Welfare Assessment Tools in Zoos: From Theory to Practice
Abstract
:1. Introduction
2. Review Methodology
3. Defining Welfare and Emotion
4. Frameworks and Protocols Utilized to Inform Welfare Assessment in Zoo Animals
4.1. Five Domains Model for Animal Welfare Assessment
4.2. The European Welfare Quality® Animal Welfare Assessment Protocol
4.3. Zoo-Specific Welfare Assessment Programs
| Assessment Framework | Features | References and Species Examined |
|---|---|---|
| Five Domains | Criteria listed under 4 physical domains:
| Multiple species [3] |
| European Welfare Quality® | 4 principles:
| Bottlenose Dolphins [36] Dorcas Gazelles [37] Pygmy Blue-tongue Skink [10] |
| Universal Animal Welfare Framework | Four components:
| None-overarching philosophy [16] |
| Opportunities to Thrive program | Flips the Five Freedoms to transform them to focus on attainment of positive affect:
| Hawaiian Endangered Birds-multiple species [38] |
| Animal Welfare Assessment Grid | Four components:
| Zoo primates and birds [43] Giraffe, Scimitar horned oryx and large felids (tigers, leopards and cheetahs) [13] Western Lowland Gorillas [44] |
5. Derivation of Welfare Indicators
5.1. Qualitative Behavior Assessment
5.2. Behavioral Diversity
5.3. Cognitive Bias Assessments
5.4. Delphi Consensus Methods
6. Important Features of Welfare Assessment Tools for Zoos
Tool Features: Validity, Reliability, Practicality
7. Factors for Consideration in Development of Welfare Assessment Tools
7.1. Animal-Based Considerations
7.2. Scoring Methodology
8. Application
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Phillips, C.; Izmirli, S.; Aldavood, S.; Alonso, M.; Choe, B.; Hanlon, A.; Handziska, A.; Illmann, G.; Keeling, L.; Kennedy, M. Students’ attitudes to animal welfare and rights in europe and asia. Anim. Welf. 2012, 21, 87. [Google Scholar] [CrossRef] [Green Version]
- Coleman, G. Public animal welfare discussions and outlooks in australia. Anim. Front. 2018, 8, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherwen, S.; Hemsworth, L.; Beausoleil, N.; Embury, A.; Mellor, D. An animal welfare risk assessment process for zoos. Animals 2018, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Maple, T.L.; Perdue, B.M. Zoo Animal Welfare; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- About AZA Accreditation|Association of Zoos & Aquariums. 2021. Available online: https://www.aza.org/what-is-accreditation (accessed on 28 April 2021).
- Zoo Aquarium Association Australasia. Home. 2021. Available online: https://www.zooaquarium.org.au/public/Home/Public/Default.aspx?hkey=72fe7386-d3c1-4f5a-9dc0-d7b565124e04 (accessed on 28 April 2021).
- Mellor, D.; Beausoleil, N.; Littlewood, K.; McLean, A.; McGreevy, P.; Jones, B.; Wilkins, C. The 2020 five domains model: Including human-animal interactions in assessments of animal welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, A.L.; Marsh, L.E. The role of behavioural assessment in determining ‘positive’ affective states in animals. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2019, 14, 1–13. [Google Scholar] [CrossRef]
- Broom, D. Animal welfare: Concepts and measurement. J. Anim. Sci. 1991, 69, 4167–4175. [Google Scholar] [CrossRef]
- Benn, A.; McLelland, D.; Whittaker, A. A review of welfare assessment methods in reptiles, and preliminary application of the welfare quality® protocol to the pygmy blue-tongue skink, tiliqua adelaidensis, using animal-based measures. Animals 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Whitham, J.C.; Wielebnowski, N. New directions for zoo animal welfare science. Appl. Anim. Behav. Sci. 2013, 147, 247–260. [Google Scholar] [CrossRef]
- Manteca, X.; Temple, D.; Salas, M. Animal-based indicators to assess welfare in zoo animals. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Wolfensohn, S.; Shotton, J.; Bowley, H.; Davies, S.; Thompson, S.; Justice, W. Assessment of welfare in zoo animals: Towards optimum quality of life. Animals 2018, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Veasey, J.S. In pursuit of peak animal welfare; the need to prioritize the meaningful over the measurable. Zoo Biol. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Kagan, R.; Carter, S.; Allard, S. A universal animal welfare framework for zoos. J. Appl. Anim. Welf. Sci. 2015, 18, S1–S10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franks, B.; Champagne, F.A.; Higgins, E.T. How enrichment affects exploration trade-offs in rats: Implications for welfare and well-being. PLoS ONE 2013, 8, e83578. [Google Scholar] [CrossRef]
- Spinka, M.; Wemelsfelder, F.; Appleby, M.C.; Mench, J.A.; Olsson, A.; Hughes, B.O. Environmental challenge and animal agency. In Animal Welfare; Appleby, M.C., Mench, J.A., Olsson, A., Hughes, B.O., Eds.; CABI Publishing: Wallingford, UK, 2011; pp. 27–43. [Google Scholar]
- OIE (World Organisation for Animal Health). Chapter 7.1: Introduction to the Recommendations for Animal Welfare. In Terrestrial Animal Health Code; Article 7.1.1. 2010. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/2018/en_chapitre_aw_introduction.htm (accessed on 23 December 2021).
- Zoological Information Management System. Available online: www.zims.Species360.org (accessed on 26 December 2020).
- Whittaker, A.L.; Barker, T.H. A consideration of the role of biology and test design as confounding factors in judgement bias tests. Appl. Anim. Behav. Sci. 2020, 232, 105126. [Google Scholar] [CrossRef]
- Hill, S.; Broom, D. Measuring zoo animal welfare: Theory and practice. Zoo Biol. 2009, 28, 531–544. [Google Scholar] [CrossRef]
- Watters, J.V.; Krebs, B.L.; Eschmann, C.L. Assessing Animal Welfare with Behavior: Onward with Caution. J. Zool. Bot. Gard. 2021, 2, 75–87. [Google Scholar] [CrossRef]
- Rochlitz, I. The Welfare of Cats Kept in Confined Environments. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 1997. [Google Scholar]
- Brambell, R. Report of the Technical Committee to Enquire into the Welfare of Animals Kept under Intensive Livestock Husbandry Systems, Cmd. (Great Britain. Parliament); H.M. Stationery Office: London, UK, 1965; pp. 1–84. [Google Scholar]
- Morton, R.; Hebart, M.L.; Ankeny, R.A.; Whittaker, A.L. Assessing the uniformity in australian animal protection law: A statutory comparison. Animals 2021, 11, 35. [Google Scholar] [CrossRef]
- Mellor, D.J. Operational details of the five domains model and its key applications to the assessment and management of animal welfare. Animals 2017, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Animal Welfare Victoria. Available online: https://agriculture.vic.gov.au/livestock-and-animals/animal-welfare-victoria/livestock-management-and-welfare/livestock-management-legislation-and-regulations (accessed on 17 December 2021).
- McCulloch, S.P. A critique of fawc’s five freedoms as a framework for the analysis of animal welfare. J. Agric. Environ. Ethics 2013, 26, 959–975. [Google Scholar] [CrossRef]
- Temple, D.; Courboulay, V.; Velarde, A.; Dalmau, A.; Manteca, X. The welfare of growing pigs in five different production systems in france and spain: Assessment of health. Anim. Welf. 2012, 21, 257–271. [Google Scholar] [CrossRef] [Green Version]
- Mononen, J.; Møller, S.H.; Hansen, S.W.; Hovland, A.; Koistinen, T.; Lidfors, L.; Malmkvist, J.; Vinke, C.; Ahola, L. The development of on-farm welfare assessment protocols for foxes and mink: The welfur project. Anim. Welf. 2012, 21, 363. [Google Scholar] [CrossRef]
- Buijs, S.; Ampe, B.; Tuyttens, F. Sensitivity of the welfare quality® broiler chicken protocol to differences between intensively reared indoor flocks: Which factors explain overall classification? Animal 2017, 11, 244–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, C.; Browne, W.; Mullan, S.; Main, D. Navigating the iceberg: Reducing the number of parameters within the welfare quality® assessment protocol for dairy cows. Animal 2014, 8, 1978–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welfare Quality®. Welfare Quality® Assessment Protocol for Poultry (Broilers, Laying Hens); Wageningen UR Livestock Research: Lelystad, The Netherlands, 2009. [Google Scholar]
- Botreau, R.; Veissier, I.; Butterworth, A.; Bracke, M.B.M.; Keeling, L.J. Definition of criteria for overall assessment of animal welfare. Anim. Welf. 2007, 16, 225–228. [Google Scholar]
- Clegg, I.; Borger-Turner, J.; Eskelinen, H. C-well: The development of a welfare assessment index for captive bottlenose dolphins (Tursiops truncatus). Anim. Welf. 2015, 24, 267–282. [Google Scholar] [CrossRef]
- Salas, M.; Manteca, X.; Abáigar, T.; Delclaux, M.; Enseñat, C.; Martínez-Nevado, E.; Quevedo, M.; Fernández-Bellon, H. Using farm animal welfare protocols as a base to assess the welfare of wild animals in captivity—Case study: Dorcas gazelles (Gazella dorcas). Animals 2018, 8, 111. [Google Scholar] [CrossRef] [Green Version]
- Greggor, A.L.; Vicino, G.A.; Swaisgood, R.R.; Fidgett, A.; Brenner, D.; Kinney, M.E.; Farabaugh, S.; Masuda, B.; Lamberski, N. Animal welfare in conservation breeding: Applications and challenges. Front. Vet. Sci. 2018, 5, 323. [Google Scholar] [CrossRef]
- Meagher, R.K. Observer ratings: Validity and value as a tool for animal welfare research. Appl. Anim. Behav. Sci. 2009, 119, 1–14. [Google Scholar] [CrossRef]
- Weiss, A.; King, J.E.; Perkins, L. Personality and subjective well-being in orangutans (Pongo pygmaeus and Pongo abelii). J. Personal. Soc. Psychol. 2006, 90, 501–511. [Google Scholar] [CrossRef]
- Whitham, J.C.; Wielebnowski, N. Animal-based welfare monitoring: Using keeper ratings as an assessment tool. Zoo Biol. 2009, 28, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Wielebnowski, N.C.; Fletchall, N.; Carlstead, K.; Busso, J.M.; Brown, J.L. Noninvasive assessment of adrenal activity associated with husbandry and behavioral factors in the north american clouded leopard population. Zoo Biol. 2002, 21, 77–98. [Google Scholar] [CrossRef]
- Justice, W.S.M.; O’Brien, M.F.; Szyszka, O.; Shotton, J.; Gilmour, J.E.M.; Riordan, P.; Wolfensohn, S. Adaptation of the animal welfare assessment grid (awag) for monitoring animal welfare in zoological collections. Vet. Rec. 2017, 181, 143. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, S.; Duchateau, M.J. Feasibility and validity of the animal welfare assessment grid to monitor the welfare of zoo-housed gorillas Gorilla gorilla gorilla. J. Zoo Aquar. Res. 2021, 9, 208–217. [Google Scholar]
- Mason, G.J.; Latham, N. Can’t stop, won’t stop: Is stereotypy a reliable animal welfare indicator? Anim. Welf. 2004, 13, S57–S69. [Google Scholar]
- Wemelsfelder, F.; Hunter, T.; Mendl, M.; Lawrence, A. Assessing the “whole animal”: A free-choice-profiling approach. Anim. Behav. 2001, 62, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Minero, M.; Dalla Costa, E.; Dai, F.; Murray, L.A.M.; Canali, E.; Wemelsfelder, F. Use of qualitative behaviour assessment as an indicator of welfare in donkeys. Appl. Anim. Behav. Sci. 2016, 174, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Andreasen, S.N.; Wemelsfelder, F.; Sandøe, P.; Forkman, B. The correlation of qualitative behavior assessments with welfare quality® protocol outcomes in on-farm welfare assessment of dairy cattle. Appl. Anim. Behav. Sci. 2013, 143, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Yon, L.; Williams, E.; Harvey, N.D.; Asher, L. Development of a behavioural welfare assessment tool for routine use with captive elephants. PLoS ONE 2019, 14, e0210783. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.J.; Vicino, G.A.; Sheftel, J.; Lauderdale, L.K. Behavioral diversity as a potential indicator of positive animal welfare. Animals 2020, 10, 1211. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Howell, C.P.; Cheyne, S.M. Complexities of using wild versus captive activity budget comparisons for assessing captive primate welfare. J. Appl. Anim. Welf. Sci. 2019, 22, 78–96. [Google Scholar] [CrossRef] [PubMed]
- Veasey, J. Concepts in the care and welfare of captive elephants. Int. Zoo Yearb. 2006, 40, 63–79. [Google Scholar] [CrossRef]
- Cronin, K.A.; Ross, S.R. Technical contribution: A cautionary note on the use of behavioural diversity (h-index) in animal welfare science. Anim. Welf. 2019, 28, 157–164. [Google Scholar] [CrossRef]
- Miller, L.J.; Pisacane, C.; Vicino, G.A. Relationship between behavioural diversity and faecal glucocorticoid metabolites: A case study with cheetahs (Acinonyx jubatus). Anim. Welf. 2016, 25, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Delfour, F.; Vaicekauskaite, R.; Garcia Parraga, D.; Pilenga, C.; Serres, A.; Isabelle, B.; Pascaud, A.; Perlado-Campos, E.; Sánchez Contreras, G.; Baumgartner, K.; et al. Behavioural diversity study in bottlenose dolphin (Tursiops truncatus) groups and its implications for welfare assessments. Animals 2021, 11, 1715. [Google Scholar] [CrossRef]
- Clegg, I.L. Cognitive bias in zoo animals: An optimistic outlook for welfare assessment. Animals 2018, 8, 104. [Google Scholar] [CrossRef] [Green Version]
- Rioja-Lang, F.C.; Connor, M.; Bacon, H.; Dwyer, C.M. Determining a welfare prioritization for horses using a delphi method. Animals 2020, 10, 647. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, N.; Hugé, J.; Sutherland, W.J.; McNeill, J.; Van Opstal, M.; Dahdouh-Guebas, F.; Koedam, N. The delphi technique in ecology and biological conservation: Applications and guidelines. Methods Ecol. Evol. 2015, 6, 1097–1109. [Google Scholar] [CrossRef] [Green Version]
- Veasey, J.S. Assessing the psychological priorities for optimising captive asian elephant (Elephas maximus) welfare. Animals 2020, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Veasey, J.S. Can zoos ever be big enough for large wild animals? A review using an expert panel assessment of the psychological priorities of the amur tiger (Panthera tigris altaica) as a model species. Animals 2020, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, A.L.; Golder-Dewar, B.; Triggs, J.L.; Sherwen, S.L.; McLelland, D.J. Identification of animal-based welfare indicators in captive reptiles: A delphi consultation survey. Animals 2021, 11, 2010. [Google Scholar] [CrossRef] [PubMed]
- Wigham, E.E.; Butterworth, A.; Wotton, S. Assessing cattle welfare at slaughter-why is it important and what challenges are faced? Meat Sci. 2018, 145, 171–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raw, Z.; Rodrigues, J.B.; Rickards, K.; Ryding, J.; Norris, S.L.; Judge, A.; Kubasiewicz, L.M.; Watson, T.L.; Little, H.; Hart, B.; et al. Equid assessment, research and scoping (ears): The development and implementation of a new equid welfare assessment and monitoring tool. Animals 2020, 10, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, D. Assessing animal welfare: Different philosophies, different scientific approaches. Zoo Biol. 2009, 28, 507–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wielebnowski, N.C. Behavioral differences as predictors of breeding status in captive cheetahs. Zoo Biol. 1999, 18, 335–349. [Google Scholar] [CrossRef]
- Wemelsfelder, F.; Hunter, E.A.; Mendl, M.T.; Lawrence, A.B. The spontaneous qualitative assessment of behavioural expressions in pigs: First explorations of a novel methodology for integrative animal welfare measurement. Appl. Anim. Behav. Sci. 2000, 67, 193–215. [Google Scholar] [CrossRef]
- Wemelsfelder, F.; Lawrence, A.B. Qualitative assessment of animal behaviour as an on-farm welfare-monitoring tool. Acta Agric. Scand. Sect. A—Anim. Sci. 2001, 51, 21–25. [Google Scholar]
- Wolfensohn, S.; Sharpe, S.; Hall, I.; Lawrence, S.; Kitchen, S.; Dennis, M. Refinement of welfare through development of a quantitative system for assessment of lifetime experience. Anim. Welf. 2015, 24, 139–149. [Google Scholar] [CrossRef]
- Diana, A.; Salas, M.; Pereboom, Z.; Mendl, M.; Norton, T. A systematic review of the use of technology to monitor welfare in zoo animals: Is there space for improvement? Animals 2021, 11, 3048. [Google Scholar] [CrossRef]
- Wark, J.D.; Cronin, K.A.; Niemann, T.; Shender, M.; Horrigan, A.; Kao, A.; Ross, M.R. Monitoring the behavior and habitat use of animals to enhance welfare using the zoomonitor app. Anim. Behav. Cogn. 2019, 6, 158–167. [Google Scholar] [CrossRef]
- Brando, S.; Buchanan-Smith, H.M. The 24/7 approach to promoting optimal welfare for captive wild animals. Behav. Processes 2018, 156, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Mason, G.J.; Mendl, M. Why is there no simple way of measuring animal welfare? Anim. Welf. 1993, 2, 301. [Google Scholar]
- Posta, B.; Huber, R.; Moore, D.E., III. The effects of housing on zoo elephant behavior: A quantitative case study of diurnal and seasonal variation. Int. J. Comp. Psychol. 2013, 26, 37–52. [Google Scholar] [CrossRef]
- Fernandez, E.J.; Yoakum, E.; Andrews, N. Seasonal and daily activity of two zoo-housed grizzly bears (Ursus Arctos Horribilis). J. Zool. Bot. Gard. 2020, 1, 1–12. [Google Scholar] [CrossRef]
- Lewton, J.; Rose, P.E. Evaluating the social structure of captive rothschild’s giraffes (Giraffa camelopardalis rothschildi): Relevance to animal management and animal welfare. J. Appl. Anim. Welf. Sci. 2020, 23, 178–192. [Google Scholar] [CrossRef]
- Collins, C.; Corkery, I.; Haigh, A.; McKeown, S.; Quirke, T.; O’Riordan, R. The effects of environmental and visitor variables on the behavior of free-ranging ring-tailed lemurs (Lemur catta) in captivity. Zoo Biol. 2017, 36, 250–260. [Google Scholar] [CrossRef]
- Ross, S.R.; Wagner, K.E.; Schapiro, S.J.; Hau, J.; Lukas, K.E. Transfer and acclimatization effects on the behavior of two species of african great ape (Pan troglodytes and Gorilla gorilla gorilla) moved to a novel and naturalistic zoo environment. Int. J. Primatol. 2011, 32, 99–117. [Google Scholar] [CrossRef]
- Zerbe, P.; Clauss, M.; Codron, D.; Bingaman Lackey, L.; Rensch, E.; Streich, J.W.; Hatt, J.M.; Müller, D.W. Reproductive seasonality in captive wild ruminants: Implications for biogeographical adaptation, photoperiodic control, and life history. Biol. Rev. 2012, 87, 965–990. [Google Scholar] [CrossRef] [Green Version]
- Claxton, A.M. The potential of the human–animal relationship as an environmental enrichment for the welfare of zoo-housed animals. Appl. Anim. Behav. Sci. 2011, 133, 1–10. [Google Scholar] [CrossRef]
- Wong, B.B.M.; Candolin, U. Behavioral responses to changing environments. Behav. Ecol. 2015, 26, 665–673. [Google Scholar] [CrossRef] [Green Version]
- Kagan, R.; Allard, S.; Carter, S. What is the future for zoos and aquariums? J. Appl. Anim. Welf. Sci. 2018, 21, 59–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlstead, K.; Mellen, J.; Kleiman, D.G. Black rhinoceros (Diceros bicornis) in U.S. Zoos: I. Individual behavior profiles and their relationship to breeding success. Zoo Biol. 1999, 18, 17–34. [Google Scholar] [CrossRef]
- Clubb, R.; Rowcliffe, M.; Lee, P.; Mar Khyne, U.; Moss, C.; Mason Georgia, J. Compromised survivorship in zoo elephants. Science 2008, 322, 1649. [Google Scholar] [CrossRef] [Green Version]
- Vickery, S.S.; Mason, G.J. Behavioral persistence in captive bears: Implications for reintroduction. Ursus 2003, 14, 35–43. [Google Scholar]
- Botreau, R.; Bonde, M.; Butterworth, A.; Perny, P.; Bracke, M.B.; Capdeville, J.; Veissier, I. Aggregation of measures to produce an overall assessment of animal welfare. Part 1: A review of existing methods. Animal 2007, 1, 1179–1187. [Google Scholar] [CrossRef] [Green Version]
- Whay, H.R.; Main, D.C.J.; Green, L.E.; Webster, A.J.F. An animal-based welfare assessment of group-housed calves on UK dairy farms. Anim. Welf. 2003, 12, 611–617. [Google Scholar]
- Milne, T.; Michael Bull, C.; Hutchinson, M.N. Fitness of the endangered pygmy blue tongue lizard tiliqua adelaidensis in artificial burrows. J. Herpetol. 2003, 37, 762–765. [Google Scholar] [CrossRef]
- Doneley, B.; Monks, D.; Johnson, R.; Carmel, B. Reptile Medicine and Surgery in Clinical Practice; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Arnold, E. Evolutionary aspects of tail shedding in lizards and their relatives. J. Nat. Hist. 1984, 18, 127–169. [Google Scholar] [CrossRef]
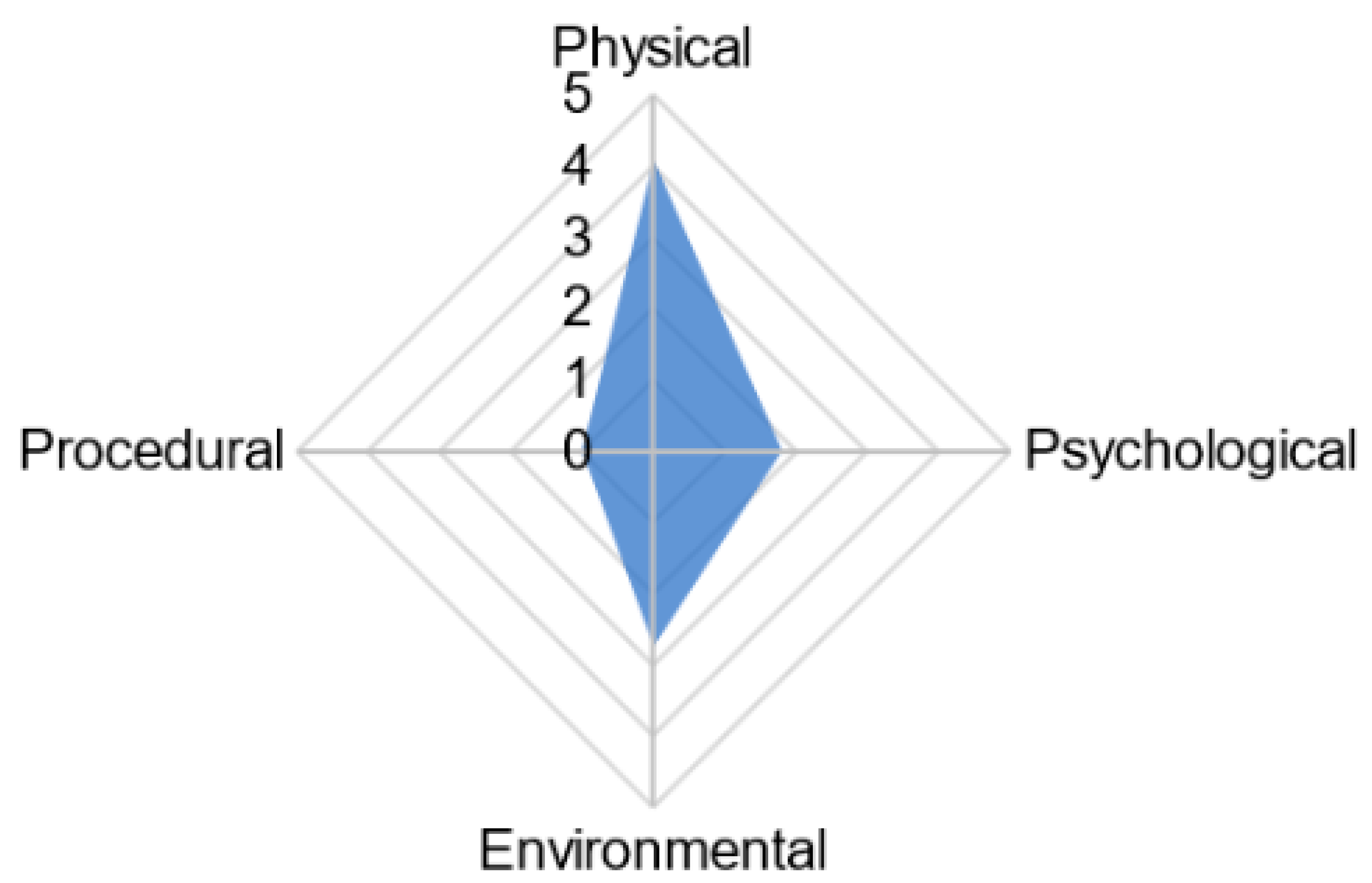
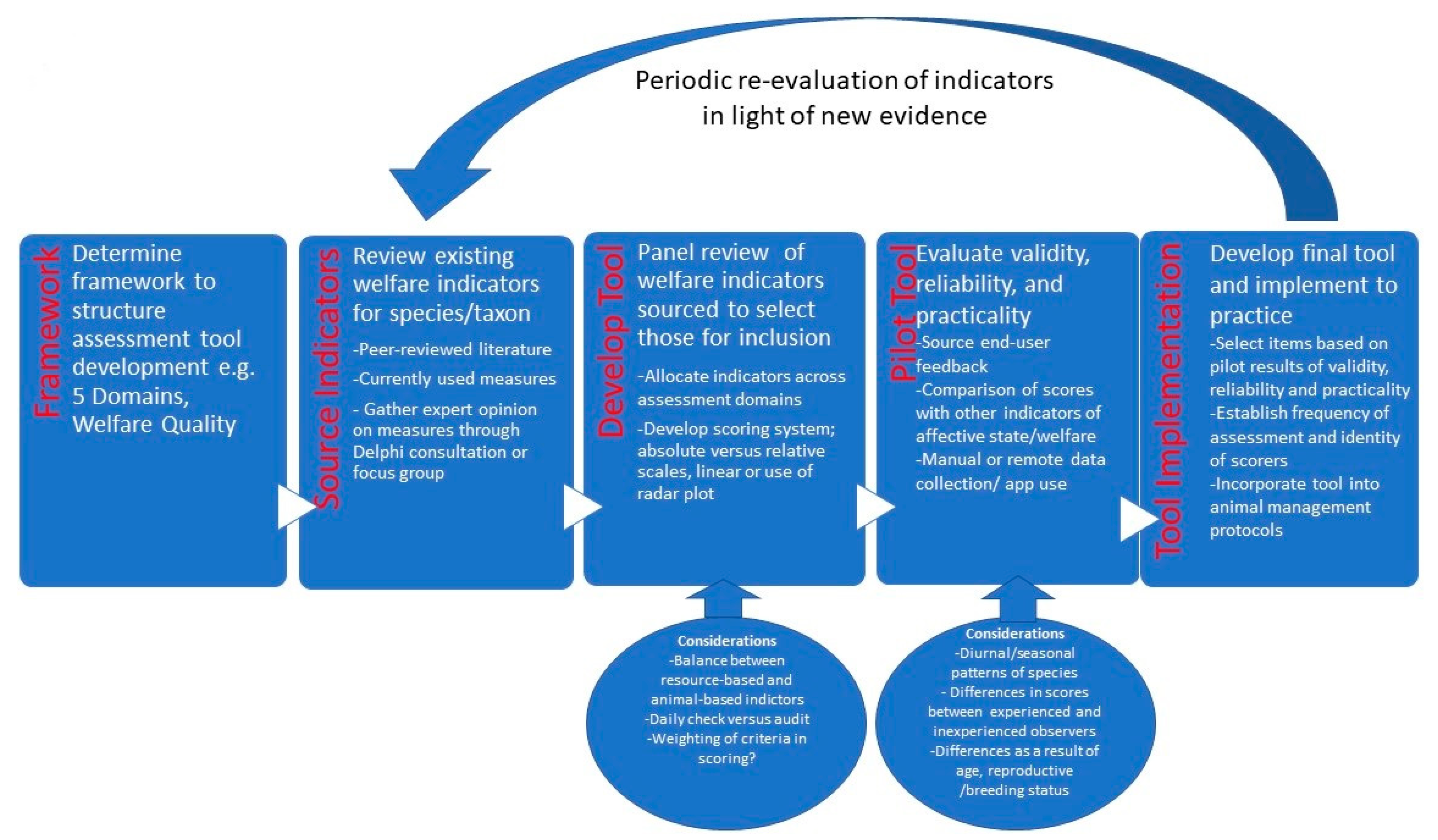
| Step in Tool Derivation Process | Process Performed | Outcome of Step | Examples of Considerations |
|---|---|---|---|
| Framework Choice | Contrasted frameworks available and selected Welfare Quality Protocol due to clear guidance on criteria provided | Selection of Welfare Quality Protocol | Frameworks considered were Five Domains and Welfare Quality due to previous experience with use |
| Source Indicators | Species- relevant criteria were derived by local consultation with keepers and veterinarians, and review of broader literature on lizards | Derived 39 animal or resource-based indicators, aligned against the 12 Welfare Quality criteria and 4 principles | Animal cleanliness was derived as a measure of comfort around resting- the literature suggests that scat piling may be disturbed if welfare poor [88] |
| The PBTS is omnivorous, and their diet should consist of 50% vegetables, 25% fruits, and 25% invertebrates such as snails. This needs consideration under the criteria “appropriate diet” for the category of ‘good feeding’ [89] | |||
| Review of indicators to determine those for inclusion | Review of derived indicators by team comprising zoo veterinarian, researchers, animal welfare officer and two reptile keepers | Removed one animal-based indicator to yield 38 indicators. Tool was made up of predominately animal-based indicators (77%) and was designed to be | Tail autotomy was initially identified but removed since in the particular genus of interest, Tiliqua, there are reduced planes and tail drop is therefore unlikely [90] |
| part of a longer audit-type assessment. | Tool primarily comprised animal-based indicators as suggested in EU Welfare Quality documentation | ||
| Adapted grading system from Sherwen at al. 2018 [3] with scoring for from 0–2 representing high risk to low risk for resource-based indicators, and poor to good welfare for animal-based measures. The overall score was determined by summation and determination of percent out of the maximum score possible. | |||
| Scores were not weighed since there was no evidence at hand to determine relative importance of the criteria in terms of indicators of animal welfare | |||
| Evaluate validity, reliability and practicality | Pilot study performed on a breeding pair of pygmy blue-tongue skinks and their enclosure through manual observation | Observation took 2 h for this pair. This is likely suitable for an audit-type assessment but would have been excessive for a daily check | Noted that some criteria could not be observed but were not necessarily a sign of compromised welfare. For example, food was not presented at the time of observation so food intake and hunting behaviour could not be assessed. This highlighted the need to consider incorporating information from records since these criteria had been observed previously the same week. |
| Observation performed in winter | Understanding that due to ectothermic physiology and lizards being dormant, behavior may differ in contrast to summer assessment. In spite of this animals scored 79% implying good welfare (based on a 60% threshold). | ||
| Develop Final Tool and Implement | Review of derived indicators by team comprising zoo veterinarian, researchers and reptile keepers | Three resource-based indicators were added following observations to yield tool with 41 indicators | Three resource-based indicators were added; enclosure cleanliness, maintenance and group size |
| Ensure welfare assessment done at a time when food available so the feeding- related criteria can be assessed | After reflective process, decided to continue work to expand the derived tool out to other reptiles with a focus at taxa level | ||
| Determine identity of scorers and that tool most appropriate for audit-style assessment | Implement and continue to refine tool based on feedback from users and considering corroboration with other indicators e.g., information from health records. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, N.; Sherwen, S.L.; Robbins, R.; McLelland, D.J.; Whittaker, A.L. Welfare Assessment Tools in Zoos: From Theory to Practice. Vet. Sci. 2022, 9, 170. https://doi.org/10.3390/vetsci9040170
Jones N, Sherwen SL, Robbins R, McLelland DJ, Whittaker AL. Welfare Assessment Tools in Zoos: From Theory to Practice. Veterinary Sciences. 2022; 9(4):170. https://doi.org/10.3390/vetsci9040170
Chicago/Turabian StyleJones, Narelle, Sally L. Sherwen, Rachel Robbins, David J. McLelland, and Alexandra L. Whittaker. 2022. "Welfare Assessment Tools in Zoos: From Theory to Practice" Veterinary Sciences 9, no. 4: 170. https://doi.org/10.3390/vetsci9040170
APA StyleJones, N., Sherwen, S. L., Robbins, R., McLelland, D. J., & Whittaker, A. L. (2022). Welfare Assessment Tools in Zoos: From Theory to Practice. Veterinary Sciences, 9(4), 170. https://doi.org/10.3390/vetsci9040170







