The Establishment of a Noninvasive Bioluminescence-Specific Viral Encephalitis Model by Pseudorabies Virus-Infected NF-κBp-Luciferase Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Amplification of the Virus
2.2. Cell Culture
2.3. Animal Model for Detecting the Activation of the NF-kB Pathway
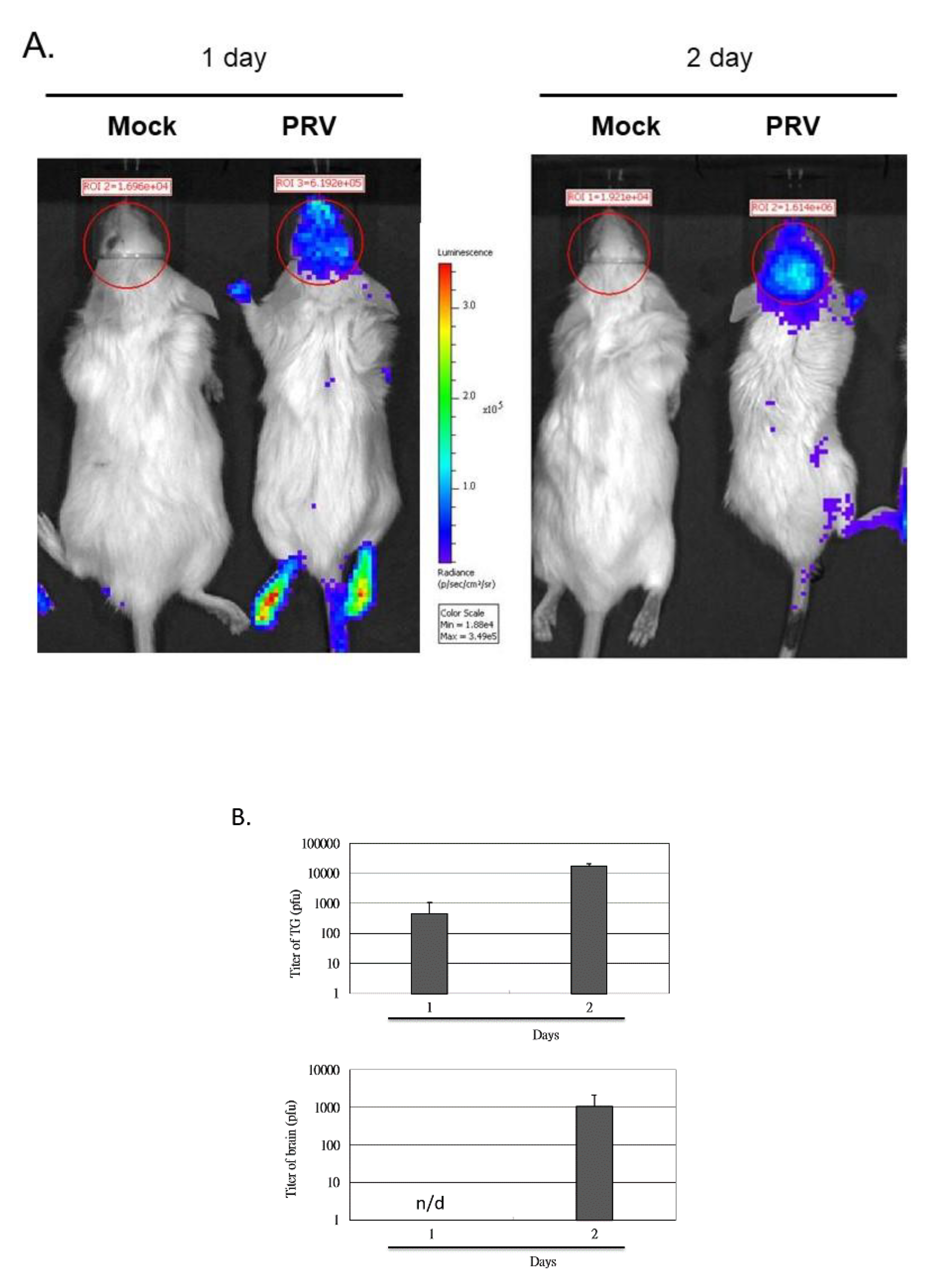
2.4. Quantification of the Virus from Both Brain and Trigeminal Ganglia (TG) of Infectied Mice by Plaque Assay
2.5. Histopathological Analysis with H&E Stain
2.6. Determination of the Expression Levels of TNF-α, IL-6 and IL-1β in a PRV-Infected Mouse Brain or BV-2 Cells by an Enzyme-Linked Immunosorbant Assay (ELISA)
2.7. Detection of Inflammory Proteins by SDS Protein Gel Electrophoresis and Western Blotting
2.8. Statistical Analysis
3. Results
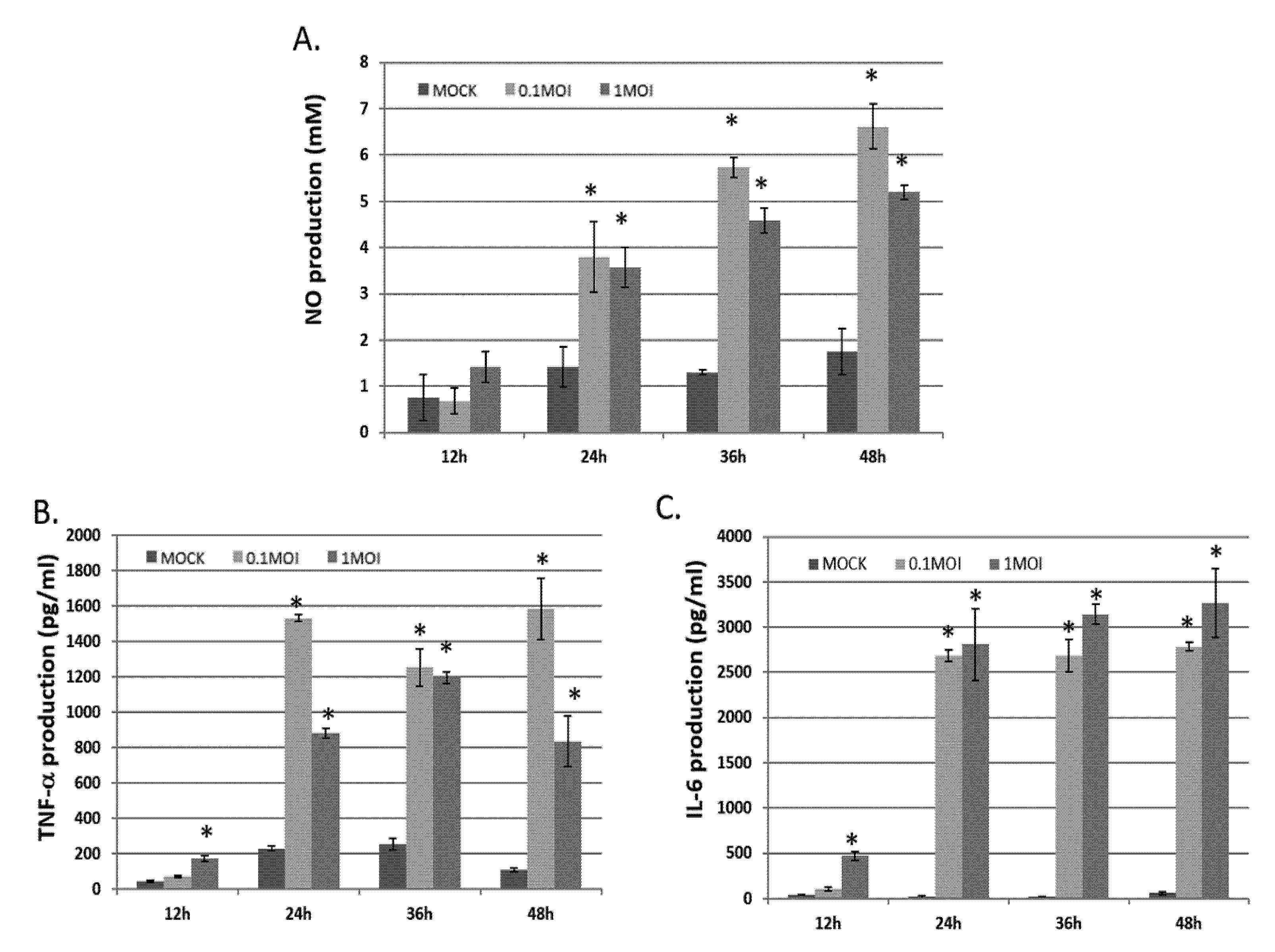
3.1. Effects of TNF-α, NO and IL-6 Generation in BV-2 Cells Infected with PRV
3.2. Induction of the NF-κB Signal Pathway In Vivo after the Inoculation of Virus
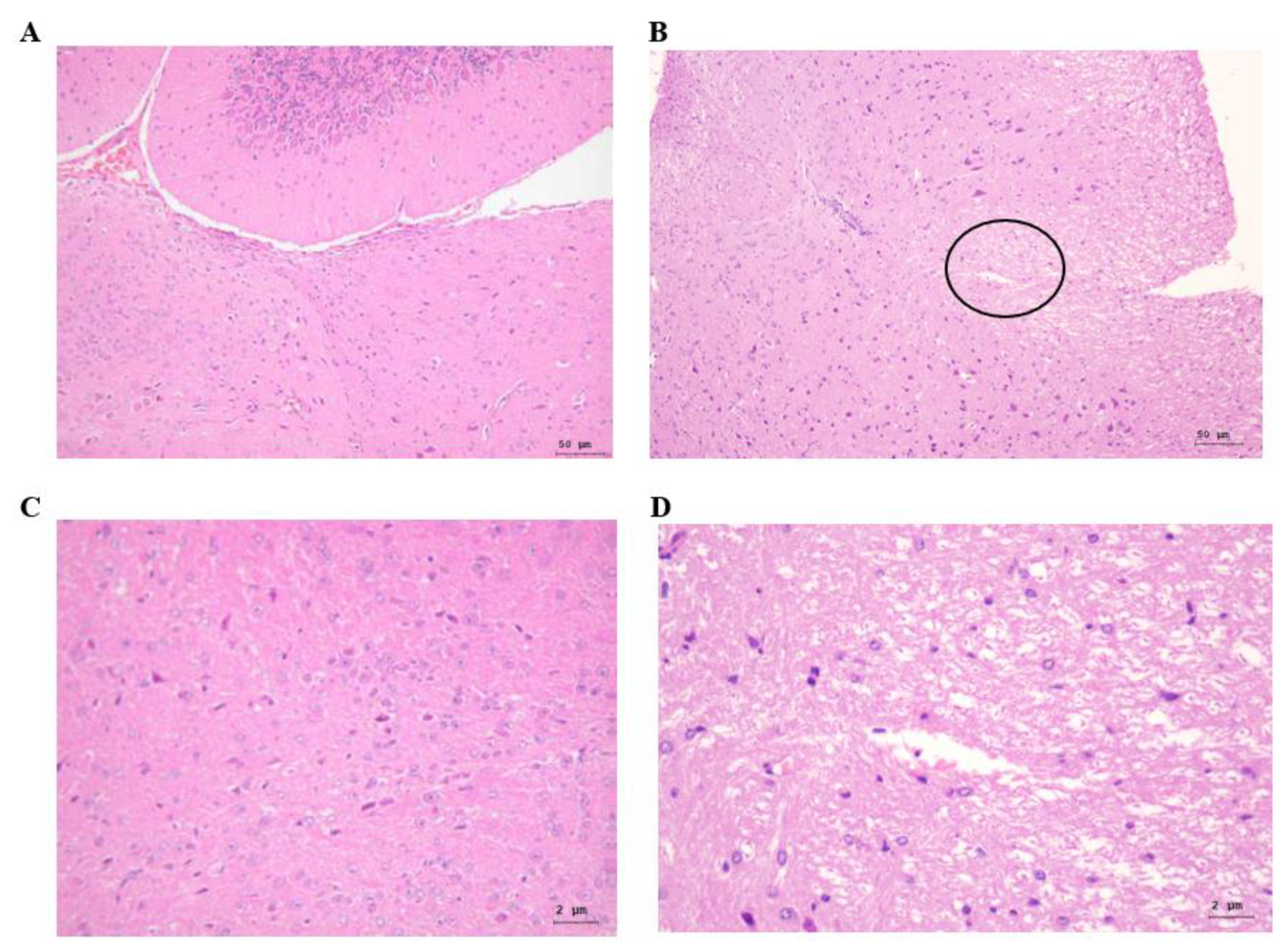
3.3. Histopathological Changes in the Brain Tissues of PRV-Infected Mice
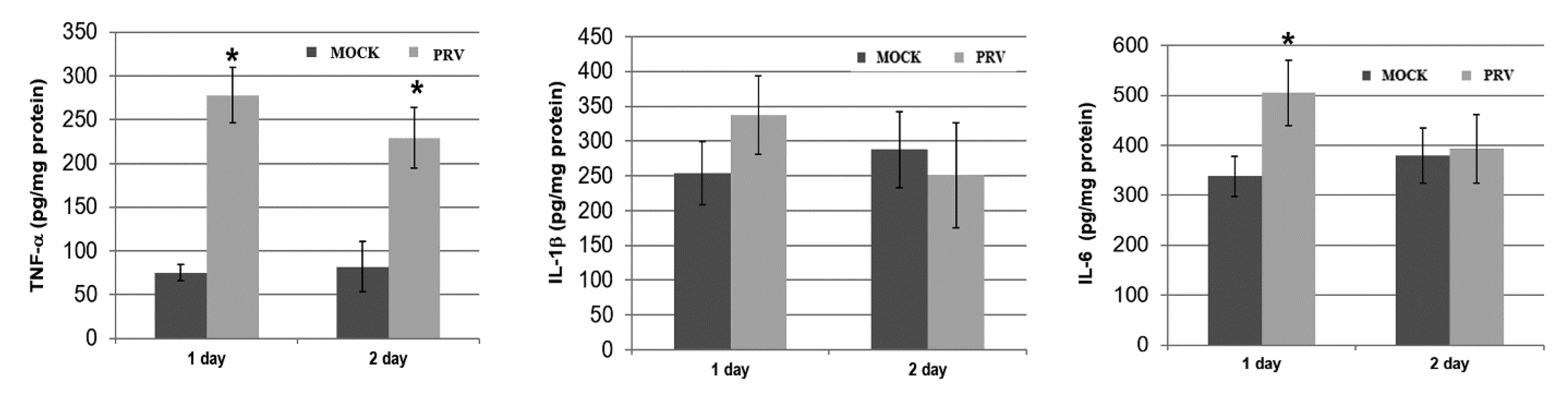
3.4. PRV-Induced TNF-α, IL-1β and-6 Expression in Mouse Brains
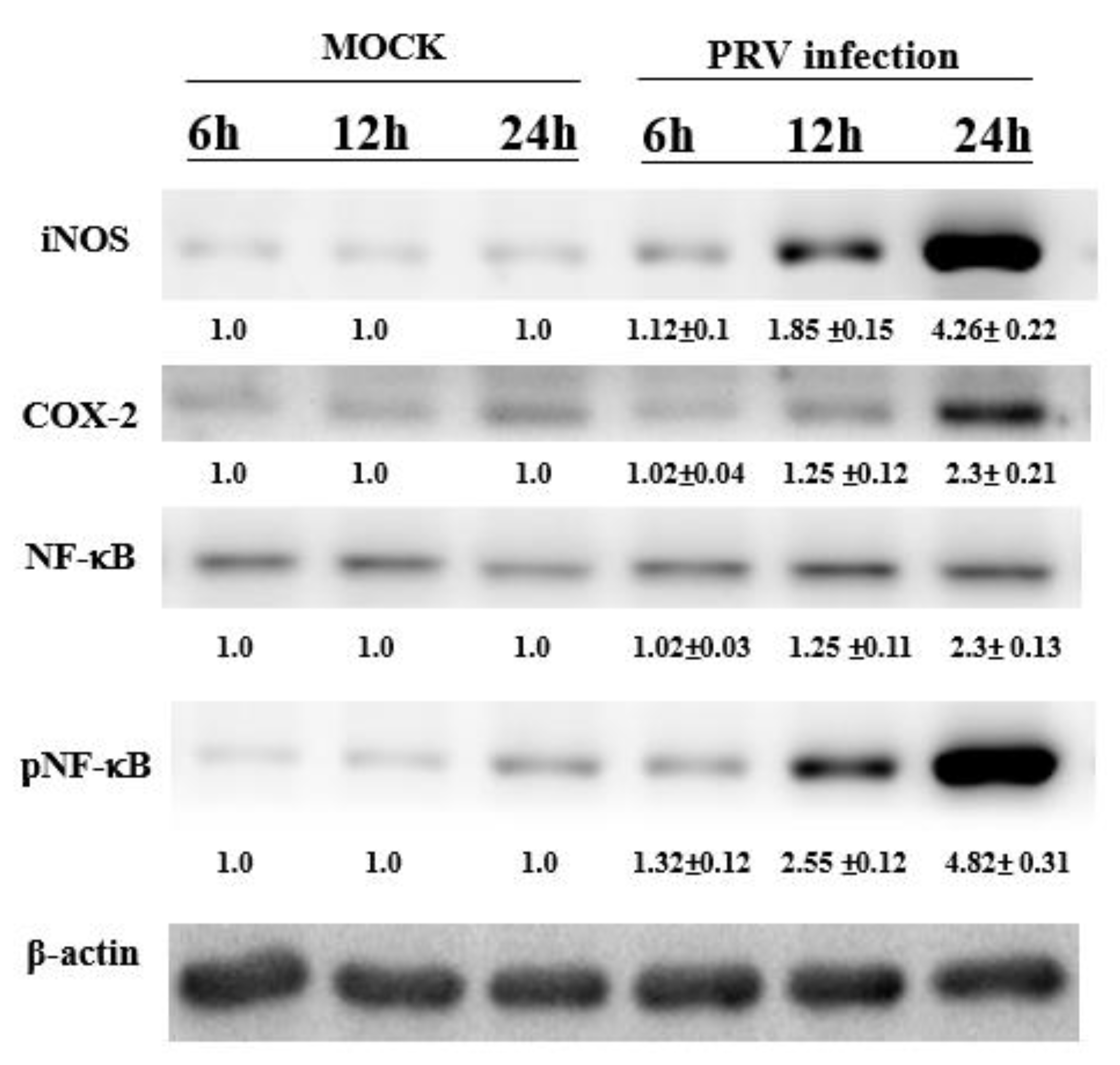
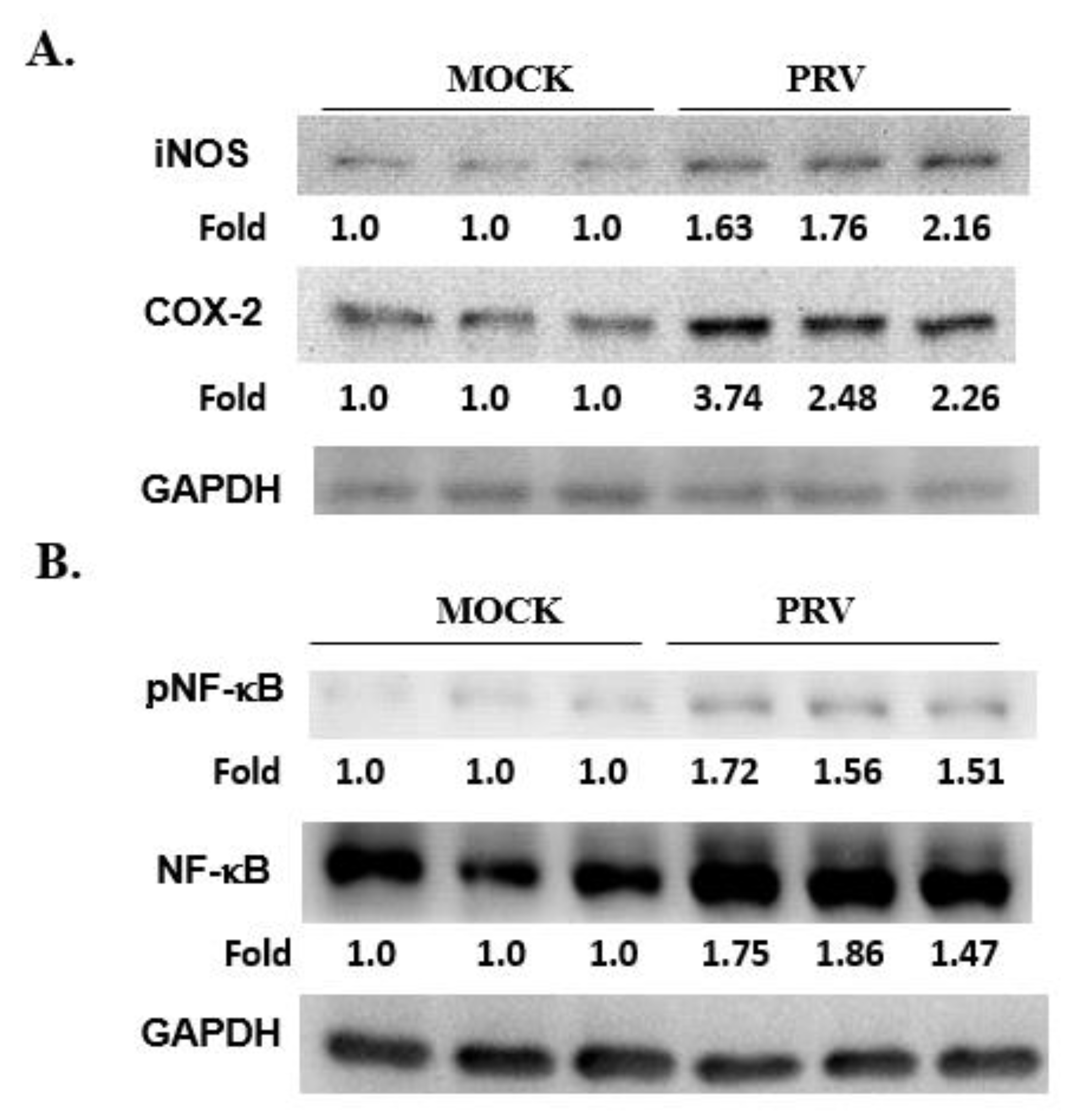
3.5. Expression of NF-κB, iNOS and COX-2 in a PRV-Infected Mouse Brain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 2005, 69, 462–500. [Google Scholar] [CrossRef] [PubMed]
- Roizman, B.; Knipe, D.M.; Whitley, R. Herpes Simplex Viruses, 5th ed.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 2501–2601. [Google Scholar]
- Brittle, E.E.; Reynolds, A.E.; Enquist, L.W. Two modes of pseudorabies virus neuroinvasion and lethality in mice. J. Virol. 2004, 78, 12951–12963. [Google Scholar] [CrossRef] [PubMed]
- Brukman, A.; Enquist, L.W. Pseudorabies virus EP0 protein counteracts an interferon-induced antiviral state in a species-specific manner. J. Virol. 2006, 80, 10871–10873. [Google Scholar] [CrossRef]
- Lin, H.W.; Chang, T.J.; Yang, D.J.; Chen, Y.C.; Wang, M.; Chang, Y.Y. Regulation of virus-induced inflammatory response by β-carotene in RAW264.7 cells. Food Chem. 2012, 134, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Lin, H.W.; Yang, D.J.; Chen, S.Y.; Tseng, J.K.; Chang, T.J.; Chang, Y.Y. Luteolin inhibits viral-induced inflammatory response in RAW264.7 cells via suppression of STAT1/3 dependent NF-κB and activation of HO-1. Free Radic. Biol. Med. 2016, 95, 180–189. [Google Scholar] [CrossRef]
- Stumpf, T.H.; Shimeld, C.; Easty, D.L.; Hill, T.J. Cytokine production in a murine model of recurrent herpetic stromal keratitis. Investig. Ophthalmol. Vis. Sci. 2001, 42, 372–378. [Google Scholar]
- Hung, Y.-P.; Ko, E.-C.; Chou, P.-H.; Chen, Y.-H.; Lin, H.-J.; Liu, Y.-H.; Tsai, H.-W.; Lee, J.-C.; Tsai, P.-J. Proton-Pump Inhibitor Exposure Aggravates Clostridium difficile-Associated Colitis: Evidence From a Mouse Model. J. Infect. Dis. 2020, 221, 168. [Google Scholar]
- Opdenakker, G.; Van den Steen, P.E.; Van Damme, J. Gelatinase B: A tuner and amplifier of immune functions. Trends Immunol. 2001, 22, 571–579. [Google Scholar] [CrossRef]
- Gu, S.M.; Park, M.H.; Hwang, C.J.; Song, H.S.; Lee, U.S.; Han, S.B.; Oh, K.W.; Ham, Y.W.; Song, M.J.; Song, D.J.; et al. Bee venom ameliorates lipopolysaccharide-induced memory loss by preventing NF-kappaB pathway. J. Neuroinflammation 2015, 12, 124. [Google Scholar] [CrossRef]
- Lin, H.W.; Chen, Y.C.; Liu, C.W.; Yang, D.J.; Chen, S.Y.; Chang, T.J.; Chang, Y.Y. Regulation of virus-induced inflammatory response by Dunaliella salina alga extract in macrophages. Food Chem. Toxicol. 2014, 71, 159–165. [Google Scholar] [CrossRef]
- Lin, H.W.; Lee, Y.J.; Yang, D.J.; Hsieh, M.C.; Chen, C.C.; Hsu, W.L.; Chang, Y.Y.; Liu, C.W. Anti-inflammatory effects of Flos Lonicerae Japonicae Water Extract are regulated by the STAT/NF-κB pathway and HO-1 expression in Virus-infected RAW264. 7 cells. Int. J. Med. Sci. 2021, 18, 2285. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Akaike, T.; Maeda, H. Role of nitric oxide in pathogenesis of herpes simplex virus encephalitis in rats. Virology 1999, 256, 203–212. [Google Scholar] [CrossRef]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar] [PubMed]
- Marcaccini, A.; López-Peña, M.; Bermúdez, R.; Quiroga, M.I.; Guerrero, F.H.; Nieto, J.M.; Alemañ, N. Pseudorabies virus induces a rapid up-regulation of nitric oxide synthases in the nervous system of swine. Vet. Microbiol. 2007, 125, 232–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ray, N.; Bisher, M.E.; Enquist, L.W. Cyclooxygenase-1 and -2 are required for production of infectious pseudorabies virus. J. Virol. 2004, 78, 12964–12974. [Google Scholar] [CrossRef]
- Hui, B.; Zhang, L.; Zhou, Q.; Hui, L. Pristimerin Inhibits LPS-Triggered Neurotoxicity in BV-2 Microglia Cells Through Modulating IRAK1/TRAF6/TAK1-Mediated NF-κB and AP-1 Signaling Pathways in Vitro. Neurotox. Res. 2018, 33, 268–283. [Google Scholar] [CrossRef]
- DiBona, V.L.; Zhu, W.; Shah, M.K.; Rafalia, A.; Ben Cheikh, H.; Crockett, D.P.; Zhang, H. Loss of Par1b/MARK2 primes microglia during brain development and enhances their sensitivity to injury. J. Neuroinflammation 2019, 16, 11. [Google Scholar] [CrossRef]
- Contag, P.R.; Olomu, I.N.; Stevenson, D.K.; Contag, C.H. Bioluminescent indicators in living mammals. Nat. Med. 1998, 4, 245–247. [Google Scholar] [CrossRef]
- Contag, C.H.; Jenkins, D.; Contag, P.R.; Negrin, R.S. Use of reporter genes for optical measurements of neoplastic disease in vivo. Neoplasia 2000, 2, 41–52. [Google Scholar] [CrossRef]
- Rehemtulla, A.; Stegman, L.D.; Cardozo, S.J.; Gupta, S.; Hall, D.E.; Contag, C.H.; Rose, B.D. Rapid and quantitative assessment of cancer treatment response using in vivo bioluminescence imaging. Neoplasia 2000, 2, 491–495. [Google Scholar] [CrossRef]
- Siragusa, G.R.; Nawotka, K.; Spilman, S.D.; Contag, P.R.; Contag, C.H. Real-time monitoring of Escherichia coli O157:H7 adherence to beef carcass surface tissues with a bioluminescent reporter. Appl. Environ. Microbiol. 1999, 65, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Luker, G.D.; Bardill, J.P.; Prior, J.L.; Pica, C.M.; Piwnica-Worms, D.; Leib, D.A. Noninvasive bioluminescence imaging of herpes simplex virus type 1 infection and therapy in living mice. J. Virol. 2002, 76, 12149–12161. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Sundaresan, G.; Iyer, M.; Gambhir, S.S. Noninvasive optical imaging of firefly luciferase reporter gene expression in skeletal muscles of living mice. Mol. Ther. 2001, 4, 297–306. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-W.; Wang, M.; Tsai, P.-J.; Lee, Y.-J.; Hsieh, M.-C.; Lu, D.-Y.; Hsu, W.-L.; Jan, M.-S.; Chang, Y.-Y. The Establishment of a Noninvasive Bioluminescence-Specific Viral Encephalitis Model by Pseudorabies Virus-Infected NF-κBp-Luciferase Mice. Vet. Sci. 2022, 9, 113. https://doi.org/10.3390/vetsci9030113
Lin H-W, Wang M, Tsai P-J, Lee Y-J, Hsieh M-C, Lu D-Y, Hsu W-L, Jan M-S, Chang Y-Y. The Establishment of a Noninvasive Bioluminescence-Specific Viral Encephalitis Model by Pseudorabies Virus-Infected NF-κBp-Luciferase Mice. Veterinary Sciences. 2022; 9(3):113. https://doi.org/10.3390/vetsci9030113
Chicago/Turabian StyleLin, Hui-Wen, Meilin Wang, Pei-Jane Tsai, Yi-Ju Lee, Ming-Chang Hsieh, Dah-Yuu Lu, Wei-Li Hsu, Ming-Shiou Jan, and Yuan-Yen Chang. 2022. "The Establishment of a Noninvasive Bioluminescence-Specific Viral Encephalitis Model by Pseudorabies Virus-Infected NF-κBp-Luciferase Mice" Veterinary Sciences 9, no. 3: 113. https://doi.org/10.3390/vetsci9030113
APA StyleLin, H.-W., Wang, M., Tsai, P.-J., Lee, Y.-J., Hsieh, M.-C., Lu, D.-Y., Hsu, W.-L., Jan, M.-S., & Chang, Y.-Y. (2022). The Establishment of a Noninvasive Bioluminescence-Specific Viral Encephalitis Model by Pseudorabies Virus-Infected NF-κBp-Luciferase Mice. Veterinary Sciences, 9(3), 113. https://doi.org/10.3390/vetsci9030113






