Kaiya, H.; Tachibana, T.; Tomonaga, S.; Denbow, D.M.; Kangawa, K.; Furuse, M. Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Reg. Pept. 2005, 125, 201–208. [Google Scholar] [CrossRef]
- Holmberg, S.K.S.; Mikko, S.; Boswell, T.; Zoorob, R.; Larhammar, D. Pharmacological characterization of cloned chicken neuropeptide Y receptors Y1 and Y5. J. Neurochem. 2002, 81, 462–471. [Google Scholar] [CrossRef]
- Kameda, Y.; Miura, M.; Nishimaki, T. Localization of neuropeptide Y mRNA and peptide in the chicken hypothalamus and their alterations after food deprivation, dehydration, and castration. J. Comp. Neurol. 2001, 436, 376–388. [Google Scholar] [CrossRef]
- Boswell, T.; Dunn, I.C.; Corr, S.A. Hypothalamic neuropeptide Y mRNA is increased after feed restriction in growing broilers. Poult. Sci. 1999, 78, 1203–1207. [Google Scholar] [CrossRef]
- Shiraishi, J.-i.; Yanagita, K.; Fujita, M.; Bungo, T. Central insulin suppresses feeding behavior via melanocortins in chicks. Domest. Anim. Endocrimol. 2008, 34, 223–228. [Google Scholar] [CrossRef]
- Dridi, S.; Swennen, Q.; Decuypere, E.; Buyse, J. Mode of leptin action in chicken hypothalamus. Brain Res. 2005, 1047, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Saarela, S.; Keith, J.S.; Hohtola, E.; Trayhurn, P. Is the “mammalian” brown fat-specific mitochondrial uncoupling protein present in adipose tissues of birds? Comp. Biochem. Physiol. Part B Comp. Biochem. 1991, 100, 45–49. [Google Scholar] [CrossRef]
- Zhang, W.; Cline, M.A.; Gilbert, E.R. Hypothalamus-adipose tissue crosstalk: Neuropeptide Y and the regulation of energy metabolism. Nut. Metab. 2014, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.E.; Kitlinska, J.B.; Tilan, J.U.; Li, L.; Baker, S.B.; Johnson, M.D.; Lee, E.W.; Burnett, M.S.; Fricke, S.T.; Kvetnansky, R. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 2007, 13, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Shipp, S.L.; Cline, M.A.; Gilbert, E.R. Recent advances in the understanding of how neuropeptide Y and α-melanocyte stimulating hormone function in adipose physiology. Adipocyte 2016, 5, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, G.; Gerrard, M.E.; Wieland, S.; Davis, M.; Cline, M.A.; Siegel, P.B.; Gilbert, E.R. Changes in adipose tissue physiology during the first two weeks posthatch in chicks from lines selected for low or high body weight. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2019, 316, R802–R818. [Google Scholar] [CrossRef]
- Liu, L.; Wang, G.; Xiao, Y.; Shipp, S.L.; Siegel, P.B.; Cline, M.A.; Gilbert, E.R. Peripheral neuropeptide Y differentially influences adipogenesis and lipolysis in chicks from lines selected for low or high body weight. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 213, 1–10. [Google Scholar] [CrossRef]
- Rojas, J.M.; Bruinstroop, E.; Printz, R.L.; Alijagic-Boers, A.; Foppen, E.; Turney, M.K.; George, L.; Beck-Sickinger, A.G.; Kalsbeek, A.; Niswender, K.D. Central nervous system neuropeptide Y regulates mediators of hepatic phospholipid remodeling and very low-density lipoprotein triglyceride secretion via sympathetic innervation. Mol. Metab. 2015, 4, 210–221. [Google Scholar] [CrossRef]
- Ding, W.-G.; Kitasato, H.; Kimura, H. Development of neuropeptide Y innervation in the liver. Microsc. Res. Tech. 1997, 39, 365–371. [Google Scholar] [CrossRef]
- Stafford, J.M.; Yu, F.; Printz, R.; Hasty, A.H.; Swift, L.L.; Niswender, K.D. Central Nervous System Neuropeptide Y Signaling Modulates VLDL Triglyceride Secretion. Diabetes 2008, 57, 1482–1490. [Google Scholar] [CrossRef]
- Bruinstroop, E.; Pei, L.; Ackermans, M.T.; Foppen, E.; Borgers, A.J.; Kwakkel, J.; Alkemade, A.; Fliers, E.; Kalsbeek, A. Hypothalamic Neuropeptide Y (NPY) Controls Hepatic VLDL-Triglyceride Secretion in Rats via the Sympathetic Nervous System. Diabetes 2012, 61, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.-X.; Foppen, E.; Abplanalp, W.; Gao, Y.; Alkemade, A.; la Fleur, S.E.; Serlie, M.J.; Fliers, E.; Buijs, R.M.; Tschöp, M.H.; et al. Glucocorticoid signaling in the arcuate nucleus modulates hepatic insulin sensitivity. Diabetes 2012, 61, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Liu, Y.; Zhang, Y.; Sun, Y.; Sun, C.; Zhang, Y.; Lv, X. Expression of neuropeptide Y is increased in an activated human HSC cell line. Sci. Rep. 2019, 9, 9500. [Google Scholar] [CrossRef] [PubMed]
- Lundell, I.; Boswell, T.; Larhammar, D. Chicken neuropeptide Y-family receptor Y4: A receptor with equal affinity for pancreatic polypeptide, neuropeptide Y and peptide YY. J. Mol. Endocrinol. 2002, 28, 225. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wang, G.; Zhang, W.; Zhang, S.; Rice, B.B.; Cline, M.A.; Gilbert, E.R. Broiler chicken adipose tissue dynamics during the first two weeks post-hatch. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 189, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Leveille, G.A.; Romsos, D.R.; Yeh, Y.; O’Hea, E.K. Lipid biosynthesis in the chick. A consideration of site of synthesis, influence of diet and possible regulatory mechanisms. Poult. Sci. 1975, 54, 1075–1093. [Google Scholar] [CrossRef]
- Zuidhof, M.J.; Schneider, B.L.; Carney, V.L.; Korver, D.R.; Robinson, F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014, 93, 2970–2982. [Google Scholar] [CrossRef]
- Petracci, M.; Mudalal, S.; Soglia, F.; Cavani, C. Meat quality in fast-growing broiler chickens. World’s Poult. Sci. J. 2015, 71, 363–374. [Google Scholar] [CrossRef]
- Moss, F.P.; Leblond, C.P. Satellite cells as the source of nuclei in muscles of growing rats. Anat. Rec. 1971, 170, 421–435. [Google Scholar] [CrossRef]
- Clark, D.L.; McCormick, J.L.; Velleman, S.G. Effect of incubation temperature on neuropeptide Y and neuropeptide Y receptors in turkey and chicken satellite cells. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 219, 58–66. [Google Scholar] [CrossRef]
- Reed, K.M.; Mendoza, K.M.; Strasburg, G.M.; Velleman, S.G. Response of Turkey Muscle Satellite Cells to Thermal Challenge. II. Transcriptome Effects in Differentiating Cells. Front. Physiol. 2017, 8, 948. [Google Scholar] [CrossRef] [PubMed]
- Department for Environment, Food & Rural Affairs. Heat Stress in Poultry: Solving the Problem; Nobel House: London, UK, 2005.
- Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 2007, 13, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Gordeladze, J.O.; Reseland, J.E. A unified model for the action of leptin on bone turnover. J. Cell. Biochem. 2003, 88, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.J.; Nguyen, A.D.; Enriquez, R.F.; Doyle, K.L.; Sainsbury, A.; Baldock, P.A.; Herzog, H. Osteoblast specific Y1 receptor deletion enhances bone mass. Bone 2011, 48, 461–467. [Google Scholar] [CrossRef]
- Baldock, P.A.; Sainsbury, A.; Couzens, M.; Enriquez, R.F.; Thomas, G.P.; Gardiner, E.M.; Herzog, H. Hypothalamic Y2 receptors regulate bone formation. J. Clin. Investig. 2002, 109, 915–921. [Google Scholar] [CrossRef]
- Baldock, P.A.; Sainsbury, A.; Allison, S.; Lin, E.J.D.; Couzens, M.; Boey, D.; Enriquez, R.; During, M.; Herzog, H.; Gardiner, E.M. Hypothalamic control of bone formation: Distinct actions of leptin and y2 receptor pathways. J. Bone Miner. Res. 2005, 20, 1851–1857. [Google Scholar] [CrossRef]
- Liu, S.; Jin, D.; Wu, J.-Q.; Xu, Z.-Y.; Fu, S.; Mei, G.; Zou, Z.-L.; Ma, S.-H. Neuropeptide Y stimulates osteoblastic differentiation and VEGF expression of bone marrow mesenchymal stem cells related to canonical Wnt signaling activating in vitro. Neuropeptides 2016, 56, 105–113. [Google Scholar] [CrossRef]
- Lee, N.J.; Doyle, K.L.; Sainsbury, A.; Enriquez, R.F.; Hort, Y.J.; Riepler, S.J.; Baldock, P.A.; Herzog, H. Critical role for Y1 receptors in mesenchymal progenitor cell differentiation and osteoblast activity. J. Bone Miner. Res. 2010, 25, 1736–1747. [Google Scholar] [CrossRef]
- Igura, K.; Haider, H.K.; Ahmed, R.P.H.; Sheriff, S.; Ashraf, M. Neuropeptide y and neuropeptide y y5 receptor interaction restores impaired growth potential of aging bone marrow stromal cells. Rejuvenation Res. 2011, 14, 393–403. [Google Scholar] [CrossRef]
- Ma, W.H.; Liu, Y.J.; Wang, W.; Zhang, Y.Z. Neuropeptide Y, substance P, and human bone morphogenetic protein 2 stimulate human osteoblast osteogenic activity by enhancing gap junction intercellular communication. Braz. J. Med. Biol. Res. 2015, 48, 299–307. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, X.; Shi, S.; Zhang, Y. Neuropeptides stimulate human osteoblast activity and promote gap junctional intercellular communication. Neuropeptides 2013, 47, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, C.C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004, 83, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Nicholls, P.K.; Claus, M.; Wu, Y.; Shi, Z.; Greene, W.K.; Ma, B. Immunofluorescence characterization of innervation and nerve-immune cell interactions in mouse lymph nodes. Eur. J. Histochem. 2019, 63, 3059. [Google Scholar] [CrossRef] [PubMed]
- Wheway, J.; Herzog, H.; Mackay, F. NPY and receptors in immune and inflammatory diseases. Curr. Top. Med. Chem. 2007, 7, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Liu, Y.-B.; Liu, W.-F.; Zhou, Y.-Y.; He, H.-F.; Lin, S. Neuropeptide Y is an immunomodulatory factor: Direct and indirect. Front. Immunol. 2020, 11, 2624. [Google Scholar] [CrossRef]
- De la Fuente, M.; Bernaez, I.; Del Rio, M.; Hernanz, A. Stimulation of murine peritoneal macrophage functions by neuropeptide Y and peptide YY. Involvement of protein kinase C. Immunology 1993, 80, 259. [Google Scholar]
- Mitić, K.; Stanojević, S.; Kuštrimović, N.; Vujić, V.; Dimitrijević, M. Neuropeptide Y modulates functions of inflammatory cells in the rat: Distinct role for Y1, Y2 and Y5 receptors. Peptides 2011, 32, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Nave, H.; Bedoui, S.; Moenter, F.; Steffens, J.; Felies, M.; Gebhardt, T.; Straub, R.H.; Pabst, R.; Dimitrijevic, M.; Stanojevic, S. Reduced tissue immigration of monocytes by neuropeptide Y during endotoxemia is associated with Y2 receptor activation. J. Neuroimmunol. 2004, 155, 1–12. [Google Scholar] [CrossRef]
- Woods, T.A.; Du, M.; Carmody, A.; Peterson, K.E. Neuropeptide Y negatively influences monocyte recruitment to the central nervous system during retrovirus infection. J. Virol. 2015, 90, 2783–2793. [Google Scholar] [CrossRef]
- Dimitrijevic, M.; Stanojevic, S.; Kustrimovic, N.; Leposavic, G. End-point effector stress mediators in neuroimmune interactions: Their role in immune system homeostasis and autoimmune pathology. Immunol. Res. 2012, 52, 64–80. [Google Scholar] [CrossRef]
- Gao, B.; Li, L.; Zhu, P.; Zhang, M.; Hou, L.; Sun, Y.; Liu, X.; Peng, X.; Gu, Y. Chronic administration of methamphetamine promotes atherosclerosis formation in ApoE−/− knockout mice fed normal diet. Atherosclerosis 2015, 243, 268–277. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Del Rıo, M.; Medina, S. Changes with aging in the modulation by neuropeptide Y of murine peritoneal macrophage functions. J. Neuroimmunol. 2001, 116, 156–167. [Google Scholar] [CrossRef]
- Hernanz, A.; Tato, E.; De la Fuente, M.; de Miguel, E.; Arnalich, F. Differential effects of gastrin-releasing peptide, neuropeptide Y, somatostatin and vasoactive intestinal peptide on interleukin-1β, interleukin-6 and tumor necrosis factor-α production by whole blood cells from healthy young and old subjects. J. Neuroimmunol. 1996, 71, 25–30. [Google Scholar] [CrossRef]
- Singer, K.; Morris, D.L.; Oatmen, K.E.; Wang, T.; DelProposto, J.; Mergian, T.; Cho, K.W.; Lumeng, C.N. Neuropeptide Y is produced by adipose tissue macrophages and regulates obesity-induced inflammation. PLoS ONE 2013, 8, e57929. [Google Scholar]
- Bahry, M.A.; Chowdhury, V.S.; Yang, H.; Tran, P.V.; Do, P.H.; Han, G.; Ikeda, H.; Cockrem, J.F.; Furuse, M. Central administration of neuropeptide Y differentially regulates monoamines and corticosterone in heat-exposed fed and fasted chicks. Neuropeptides 2017, 62, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Eltahan, H.M.; Bahry, M.A.; Yang, H.; Han, G.; Nguyen, L.T.N.; Ikeda, H.; Ali, M.N.; Amber, K.A.; Furuse, M.; Chowdhury, V.S. Central NPY-Y5 sub-receptor partially functions as a mediator of NPY-induced hypothermia and affords thermotolerance in heat-exposed fasted chicks. Physiol. Rep. 2017, 5, e13511. [Google Scholar] [CrossRef] [PubMed]
- Bohler, M.; Gilbert, E.R.; Cline, M.A. Reduced food intake during exposure to high ambient temperatures is associated with molecular changes in the nucleus of the hippocampal commissure and the paraventricular and arcuate hypothalamic nuclei. Gen. Comp. Endocrinol. 2020, 298, 113576. [Google Scholar] [CrossRef]
- Jackerott, M.; Larsson, L.-I. Immunocytochemical localization of the NPY/PYY Y1 receptor in enteric neurons, endothelial cells, and endocrine-like cells of the rat intestinal tract. J. Histochem. Cytochem. 1997, 45, 1643–1650. [Google Scholar] [CrossRef]
- Cox, H.M. Neuropeptide Y receptors; antisecretory control of intestinal epithelial function. Auton. Neurosci. 2007, 133, 76–85. [Google Scholar] [CrossRef]
- Ishiguchi, T.; Amano, T.; Matsubayashi, H.; Tada, H.; Fujita, M.; Takahashi, T. Centrally administered neuropeptide Y delays gastric emptying via Y2 receptors in rats. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2001, 281, R1522–R1530. [Google Scholar] [CrossRef]
- Ishiguchi, T.; Nakajima, M.; Sone, H.; Tada, H.; Kumagai, A.K.; Takahashi, T. Gastric distension-induced pyloric relaxation: Central nervous system regulation and effects of acute hyperglycaemia in the rat. J. Physiol. 2001, 533, 801. [Google Scholar] [CrossRef] [PubMed]
- Holzer-Petsche, U.; Petritsch, W.; Hinterleitner, T.; Eherer, A.; Sperk, G.; Krejs, G.J. Effect of neuropeptide Y on jejunal water and ion transport in humans. Gastroenterology 1991, 101, 325–330. [Google Scholar] [CrossRef]
- Tough, I.R.; Forbes, S.; Tolhurst, R.; Ellis, M.; Herzog, H.; Bornstein, J.C.; Cox, H.M. Endogenous peptide YY and neuropeptide Y inhibit colonic ion transport, contractility and transit differentially via Y1 and Y2 receptors. Br. J. Pharmacol. 2011, 164, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Hubel, K.A.; Renquist, K.S. Effect of neuropeptide Y on ion transport by the rabbit ileum. J. Pharmacol. Exper. Ther. 1986, 238, 167–169. [Google Scholar]
- Saksena, S.; Tyagi, S.; Goyal, S.; Gill, R.K.; Alrefai, W.A.; Ramaswamy, K.; Dudeja, P.K. Stimulation of apical Cl−/HCO3−(OH−) exchanger, SLC26A3 by neuropeptide Y is lipid raft dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1334–G1343. [Google Scholar] [CrossRef]
- Hansen, C.J.; Burnell, K.K.; Brogden, K.A. Antimicrobial activity of Substance P and Neuropeptide Y against laboratory strains of bacteria and oral microorganisms. J. Neuroimmunol. 2006, 177, 215–218. [Google Scholar] [CrossRef]
- El Karim, I.A.; Linden, G.J.; Orr, D.F.; Lundy, F.T. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J. Neuroimmunol. 2008, 200, 11–16. [Google Scholar] [CrossRef]
- Vouldoukis, I.; Shai, Y.; Nicolas, P.; Mor, A. Broad spectrum antibiotic activity of skin-PYY. FEBS Lett. 1996, 380, 237–240. [Google Scholar] [CrossRef]
- Thomas, L.; Scheidt, H.A.; Bettio, A.; Beck-Sickinger, A.G.; Huster, D.; Zschörnig, O. The interaction of neuropeptide Y with negatively charged and zwitterionic phospholipid membranes. Eur. Biophys. J. 2009, 38, 663–677. [Google Scholar] [CrossRef]
- Thomas, L.; Scheidt, H.A.; Bettio, A.; Huster, D.; Beck-Sickinger, A.G.; Arnold, K.; Zschörnig, O. Membrane interaction of neuropeptide Y detected by EPR and NMR spectroscopy. BBA Biomembr. 2005, 1714, 103–113. [Google Scholar] [CrossRef]
- Sato, H.; Feix, J.B. Peptide–membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. BBA Biomembr. 2006, 1758, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Houser, M.C.; Tansey, M.G. The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinson’s Dis. 2017, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Boswell, T.; Li, Q.; Takeuchi, S. Neurons expressing neuropeptide Y mRNA in the infundibular hypothalamus of Japanese quail are activated by fasting and co-express agouti-related protein mRNA. Mol. Brain Res. 2002, 100, 31–42. [Google Scholar] [CrossRef]
- Zhou, W.; Murakami, M.; Hasegawa, S.; Yoshizawa, F.; Sugahara, K. Neuropeptide Y content in the hypothalamic paraventricular nucleus responds to fasting and refeeding in broiler chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 141, 146–152. [Google Scholar] [CrossRef]
- Yuan, L.; Ni, Y.; Barth, S.; Wang, Y.; Grossmann, R.; Zhao, R. Layer and broiler chicks exhibit similar hypothalamic expression of orexigenic neuropeptides but distinct expression of genes related to energy homeostasis and obesity. Brain Res. 2009, 1273, 18–28. [Google Scholar] [CrossRef]
- Ka, S.; Lindberg, J.; Strömstedt, L.; Fitzsimmons, C.; Lindqvist, N.; Lundeberg, J.; Siegel, P.B.; Andersson, L.; Hallböök, F. Extremely different behaviours in high and low body weight lines of chicken are associated with differential expression of genes involved in neuronal plasticity. J. Neuroendocrinol. 2009, 21, 208–216. [Google Scholar] [CrossRef]
- Sakkou, M.; Wiedmer, P.; Anlag, K.; Hamm, A.; Seuntjens, E.; Ettwiller, L.; Tschöp, M.H.; Treier, M. A role for brain-specific homeobox factor Bsx in the control of hyperphagia and locomotory behavior. Cell Metab. 2007, 5, 450–463. [Google Scholar] [CrossRef]
- Dridi, S.; Ververken, C.; Hillgartner, F.B.; Lutgarde, A.; Van der Gucht, E.; Cnops, L.; Decuypere, E.; Buyse, J. FAS inhibitor cerulenin reduces food intake and melanocortin receptor gene expression without modulating the other (an)orexigenic neuropeptides in chickens. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2006, 291, R138–R147. [Google Scholar] [CrossRef]
- Blankenship, K.; Gilley, A.; Piekarski, A.; Orlowski, S.; Greene, E.; Bottje, W.; Anthony, N.; Dridi, S. Differential expression of feeding-related hypothalamic neuropeptides in the first generation of quails divergently selected for low or high feed efficiency. Neuropeptides 2016, 58, 31–40. [Google Scholar] [CrossRef]
- Xu, A.W.; Kaelin, C.B.; Takeda, K.; Akira, S.; Schwartz, M.W.; Barsh, G.S. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J. Clin. Investig. 2005, 115, 951–958. [Google Scholar] [CrossRef]
- Zakrzewska, K.E.; Sainsbury, A.; Cusin, I.; Rouru, J.; Jeanrenaud, B.; Rohner-Jeanrenaud, F. Selective dependence of intracerebroventricular neuropeptide Y-elicited effects on central glucocorticoids. Endocrinology 1999, 140, 3183–3187. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Song, Z.; Jiao, H.; Lin, H. Glucocorticoids increase NPY gene expression via hypothalamic AMPK signaling in broiler chicks. Endocrinology 2014, 155, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Denbow, D.M.; Meade, S.; Robertson, A.; McMurtry, J.P.; Richards, M.; Ashwell, C. Leptin-induced decrease in food intake in chickens. Physiol. Behav. 2000, 69, 359–362. [Google Scholar] [CrossRef]
- Liu, L.; Xu, S.; Wang, X.; Jiao, H.; Zhao, J.; Lin, H. Effect of dexamethasone on hypothalamic expression of appetite-related genes in chickens under different diet and feeding conditions. J. Anim. Sci. Biotechnol. 2016, 7, 23. [Google Scholar] [CrossRef]
- Shiraishi, J.-i.; Yanagita, K.; Fukumori, R.; Sugino, T.; Fujita, M.; Kawakami, S.-I.; McMurtry, J.P.; Bungo, T. Comparisons of insulin related parameters in commercial-type chicks: Evidence for insulin resistance in broiler chicks. Physiol. Behav. 2011, 103, 233–239. [Google Scholar] [CrossRef]
- Honda, K.; Kamisoyama, H.; Saneyasu, T.; Sugahara, K.; Hasegawa, S. Central administration of insulin suppresses food intake in chicks. Neurosci. Lett. 2007, 423, 153–157. [Google Scholar] [CrossRef]
- Yousefvand, S.; Hamidi, F.; Zendehdel, M.; Parham, A. Survey the Effect of Insulin on Modulating Feed Intake Via NPY Receptors in 5-Day-Old Chickens. Int. J. Pept. Res. Ther. 2020, 26, 467–476. [Google Scholar] [CrossRef]
- Yousefvand, S.; Hamidi, F.; Zendehdel, M.; Parham, A. Hypophagic effects of insulin are mediated via NPY1/NPY2 receptors in broiler cockerels. Can. J. Physiol. Pharmacol. 2018, 96, 1301–1307. [Google Scholar] [CrossRef]
- Tu, W.-L.; Cheng, C.-Y.; Wang, S.-H.; Tang, P.-C.; Chen, C.-F.; Chen, H.-H.; Lee, Y.-P.; Chen, S.-E.; Huang, S.-Y. Profiling of differential gene expression in the hypothalamus of broiler-type Taiwan country chickens in response to acute heat stress. Theriogenology 2016, 85, 483–494. [Google Scholar] [CrossRef]
- Ito, K.; Bahry, M.A.; Hui, Y.; Furuse, M.; Chowdhury, V.S. Acute heat stress up-regulates neuropeptide Y precursor mRNA expression and alters brain and plasma concentrations of free amino acids in chicks. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 187, 13–19. [Google Scholar] [CrossRef]
- Lei, L.; Hepeng, L.; Xianlei, L.; Hongchao, J.; Hai, L.; Sheikhahmadi, A.; Yufeng, W.; Zhigang, S. Effects of acute heat stress on gene expression of brain–gut neuropeptides in broiler chickens (Gallus gallus domesticus). J. Anim. Sci. 2013, 91, 5194–5201. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, T.; Saito, S.; Tomonaga, S.; Takagi, T.; Saito, E.-S.; Nakanishi, T.; Koutoku, T.; Tsukada, A.; Ohkubo, T.; Boswell, T. Effect of central administration of prolactin-releasing peptide on feeding in chicks. Physiol. Behav. 2004, 80, 713–719. [Google Scholar] [CrossRef] [PubMed]
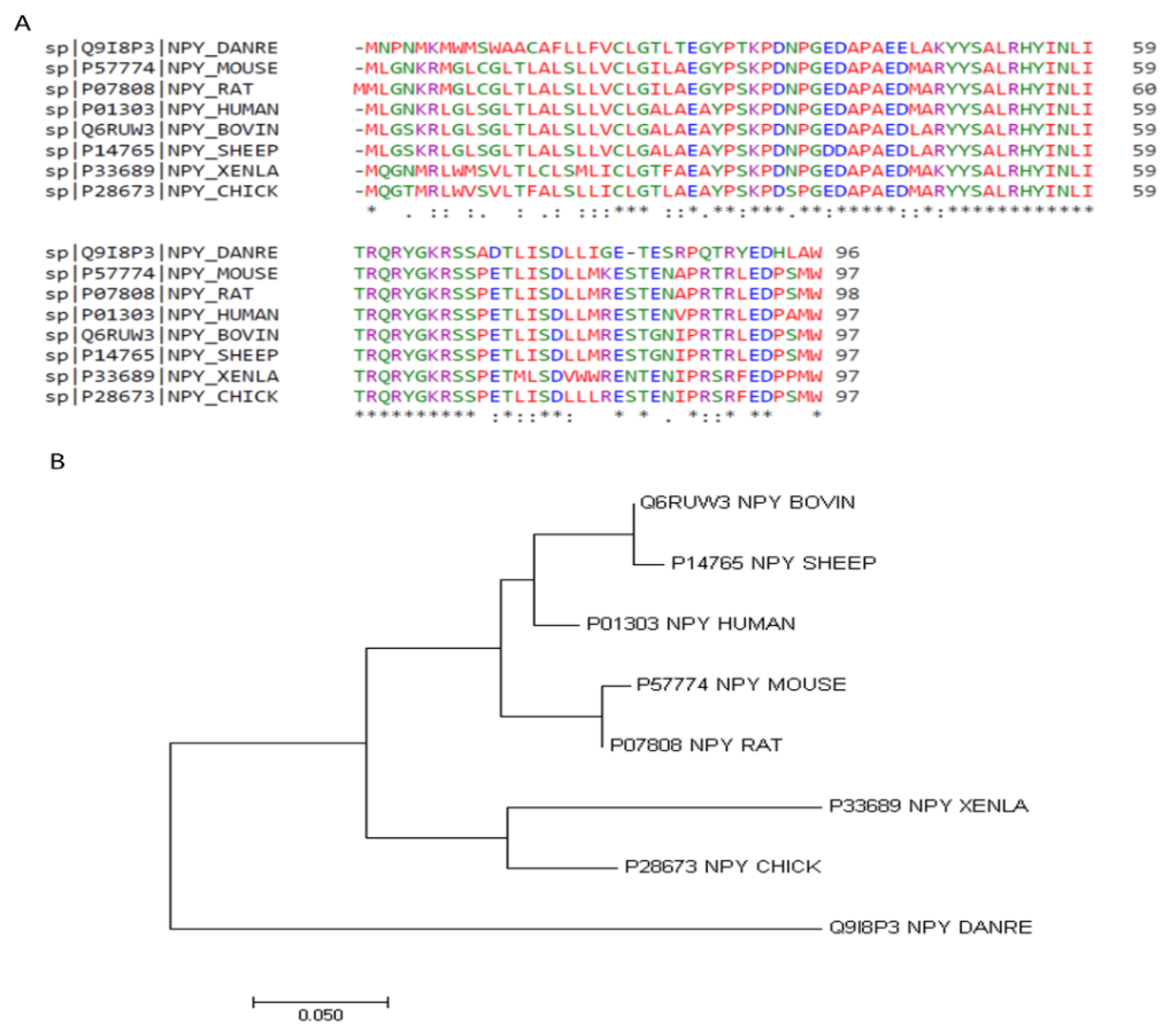
| Zebrafish | Mouse | Rat | Human | Bovine | Sheep | Xenopus | Chicken | |
|---|---|---|---|---|---|---|---|---|
| Zebrafish | 100.00 | 67.71 | 67.71 | 66.67 | 65.62 | 64.58 | 62.50 | 65.62 |
| Mouse | 67.71 | 100.00 | 98.97 | 92.78 | 90.72 | 89.69 | 74.23 | 81.44 |
| Rat | 67.71 | 98.97 | 100.00 | 93.81 | 91.75 | 90.72 | 75.26 | 82.47 |
| Human | 66.67 | 92.78 | 93.81 | 100.00 | 94.85 | 93.81 | 78.35 | 84.54 |
| Bovine | 65.62 | 90.72 | 91.75 | 94.85 | 100.00 | 98.97 | 76.29 | 84.54 |
| Sheep | 64.58 | 89.69 | 90.72 | 93.81 | 98.97 | 100.00 | 75.26 | 83.51 |
| Xenopus | 62.50 | 74.23 | 75.26 | 78.35 | 76.29 | 75.26 | 100.00 | 84.54 |
| Chicken | 65.62 | 81.44 | 82.47 | 84.54 | 84.54 | 83.51 | 84.54 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greene, E.S.; Abdelli, N.; Dridi, J.S.; Dridi, S. Avian Neuropeptide Y: Beyond Feed Intake Regulation. Vet. Sci. 2022, 9, 171. https://doi.org/10.3390/vetsci9040171
Greene ES, Abdelli N, Dridi JS, Dridi S. Avian Neuropeptide Y: Beyond Feed Intake Regulation. Veterinary Sciences. 2022; 9(4):171. https://doi.org/10.3390/vetsci9040171
Chicago/Turabian StyleGreene, Elizabeth S., Nedra Abdelli, Jalila S. Dridi, and Sami Dridi. 2022. "Avian Neuropeptide Y: Beyond Feed Intake Regulation" Veterinary Sciences 9, no. 4: 171. https://doi.org/10.3390/vetsci9040171
APA StyleGreene, E. S., Abdelli, N., Dridi, J. S., & Dridi, S. (2022). Avian Neuropeptide Y: Beyond Feed Intake Regulation. Veterinary Sciences, 9(4), 171. https://doi.org/10.3390/vetsci9040171






