Abstract
Nerve growth factor (NGF), a member of the neurotrophin family, has emerged as an active mediator in different crucial events in the peripheral and central nervous system. At the same time, several studies showed that this neurotrophin can also play a role in non-neuronal tissues (e.g., among gonads). In spite of a large number of studies present in mammals, investigations devoted to NGF and its receptor TrkA in the reproductive system of other animal models, such as teleost fish, are scarce. To increase our knowledge of NGF and its receptor in a vertebrate gonads model, the present report describes the expression patterns of ngf and trka mRNA in the testis and ovary of adult zebrafish. By using chromogenic and fluorescence in situ hybridization, we demonstrate that in the testis of adult zebrafish, ngf and its receptor trka are mainly expressed in spermatogony B and spermatocytes. In the ovary of this fish, ngf and trka are expressed at different stages of oocyte development. Altogether, these results show that this neurotrophin and its receptor have an important role in the reproductive system that is conserved during vertebrate evolution.
1. Introduction
Nerve growth factor (NGF) has been the first discovered member of the neurotrophin family [1,2]. Additional components of this group are brain derived neurotrophic factor (BDNF); neurotrophin 3 (NT3); neurotrophin 4/5 (NT4); and neurotrophin 6/7 (NT6/7) [3,4,5,6]. All neurotrophins can be synthesizes as precursors. Indeed, NGF also presents a precursor form (pro-NGF) and is either secreted outside the cells, as pro- and mature NGF, or is cleaved intracellularly to mature NGF. Both forms are active [7] and homodimers [8]. The ngf gene, as with other neurotrophins, is well conserved across vertebrate evolution, suggesting conserved functions [9,10,11,12,13]. It can bind a tyrosine kinase receptor TrkA and/or p75 neurotrophin receptor [14,15]. The binding with TrkA receptor is essential to ensure the survival of neurons and induce their growth in the peripheral and central nervous systems [16]. Otherwise, binding with p75 induces cell death. In vertebrates, this neurotrophin and TrkA receptor are also important in peripheral tissues [17,18,19,20,21,22], as reported by their presence in several organs (such as the reproductive system) [23,24]. Previous studies from human, rat, mice, wild ground squirrel, and Japanese quail have shown that this neurotrophin and TrkA receptor can fill a crucial function in the integrity of ovarian sympathetic innervation [25,26,27,28,29]. In these species, NGF and its receptor have been detected in different populations of ovary constituent cells, such as oocytes; interstitial cells within the ovary; theca; and granulosa cells [30,31,32]. It has been widely reported that NGF/TRKA signaling pathway is required to facilitate the ovulatory process and ensure the development of primordial ovarian follicles [33]. In non-mammalian vertebrates, NGF and its receptor have been found in Xenopus ovaries. Further functional studies reported that NGF treatment induced meiotic maturation of Xenopus oocytes overexpressing TrkA receptor [34]. At the same time, it has been shown that this signaling pathway appears to be involved in spermatogenesis [35]. In the testes of several mammal species, NGF has been observed in elongated spermatids, pachytene, and primary spermatocytes [36,37,38]. Differently, its receptor TrkA is present only in elongated spermatids and membranes of Leydig cells. Concerning the functional role during spermatogenesis [39] and testicular development, recent studies have shown that NGF and its receptor play an important role promoting testosterone production, proliferation, and differentiation of Leydig cells [40]. Low levels of ngf mRNA in the sperm have been associated with azoospermia in human patients. These results have been confirmed by functional investigations in rabbit and mice [41], in which the inhibition of the NGF/TrKA signaling pathway induced a specific decrease of sperm motility. More recent studies have shown that the administration of this neurotrophin can be used as a potential therapy to restore spermatogenesis in a new mice model affected by severe testicular atrophy of the seminiferous tubules [42]. In spite of a large number of studies present in mammals, investigations concerning this neurotrophin and its specific receptor in the reproductive system of other animal models, such as fish, are scarce. In the present study, we report for the first time the expression patterns of ngf and trka in the reproductive system of Danio rerio (i.e., zebrafish). This fish presents different features (small size, rapid fertilization, and fast embryonic development) making it an excellent animal model for biomedical and veterinary research. This fascinating model can be utilized in mutagenesis analyses and drug screening tests. It has been used to conduct experiments related to different research fields such as embryology, genetics, cancer, cardiovascular, and organ and tissue regeneration [43,44,45,46]. In the present report, firstly, in order to identify different cell populations in adult zebrafish testis and ovary, hematoxylin-eosin staining on paraffin sections has been carried out. Next, by using chromogenic and fluorescence in situ hybridization, it has been shown that ngf and trka mRNA were highly expressed during spermatogenesis and oogenesis of adult zebrafish, confirming their role during vertebrate evolution.
2. Material and Methods
2.1. Animals and Gonads Dissection
All experiments have been performed according to the Italian Decree 26/2014, and approved (n°2/2020-PR), by the Committee of the University of Naples Federico II. Adult females and males (1 year) of Danio rerio were anesthetized using 0.3% aminobenzoic acid-ethyl-methylester (MS222, Sigma, St. Louis, MO, USA). The ovary and testis were dissected and fixed for one day in 4% paraformaldeyde, after dehydration, included in paraffin and then sectioned. The sections were used for hematoxylin-eosin staining and in situ hybridization (for detail see Section 2.2 and Section 2.3).
2.2. Histology (Haematoxylin and Eosin Staining)
Ovary and testis were fixed in 4% paraformaldehyde in PBS for 24 h at 4 °C. Next, the tissues were washed and stored in ethanol (70%) at 4 °C. After, ovary and testis were embedded in paraffin and sectioned using a rotary microtome. The paraffin section were mounted on slides. To deparaffinize, all paraffin sections were immersed in xylene, and rehydrated in ethanol 100%, 95%, 80%, 70%, and 50%. Next, the paraffin sections were washed three times in distilled H2O (1 min). Based on the morphological features described by previous studies [47,48], to examine cell and tissue morphology of ovary and testis, it has been used hematoxylin and eosin (HE) staining.
2.3. Chromogenic and Fluorescence In Situ Hybridization (ISH)
Digoxigenin (DIG)-labeled antisense riboprobes, were prepared as reported in previous studies [49,50,51]. In detail, ngf and trka expressions were detected using in situ hybridization (ISH). To generate ngf and trka probes, have been linearized the vectors ZeroBlunt and or TOPO-TA holding the product amplified using the polymerase chain reaction. Next to produce antisense and sense ribo-probes, the plasmids were linearized with the specific enzymes. After plasmid linearization, it has been performed in vitro transcription, using SP6 and or T7 polymerase (both from Roche-Diagnostic, Chicago, IL, USA), adding the label mix digoxigenin RNA. Next, to validate the specificity of the riboprobes, the anti and sense probes were hybridized on adjacent sections (see Supplementary Figures S1 and S2). To generate ngf and trka riboprobes, have been used the following primers:
- ngf F: GGAGCACAGGAGATCTACGC and R: CGTGGAAAAACCCAACTCAT;
- trka F: AGTTGTTGCTTGCAGGGTGG and R: TGGGTCAATCATGACCTCAG.
After the production of the specific riboprobes, ovary and testis were quickly dissected and fixed in 4% paraformaldehyde, overnight at 4 °C. After 24 h, they were processed for paraffin. The slides (10 µm) were obtained using a rotary microtome and mounted on slides. To deparaffinize, all paraffin sections were immersed in xylene two times (3 min), and rehydrated in ethanol 100%, 95%, 80%, 70%, 50% (3 min each). To fix, the sections were immersed for 20 min in 4% paraformaldehyde. Then, the sections of ovary and testis immersed in PBS and adding the proteinase K diluted 2 mg/mL at room temperature for 7 min. Next, all slides were processed as follows: fixed in 4% paraformaldehyde for 20 min, washed in PBS and standard saline sodium citrate (SSC 2x) 2 times (10 min each). Next, the slides were incubated at 63 °C for 24 h, using a moist chamber with the probes (2 µg/mL) diluted in a specific medium (Denhart 5x; SSC 2x; 50% formamide; ethylenediamine-tetra acetic acid 4 mM; 5% dextran sulfate; yeast tRNA 50 µg/mL). After 24 h, the section was washed with SCC 2x; 50% formamide/SCC 2x; SSC 0.2x and SSC 0.1x. The sections were immersed in a buffer Tris-HCl/NaCl (mixing 100 mM of Tris-HCl pH 7.5 and 150 mM NaCl) and washed in the same buffer containing 0.5% milk powder and adding 0.1% Triton.
For chromogenic ISH, during the second day, all sections have been incubated with anti-digoxigenin alkaline phosphatase Fab fragments, dilution 1:5000 (Roche Diagnostic company, Chicago, IL, USA), overnight at room temperature (RT). After 24 h, all sections were washed in Tris-HCl/NaCl buffer and with 110 mM HCl-Tris (pH 8) containing 10 mM MgCl2 and 110 mM NaCl. Staining was performed using NBT/BCIP buffer (pH 9.5).
For fluorescence ISH, all slides were immersed in anti-DIG POD antibody (Roche) 1:200 dilution in the above blocking solution at room temperature for 24 h. Next, the slides were washed as follow: 4 times in maleic acid buffer (20 min each); 4 times in PBS (10 min each). Next, the sections were incubated for 1 h in a buffer for amplification (PerkinElmer company, Life Sciences, Boston, USA). For the reaction, it has been prepared Cy3 tyramide (TSA plus Cyanine 3, PerkinElmer company, Life Sciences, Boston, USA) reagent 1:100 in amplification buffer and AlexaFluor 488 reagent (TSA Reagent, Alexa Fluor 488 Tyramide Reagent, Invitrogen™, Boston, MA, USA). Next the slides have been immersed several times (10 min each), mounted with DAPI and coverslide and then observed with a confocal Nikon Eclipse 90i microscope. All pictures have been acquired using a software (NIS-Elements 4.2, Nikon, Milan, Italy).
3. Results
3.1. Morphological Analysis of Adult Zebrafish Testis
As reported also in previous morphological studies, spermatogenesis in zebrafish occurs in cysts (Figure 1a–c).
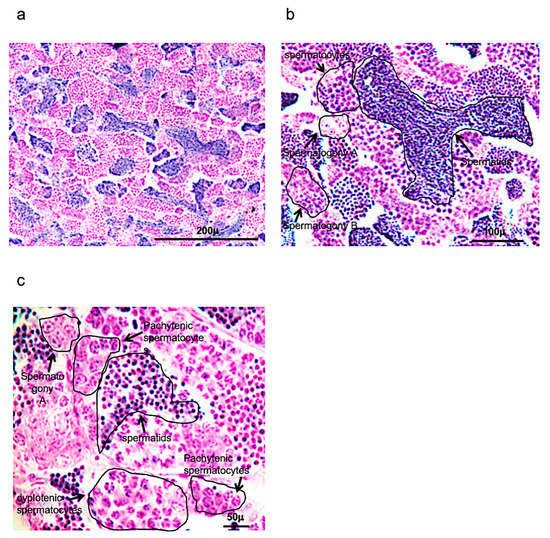
Figure 1.
Hematoxylin-eosin staining of adult zebrafish testis. (a) Overview of adult zebrafish testis by hematoxylin-eosin staining. (b) Spermatogony A-B, spermatocytes and spermatids in adult zebrafish testis by hematoxylin-eosin staining. (c) High magnification of spermatogony A, spermatocytes (different phases of meiosis) and spermatids in adult zebrafish testis by hematoxylin-eosin staining. Scale bars are: 200 µ (a); 100 µ (b); and 50 µ (c).
These cysts can be formed after several Sertoli cells are positioned around the spermatogonium in the cytoplasm. Numerous cysts can be observed, characterized by spermatogonia A (round shape), presenting a different number of nucleoli (one, two or more); spermatogonia B, presenting a rising presence of heterochromatin in elongated and/or round nuclei (it can also be present small nucleoli); spermatocytes, which can be identified by several steps of meiotic division, observing the size of the nucleus and chromosome condensation; and early, intermediate, and mature spermatids, which present a significant decrease of the cellular and nuclear volumes (Figure 1a–c).
3.2. Ngf and Its Receptor trka Are Expressed in Adult Zebrafish Testis
Next, it has been performed chromogenic and fluorescence in situ hybridization, to identify the expression patterns of ngf and its receptor trka, in adult zebrafish testis.
Ngf was mainly expressed in spermatogony B and spermatocytes (dyplotenic phase) (Figure 2a–f). These results have been confirmed by using confocal microscopy with high magnification (Figure 3a,b).
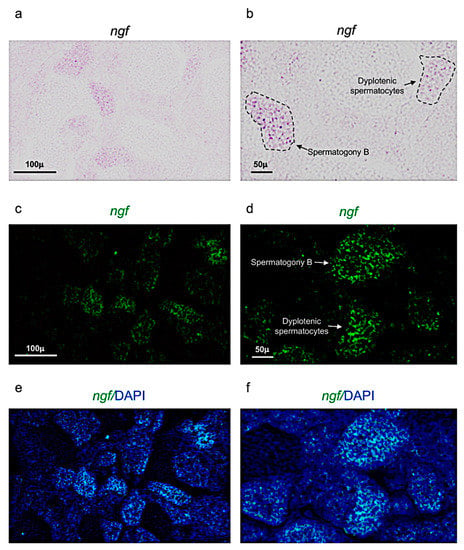
Figure 2.
Ngf expression pattern in adult zebrafish testis. (a,b) Chromogenic in situ hybridization of ngf in adult zebrafish testis. (c,d) Fluorescence in situ hybridization of ngf in adult zebrafish testis. (e,f) Fluorescence in situ hybridization of ngf and cell nuclei (DAPI), in adult zebrafish testis. Scale bars are: 100 µ (a,c,e); 50 µ (b,d,f).
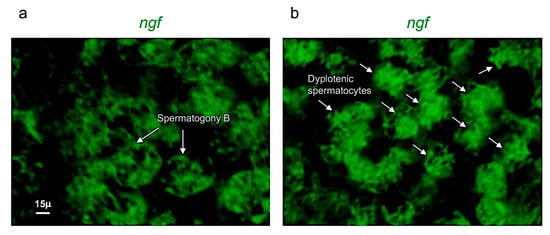
Figure 3.
Ngf is specifically expressed in spermatogony B and dyplotenic spermatocytes. High magnification, fluorescence in situ hybridization of ngf, specifically expressed in (a) spermatogony B and in (b) dyplotenic spermatocytes in adult zebrafish testis. Scale bar: 15 µ (a,b).
Concerning the receptor trka, it was expressed in three different cell populations: spermatogony type A, B and dyplotenic spermatocytes of adult zebrafish testis (Figure 4a–f). These results were confirmed by using confocal microscopy with high magnification (Figure 5a–c).
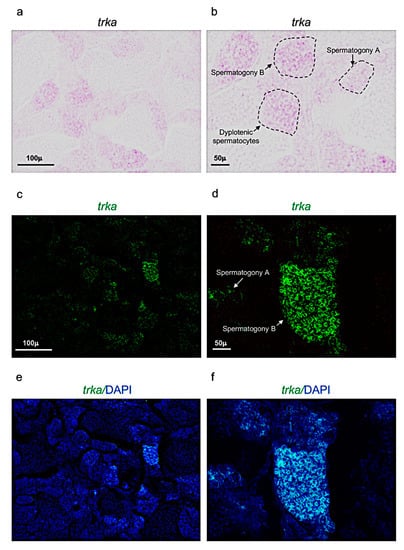
Figure 4.
Trka expression pattern in adult zebrafish testis. (a,b) Chromogenic in situ hybridization of trka in adult zebrafish testis. (c,d) Fluorescence in situ hybridization of trka in adult zebrafish testis. (e,f) Fluorescence in situ hybridization of trka and cell nuclei (DAPI) in adult zebrafish testis. Scale bars: 100 µ (a,c,e); 50 µ (b,d,f).
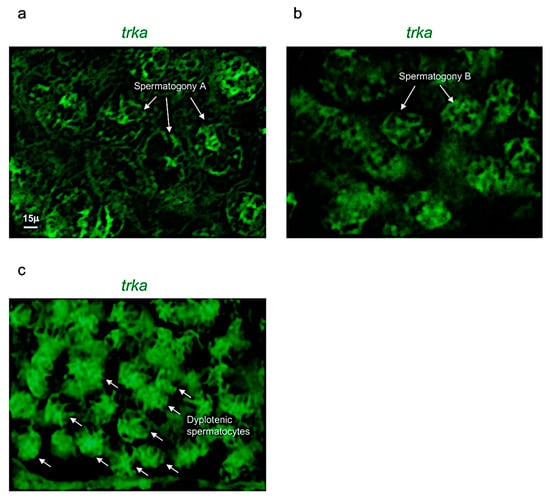
Figure 5.
Trka is specifically expressed in spermatogony A, B and dyplotenic spermatocytes. High magnification, fluorescence in situ hybridization of ngf, specifically expressed in (a) spermatogony A; (b) spermatogony B; and (c) dyplotenic spermatocytes in adult zebrafish testis. Scale bar: 15 µ (a–c).
3.3. Morphological Analysis of Zebrafish Ovary
As reported in previous studies, one can identify five different oocyte stages in zebrafish ovaries. (Figure 6a–h).
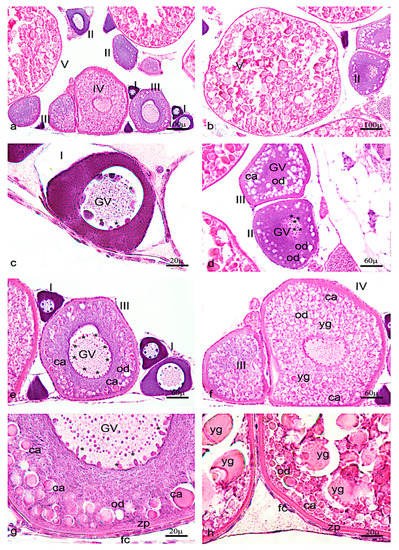
Figure 6.
Hematoxylin-eosin staining of adult zebrafish ovary. (a) Overview of adult zebrafish ovary by hematoxylin-eosin staining, five oocyte stages (I-II-II-IV-V). (b) Oocyte stages II and V. (c) Oogonia characterized by large euchromatic germinal vescicle (GV), and several nucleoli peripherically located. (d) Oocyte during primary growth (stages II–III), characterized by an increase of nucleoli in GV, and in ooplasma were present oil droplets (od) around the GV. (e) Oocyte (stages III) characterized by numerous nucleoli at the periphery of GV. (f) numerous oil droplets and cortical alveoli (ca). (g) The oocyte (stages IV) is enveloped by zona pellucida (zp) and a single layer of follicular cells (fc). (h) Oocyte (stage V) characterized by a significant increase of number and size of the yolk globules (yg). Significant increase in the thickness of the zona pellucida and appearance of thecal layer. Scale bars are: 100 µ (a,b); 60 µ (d–f); 20 µ (c,g,h).
3.4. Ngf and Its Receptor Trka Are Expressed in Adult Zebrafish Ovary
Next, chromogenic and fluorescence in situ hybridization has been used to identify the expression patterns of ngf and its receptor trka in adult zebrafish ovaries.
Ngf was mainly expressed in the perinuclear cytoplasm of oocytes at the stage I, II, and III (Figure 7a,b). At the stage IV and V, ngf was expressed in the follicular cells located around the oocyte (Figure 7c,d). The receptor trka was more expressed in the perinuclear cytoplasm of oocytes at the stages II and III (Figure 8a,b). As ngf, the receptor trka was also expressed in follicular cells of oocyte at the stage V (Figure 8c,d).
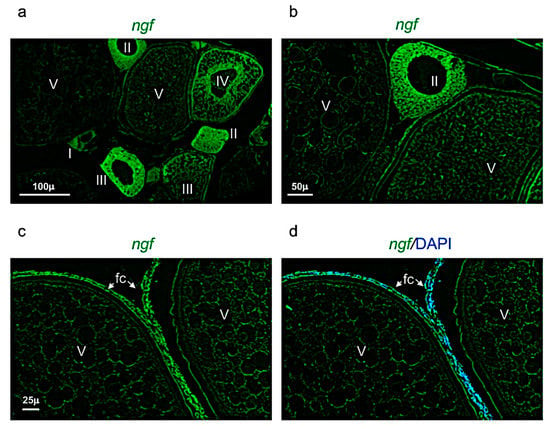
Figure 7.
Ngf expression pattern in adult zebrafish ovary. (a) Fluorescence in situ hybridization of ngf in adult zebrafish ovary, mainly expressed in oocyte at different stages: II-III-IV and in follicular cells of stage V. (b) Fluorescence in situ hybridization of ngf in oocytes at II and V stages. (c) Fluorescence in situ hybridization of ngf, high magnification of follicular cells in oocyte at stage V. (d) Fluorescence in situ hybridization of ngf and cell nuclei (DAPI), high magnification of follicular cells in oocyte at stage V. Scale bars: 100 µ (a); 50 µ (b); and 25 µ (c,d).
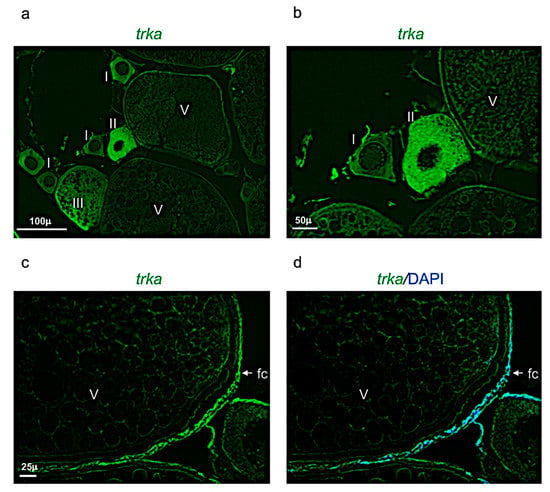
Figure 8.
Trka expression pattern in adult zebrafish ovary. (a) Fluorescence in situ hybridization of trka in adult zebrafish ovary, mainly expressed in oocytes at different stages: II, III, and in follicular cells of stage V. (b) Fluorescence in situ hybridization of trka in oocytes at the stages I, II, and V. (c) Fluorescence in situ hybridization of trka, high magnification of follicular cells in oocyte at stage V. (d) Fluorescence in situ hybridization of ngf and cell nuclei (DAPI), high magnification of follicular cells in oocyte at stage V. Scale bars: 100 µ (a); 50 µ (b); and 25 µ (c,d).
4. Discussion
For the first time, in the present report, the expression patterns of ngf and its receptor trka in the reproductive system of adult zebrafish have been described. Firstly, it has been shown that ngf and trka are specifically expressed in spermatogony A and/or B and dyplotenic spermatocytes. Therefore, assuming that they have an important role during the spermatogenesis in the testis of adult zebrafish. Previous observations in rat and mice models reported that NGF was expressed in elongated spermatids, pachytene, and primary spermatocytes. In contrast, its receptor TrkA has been detected only in the membranes of Leydig cells and elongated spermatids [52,53,54]. In addition, recent studies reported that NGF and TrkA can stimulate sperm motility and induce sperm cell acrosome reactions. Furthermore, NGF plays an important role promoting testosterone production, proliferation and differentiation of Leydig cells [55,56]. Similar findings were also reported in human, monkey and rabbit. Differently, in mature alpacas, NGF protein was mainly express in the perinuclear cytoplasm of stromal cells, Sertoli cells and germ cells [57]. On the evidence that NGF and its receptor TrkA have been specifically detected in the germinal and/or endocrine cells of the testis, it has been speculated that NGF can play an autocrine and/or paracrine signaling during testicular development and spermatogenesis. In detail, it has been reported that the synthesis of NGF occurs in somatic cells (Leyding), after this neurotrophin can bind the receptor TrkA expressed in germ cells, such as; spermatocytes and spermatids, regulating the different stages of the spermatogenesis in rat and ground squirrel. This result has been observed also in patients affected by azoospermia, all of whom presented low levels of NGF [58,59,60]. These findings have been confirmed by functional investigations in rabbit and mice [61], in which the inhibition of the NGF/TrKA signaling pathway induced a specific decrease of sperm motility. More recent study also reported that the administration of this neurotrophin can be used as a potential therapy to restore spermatogenesis in a new mice model affected by severe testicular atrophy (and characterized by the loss of germ cells).
With regard to the expression of ngf and trka in the ovaries of adult zebrafish, the results show that they are expressed in oocytes at different stages. In particular, ngf and its receptor are detected in oocytes at stages II–III and in follicular cells of oocytes at stages IV and/or V. Previous studies in different species show that NGF and its receptor play a pivotal role during the differentiation of primordial ovarian follicles and the growth of the latter in secondary follicles. In detail, it has been shown that NGF/TrkA signaling pathway induced the proliferation of theca and granulosa cells, increasing the synthesis of the follicular stimulating hormone (FSH) receptor in granulosa cells. Concerning the ovulation, the NGF/TrkA pathway is highly involved in this process. Indeed, this pathway, in pre-ovulatory follicle, can modulate the release of prostaglandin-E2 (PGE2), inhibiting the expression of gap-junctions and increasing the proliferation of theca cell [62,63,64]. High levels of NGF were reported in goat and wild ground squirrel ovaries. In human ovary [65], NGF was detected in the oocytes and granulosa cells of preantral follicles, from the primordial to the secondary stages [66]. Further studies showed that human fetal oocytes presented mostly full cytoplasmic staining for NGF, whereas oocytes from adults exhibited mostly partial cytoplasmic and nuclear staining. At the same time, in vitro studies showed that treatment can inhibit the apoptosis by down-regulating apoptotic genes in mouse ovary. The dynamic expression of NGF and its receptor TrkA, have been associated with a luteotropic effect of ovulation-inducing factor in bovine ovary [24]. These results have been confirmed also in other species such as cows and pigs [67]. Most important is that these findings have been also confirmed in non-mammalian vertebrates (such as Xenopus). In this species, NGF and its receptor have been detected in the ovary, during early stages of oocytes maturation [34]. Indeed, functional studies showed that NGF administration induced meiotic maturation of Xenopus oocytes overexpressing TrkA receptor.
5. Conclusions
This study, for the first time, provides a precise and comprehensive description of the expression patterns of ngf and its receptor trka in the reproductive systems of an adult teleost fish, zebrafish. This report showed that ngf and its receptor are mainly stored in spermatogonia and spermatocytes in this fish, as reported in previous data from different mammal species. At the same time, this study provides new results concerning ngf and trka, both are mostly expressed during early stages and in follicular cells (stage V) of oogenesis in fish. Taken altogether these results show that this neurotrophin and its receptor have an important role in the reproductive system which is conserved during vertebrate evolution.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci9050225/s1, Figure S1: ngf and trks sense probes staining in testi; Figure S2: ngf and trka sense probe staining in ovary.
Funding
This research received no external funding. The open access is supported by the reviewer activity of Pietro Cacialli.
Institutional Review Board Statement
All experiments have been performed according to the Italian Decree 26/2014, and approved (approved number, n°2/2020-PR) by the Committee of the University of Naples Federico II.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author thanks Carla Lucini, Paolo de Girolamo and Livia D’angelo for reading the manuscript.
Conflicts of Interest
The author declares no conflict of interest.
References
- Montalcini, R.L. Laboratory of Cellular Biology. Department of Neurobiology, Rome. Ric. Sci. 1970, 40, 296–298. [Google Scholar] [PubMed]
- Aloe, L. Rita Levi-Montalcini and the discovery of NGF, the first nerve cell growth factor. Arch. Ital. Biol. 2011, 149, 175–181. [Google Scholar] [PubMed]
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- McPhee, G.M.; Downey, L.A.; Stough, C. Neurotrophins as a reliable biomarker for brain function, structure and cognition: A systematic review and meta-analysis. Neurobiol. Learn. Mem 2020, 175, 107298. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Chao, M.V. Dependence receptors: What is the mechanism? Sci. STKE 2003, 2003, PE38. [Google Scholar] [CrossRef]
- Chao, M.V. Retrograde transport redux. Neuron 2003, 39, 1–2. [Google Scholar] [CrossRef]
- Hallbook, F.; Wilson, K.; Thorndyke, M.; Olinski, R.P. Formation and evolution of the chordate neurotrophin and Trk receptor genes. Brain Behav. Evol. 2006, 68, 133–144. [Google Scholar] [CrossRef]
- Hallbook, F. Evolution of the vertebrate neurotrophin and Trk receptor gene families. Curr. Opin. Neurobiol. 1999, 9, 616–621. [Google Scholar] [CrossRef]
- Cacialli, P.; Gatta, C.; D’Angelo, L.; Leggieri, A.; Palladino, A.; de Girolamo, P.; Pellegrini, E.; Lucini, C. Nerve growth factor is expressed and stored in central neurons of adult zebrafish. J. Anat. 2019, 235, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Cacialli, P.; Lucini, C. Adult neurogenesis and regeneration in zebrafish brain: Are the neurotrophins involved in? Neural Regen. Res. 2019, 14, 2067–2068. [Google Scholar] [PubMed]
- Cacialli, P. Neurotrophins Time Point Intervention after Traumatic Brain Injury: From Zebrafish to Human. Int. J. Mol. Sci. 2021, 22, 1585. [Google Scholar] [CrossRef] [PubMed]
- Hartman, D.S.; McCormack, M.; Schubenel, R.; Hertel, C. Multiple trkA proteins in PC12 cells bind NGF with a slow association rate. J. Biol. Chem. 1992, 267, 24516–24522. [Google Scholar] [CrossRef]
- Jung, K.M.; Tan, S.; Landman, N.; Petrova, K.; Murray, S.; Lewis, R.; Kim, P.K.; Kim, D.S.; Ryu, S.H.; Chao, M.V.; et al. Regulated intramembrane proteolysis of the p75 neurotrophin receptor modulates its association with the TrkA receptor. J. Biol. Chem. 2003, 278, 42161–42169. [Google Scholar] [CrossRef]
- Allsopp, T.E.; Robinson, M.; Wyatt, S.; Davies, A.M. TrkA mediates an NGF survival response in NGF-independent sensory neurons but not in parasympathetic neurons. Gene Ther. 1994, 1 (Suppl. S1), S59. [Google Scholar] [CrossRef]
- Arcamone, N.; Lucini, C.; Borzacchiello, G.; Castaldo, L.; Gargiulo, G.; De Girolamo, P. Distribution of NGF and NT-3-like protein immunoreactivity in the teleost kidney. Microsc. Res. Tech. 2005, 66, 17–24. [Google Scholar] [CrossRef]
- Russo, M.A.; Giustizieri, M.L.; Farini, D.; Siracusa, G. Expression of neurotrophin receptors in the developing and adult testis. Ital. J. Anat. Embryol. 1995, 100 (Suppl. S1), 543–551. [Google Scholar]
- Ji, A.; Shu, S.; Li, M.; Bao, X.; Zou, H.; Zhang, Z. Expression of recombinant rat Neurotrophin-3 in Chinese hamster ovary cells. Sci. China C Life Sci. 1999, 42, 655–662. [Google Scholar] [CrossRef]
- Iwane, M.; Watanabe, T.; Shintani, A.; Kaisho, Y.; Matsumoto, S.; Sasada, R.; Igarashi, K. Purification and characterization of biologically active recombinant human neurotrophin-3 produced by expression of a chimera gene in Chinese hamster ovary cells. Appl. Microbiol. Biotechnol. 1994, 41, 225–232. [Google Scholar] [CrossRef]
- Russo, M.A.; Giustizieri, M.L.; Farini, D.; Campagnolo, L.; De Felici, M.; Siracusa, G. Expression of the p75 neurotrophin receptor in the developing and adult testis of the rat. Int. J. Dev. Biol. 2001, 40 (Suppl. S1), 227S–228S. [Google Scholar]
- D’Angelo, L.; de Girolamo, P.; Cellerino, A.; Tozzini, E.T.; Castaldo, L.; Lucini, C. Neurotrophin Trk receptors in the brain of a teleost fish, Nothobranchius furzeri. Microsc. Res. Tech. 2012, 75, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Persson, H. NGF remove grower factors and neurotransmitters in the testis--new discoveries in reproduction biology. Lakartidningen 1990, 87, 4193–4196. [Google Scholar] [PubMed]
- Carrasco, R.; Singh, J.; Adams, G.P. The dynamics of trkA expression in the bovine ovary are associated with a luteotrophic effect of ovulation-inducing factor/nerve growth factor (OIF/NGF). Reprod. Biol. Endocrinol. 2016, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Julio-Pieper, M.; Lara, H.E.; Bravo, J.A.; Romero, C. Effects of nerve growth factor (NGF) on blood vessels area and expression of the angiogenic factors VEGF and TGFbeta1 in the rat ovary. Reprod. Biol. Endocrinol. 2006, 4, 57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bao, L.; Li, Q.; Liu, Y.; Li, B.; Sheng, X.; Han, Y.; Weng, Q. Immunolocalization of NGF and its receptors in ovarian surface epithelium of the wild ground squirrel during the breeding and nonbreeding seasons. Eur. J. Histochem. 2014, 58, 2363. [Google Scholar] [CrossRef]
- Abir, R.; Fisch, B.; Jin, S.; Barnnet, M.; Ben-Haroush, A.; Felz, C.; Kessler-Icekson, G.; Feldberg, D.; Nitke, S.; Ao, A. Presence of NGF and its receptors in ovaries from human fetuses and adults. Mol. Hum. Reprod. 2005, 11, 229–236. [Google Scholar] [CrossRef]
- Maruccio, L.; Lucini, C.; de Girolamo, P.; Avallone, L.; Solcan, C.; Nechita, L.E.; Castaldo, L. Neurotrophins and Trk receptors in the developing and adult ovary of Coturnix coturnix japonica. Ann. Anat. 2018, 219, 35–43. [Google Scholar] [CrossRef]
- Schultz, R.; Metsis, M.; Hokfelt, T.; Parvinen, M.; Pelto-Huikko, M. Expression of neurotrophin receptors in rat testis. Upregulation of TrkA mRNA with hCG treatment. Mol. Cell Endocrinol. 2001, 182, 121–127. [Google Scholar] [CrossRef]
- Dees, W.L.; Hiney, J.K.; Schultea, T.D.; Mayerhofer, A.; Danilchik, M.; Dissen, G.A.; Ojeda, S.R. The primate ovary contains a population of catecholaminergic neuron-like cells expressing nerve growth factor receptors. Endocrinology 1995, 136, 5760–5768. [Google Scholar] [CrossRef]
- Zhu, G.; Fang, C.; Mo, C.; Wang, Y.; Huang, Y.; Li, J. Transcriptomic analysis of granulosa cell populations proximal and distal to the germinal disc of chicken preovulatory follicles. Sci. Rep. 2021, 11, 4683. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, M.; Arancibia, S.; Fiedler, J.L.; Lara, H.E. Chronic intermittent cold stress activates ovarian sympathetic nerves and modifies ovarian follicular development in the rat. Biol. Reprod. 2003, 68, 2038–2043. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y. Effect of nerve growth factor (NGF) on the development of preimplantation rabbit embryos in vitro. Vet. Res. Commun. 2010, 34, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Nebreda, A.R.; Martin-Zanca, D.; Kaplan, D.R.; Parada, L.F.; Santos, E. Induction by NGF of meiotic maturation of Xenopus oocytes expressing the trk proto-oncogene product. Science 1991, 252, 558–561. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Zhang, J.; Wang, L.; Li, Q.; Sheng, X.; Han, Y.; Yuan, Z.; Weng, Q. Testicular expression of NGF, TrkA and p75 during seasonal spermatogenesis of the wild ground squirrel (Citellus dauricus Brandt). Eur. J. Histochem. 2015, 59, 2522. [Google Scholar] [CrossRef]
- Schachter, M.; Peret, M.W.; Moriwaki, C.; Wheeler, G.D.; Matthews, R.W.; Mehta, J.G.; Labedz, T. The varied localization and functional significance of kallikrein-like enzymes in salivary glands, pancreas, colon, sex glands and spermatozoa, including evidence for the presence of nerve growth factor (NGF) in bull sperm acrosome. Adv. Exp. Med. Biol. 1986, 198 Pt A, 1–10. [Google Scholar]
- Li, C.; Sun, Y.; Yi, K.; Ma, Y.; Sun, Y.; Zhang, W.; Zhou, X. Detection of nerve growth factor (NGF) and its specific receptor (TrkA) in ejaculated bovine sperm, and the effects of NGF on sperm function. Theriogenology 2010, 74, 1615–1622. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, A.; Arias-Alvarez, M.; Timon, P.; Bautista, J.M.; Rebollar, P.G.; Lorenzo, P.L.; Garcia-Garcia, R.M. Characterization of beta-Nerve Growth Factor-TrkA system in male reproductive tract of rabbit and the relationship between beta-NGF and testosterone levels with seminal quality during sexual maturation. Theriogenology 2019, 126, 206–213. [Google Scholar] [CrossRef]
- Castellini, C.; Mattioli, S.; Dal Bosco, A.; Mancinelli, A.C.; Rende, M.; Stabile, A.M.; Pistilli, A. Role of NGF on sperm traits: A review. Theriogenology 2020, 150, 210–214. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Yang, Y.; Liu, H.; Zhang, Q.; Xiang, Q.; Ge, R.; Su, Z.; Huang, Y. NGF induces adult stem Leydig cells to proliferate and differentiate during Leydig cell regeneration. Biochem. Biophys. Res. Commun. 2013, 436, 300–305. [Google Scholar] [CrossRef]
- Castellini, C.; Mattioli, S.; Cotozzolo, E.; Pistilli, A.; Rende, M.; Bartolini, D.; Di Sante, G.; Menchetti, L.; Dal Bosco, A.; Stabile, A.M. The Effect of Interaction NGF/p75(NTR) in Sperm Cells: A Rabbit Model. Cells 2022, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, Y.; Ji, X.; He, W.; Fan, J.; Huang, Y.; Wang, Y. NGF Rescues Spermatogenesis in Azoospermic Mice. Reprod. Sci. 2021, 28, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Confino, S.; Dor, T.; Tovin, A.; Wexler, Y.; Ben-Moshe Livne, Z.; Kolker, M.; Pisanty, O.; Park, S.K.; Geyer, N.; Reiter, J.; et al. A Zebrafish Model for a Rare Genetic Disease Reveals a Conserved Role for FBXL3 in the Circadian Clock System. Int. J. Mol. Sci. 2022, 23, 2373. [Google Scholar] [CrossRef] [PubMed]
- Bowley, G.; Kugler, E.; Wilkinson, R.; Lawrie, A.; van Eeden, F.; Chico, T.J.A.; Evans, P.C.; Noel, E.S.; Serbanovic-Canic, J. Zebrafish as a tractable model of human cardiovascular disease. Br. J. Pharmacol. 2022, 179, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, G.; Usai, A.; Funel, N.; Palmeri, M.; Montesanti, I.E.R.; Bianchini, M.; Gianardi, D.; Furbetta, N.; Guadagni, S.; Vasile, E.; et al. Use of zebrafish embryos as avatar of patients with pancreatic cancer: A new xenotransplantation model towards personalized medicine. World J. Gastroenterol. 2020, 26, 2792–2809. [Google Scholar] [CrossRef] [PubMed]
- Cacialli, P.; D’Angelo, L.; Kah, O.; Coumailleau, P.; Gueguen, M.M.; Pellegrini, E.; Lucini, C. Neuronal expression of brain derived neurotrophic factor in the injured telencephalon of adult zebrafish. J. Comp. Neurol. 2018, 526, 569–582. [Google Scholar] [CrossRef]
- Menke, A.L.; Spitsbergen, J.M.; Wolterbeek, A.P.; Woutersen, R.A. Normal anatomy and histology of the adult zebrafish. Toxicol. Pathol. 2011, 39, 759–775. [Google Scholar] [CrossRef]
- Cacialli, P.; D’Angelo, L.; de Girolamo, P.; Avallone, L.; Lucini, C.; Pellegrini, E.; Castaldo, L. Morpho-Functional Features of the Gonads of Danio rerio: The Role of Brain-Derived Neurotrophic Factor. Anat Rec. 2018, 301, 140–147. [Google Scholar] [CrossRef]
- Cacialli, P.; Mahony, C.B.; Petzold, T.; Bordignon, P.; Rougemont, A.L.; Bertrand, J.Y. A connexin/ifi30 pathway bridges HSCs with their niche to dampen oxidative stress. Nat. Commun. 2021, 12, 4484. [Google Scholar] [CrossRef]
- Mahony, C.B.; Cacialli, P.; Pasche, C.; Monteiro, R.; Savvides, S.N.; Bertrand, J.Y. Hapln1b, a central organizer of the ECM, modulates kit signaling to control developmental hematopoiesis in zebrafish. Blood Adv. 2021, 5, 4935–4948. [Google Scholar] [CrossRef]
- Russo, B.; Borowczyk, J.; Cacialli, P.; Moguelet, P.; Truchetet, M.E.; Modarressi, A.; Brembilla, N.C.; Bertrand, J.; Boehncke, W.H.; Chizzolini, C. IL-25 participates in keratinocyte-driven dermal matrix turnover and is reduced in Systemic Sclerosis epidermis. Rheumatology 2022, keac044. [Google Scholar] [CrossRef] [PubMed]
- Perrard, M.H.; Chassaing, E.; Montillet, G.; Sabido, O.; Durand, P. Cytostatic factor proteins are present in male meiotic cells and beta-nerve growth factor increases mos levels in rat late spermatocytes. PLoS ONE 2009, 4, e7237. [Google Scholar] [CrossRef] [PubMed]
- Sari, L.M.; Zampini, R.; Arganaraz, M.E.; Carretero, M.I.; Fumuso, F.G.; Barraza, D.E.; Ratto, M.; Apichela, S.A. Expression of beta-NGF and high-affinity NGF receptor (TrKA) in llama (Lama glama) male reproductive tract and spermatozoa. Mol. Reprod. Dev. 2018, 85, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Perrard, M.H.; Durand, P. Redundancy of the effect of TGFbeta1 and beta-NGF on the second meiotic division of rat spermatocytes. Microsc. Res. Tech. 2009, 72, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Jiang, Y.W.; Chen, S.X.; Li, H.J.; Chen, L.; Liu, Y.T.; Gao, S.; Zhao, Y.; Zhu, X.L.; Wang, H.T.; et al. 4-Methylcatechol inhibits cell growth and testosterone production in TM3 Leydig cells by reducing mitochondrial activity. Andrologia 2017, 49, e12581. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Liu, Y.H.; Huang, S.Y.; Zang, Z.J. Insights into the Regulation on Proliferation and Differentiation of Stem Leydig Cells. Stem Cell Rev. Rep. 2021, 17, 1521–1533. [Google Scholar] [CrossRef]
- Silva, M.; Paiva, L.; Ratto, M.H. Ovulation mechanism in South American Camelids: The active role of beta-NGF as the chemical signal eliciting ovulation in llamas and alpacas. Theriogenology 2020, 150, 280–287. [Google Scholar] [CrossRef]
- Saeednia, S.; Bahadoran, H.; Amidi, F.; Asadi, M.H.; Naji, M.; Fallahi, P.; Nejad, N.A. Nerve growth factor in human semen: Effect of nerve growth factor on the normozoospermic men during cryopreservation process. Iran J. Basic Med. Sci. 2015, 18, 292–299. [Google Scholar]
- Saeednia, S.; Shabani Nashtaei, M.; Bahadoran, H.; Aleyasin, A.; Amidi, F. Effect of nerve growth factor on sperm quality in asthenozoosprmic men during cryopreservation. Reprod. Biol. Endocrinol. 2016, 14, 29. [Google Scholar] [CrossRef]
- Li, C.; Zheng, L.; Wang, C.; Zhou, X. Absence of nerve growth factor and comparison of tyrosine kinase receptor A levels in mature spermatozoa from oligoasthenozoospermic, asthenozoospermic and fertile men. Clin. Chim. Acta 2010, 411, 1482–1486. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, A.; Abad, P.; Arias-Alvarez, M.; Rebollar, P.G.; Bautista, J.M.; Lorenzo, P.L.; Garcia-Garcia, R.M. Recombinant rabbit beta nerve growth factor production and its biological effects on sperm and ovulation in rabbits. PLoS ONE 2019, 14, e0219780. [Google Scholar]
- Garrido, M.P.; Vallejos, C.; Girardi, S.; Gabler, F.; Selman, A.; Lopez, F.; Vega, M.; Romero, C. NGF/TRKA Promotes ADAM17-Dependent Cleavage of P75 in Ovarian Cells: Elucidating a Pro-Tumoral Mechanism. Int. J. Mol. Sci. 2022, 23, 2124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, W.; Du, J.; Yu, Y.; Liang, N.; Liang, M.; Yao, G.; Cui, S.; Huang, H.; Sun, F. NGF promotes mouse granulosa cell proliferation by inhibiting ESR2 mediated down-regulation of CDKN1A. Mol. Cell Endocrinol. 2015, 406, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sheng, X.; Bao, L.; Huang, S.; Li, Q.; Liu, Y.; Han, Y.; Watanabe, G.; Taya, K.; Weng, Q. Seasonal changes in expression of nerve growth factor and its receptors TrkA and p75 in the ovary of wild ground squirrel (Citellus dauricus Brandt). J. Ovarian Res. 2014, 7, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salas, C.; Julio-Pieper, M.; Valladares, M.; Pommer, R.; Vega, M.; Mastronardi, C.; Kerr, B.; Ojeda, S.R.; Lara, H.E.; Romero, C. Nerve growth factor-dependent activation of trkA receptors in the human ovary results in synthesis of follicle-stimulating hormone receptors and estrogen secretion. J. Clin. Endocrinol. Metab. 2006, 91, 2396–2403. [Google Scholar] [CrossRef]
- Valderrama, X.; Ulloa-Leal, C.; Silva, M.E.; Goicochea, J.; Apichela, S.; Arganaraz, M.; Sari, L.; Paiva, L.; Ratto, V.F.; Ratto, M.H. beta-NGF Stimulates Steroidogenic Enzyme and VEGFA Gene Expression, and Progesterone Secretion via ERK 1/2 Pathway in Primary Culture of Llama Granulosa Cells. Front. Vet. Sci. 2020, 7, 586265. [Google Scholar] [CrossRef]
- Levanti, M.B.; Germana, A.; Abbate, F.; Montalbano, G.; Vega, J.A.; Germana, G. TrkA and p75NTR in the ovary of adult cow and pig. J. Anat. 2005, 207, 93–96. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).