Molecular Genetic Investigation of Digital Melanoma in Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Histopathology
2.2. Molecular Genetic Methods
2.2.1. BRAF Mutation, c-kit Gene Mutation
2.2.2. NRAS/KRAS Mutation Analysis
2.2.3. Copy Number Variation Analysis of KITLG
2.3. Statistical Analysis
3. Results
3.1. Case Description, Clinical Data, and Survival Time
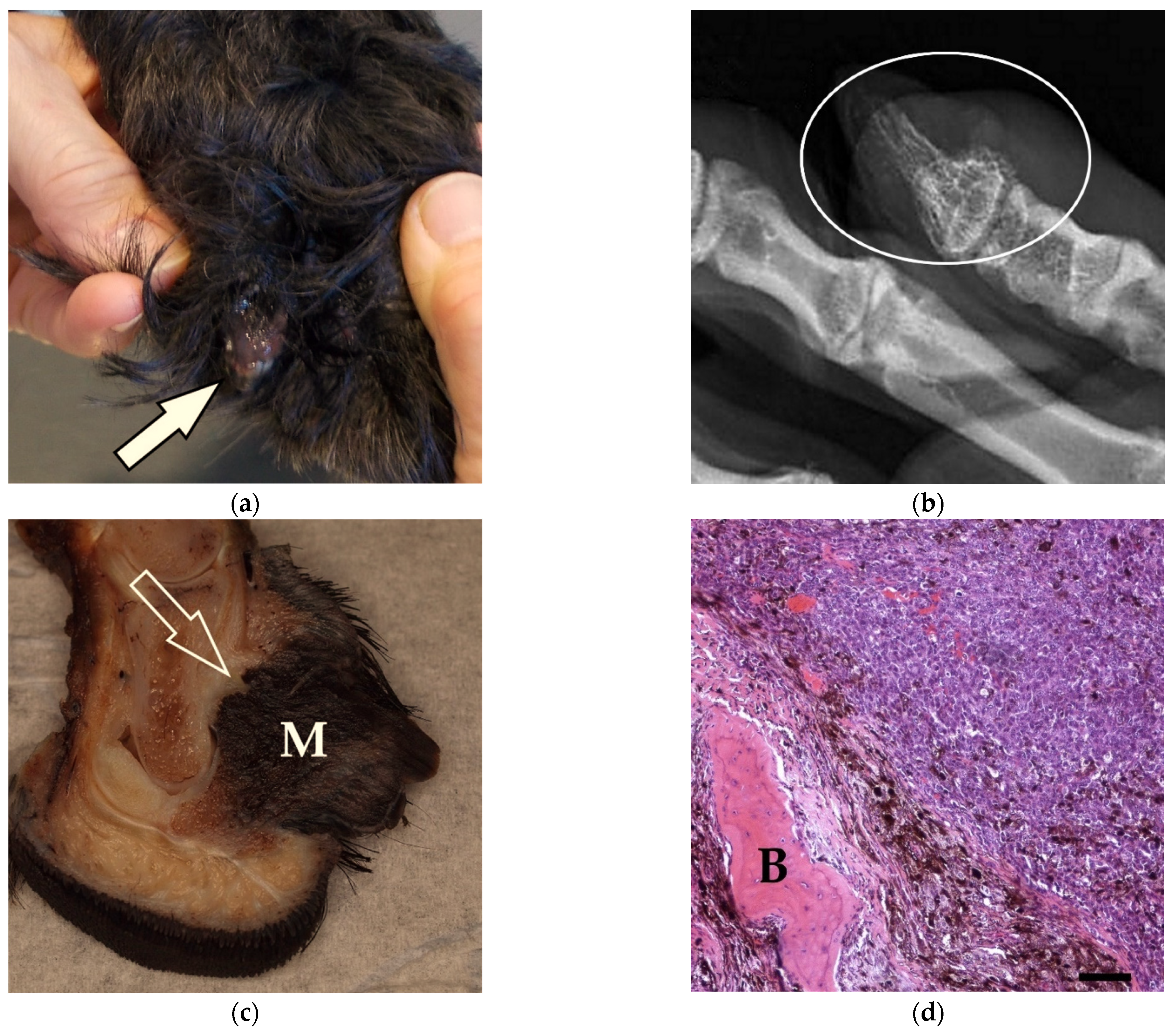
3.2. Pathological Findings
3.3. Genetic Analysis
3.4. Copy Number Variations (CNV) of KITLG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishiya, A.T.; Massoco, C.O.; Felizzola, C.R.; Perlmann, E.; Batschinski, K.; Tedardi, M.V.; Garcia, J.S.; Mendonça, P.P.; Teixeira, T.F.; Zaidan Dagli, M.L. Comparative Aspects of Canine Melanoma. Vet. Sci. 2016, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Gillard, M.; Cadieu, E.; de Brito, C.; Abadie, J.; Vergier, B.; Devauchelle, P.; Degorce, F.; Dréano, S.; Primot, A.; Dorso, L.; et al. Naturally occurring melanomas in dogs as models for non-UV pathways of human melanomas. Pigment Cell Melanoma Res. 2014, 27, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Spangler, W.L.; Kass, P.H. The histologic and epidemiologic bases for prognostic considerations in canine melanocytic neoplasia. Vet. Pathol. 2006, 43, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Bostock, D.E. Prognosis after surgical excision of canine melanomas. Vet. Pathol. 1979, 16, 32–40. [Google Scholar] [CrossRef]
- Henry, C.J.; Brewer, W.G., Jr.; Whitley, E.M.; Tyler, J.W.; Ogilvie, G.K.; Norris, A.; Fox, L.E.; Morrison, W.B.; Hammer, A.; Vail, D.M.; et al. Canine Digital Tumors: A Veterinary Cooperative Oncology Group Retrospective Study of 64 Dogs. J. Vet. Intern. Med. 2005, 19, 720–724. [Google Scholar] [CrossRef]
- Smith, S.H.; Goldschmidt, M.H.; McManus, P.M. A comparative review of melanocytic neoplasms. Vet. Pathol. 2002, 39, 651–678. [Google Scholar] [CrossRef]
- Grassinger, J.M.; Floren, A.; Müller, T.; Cerezo-Echevarria, A.; Beitzinger, C.; Conrad, D.; Törner, K.; Staudacher, M.; Aupperle-Lellbach, H. Digital Lesions in Dogs: A Statistical Breed Analysis of 2912 Cases. Vet. Sci. 2021, 8, 136. [Google Scholar] [CrossRef]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef]
- Mochizuki, H.; Breen, M. Comparative Aspects of BRAF Mutations in Canine Cancers. Vet. Sci. 2015, 2, 231–245. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Brose, M.S.; Volpe, P.; Feldman, M.; Kumar, M.; Rishi, I.; Gerrero, R.; Einhorn, E.; Herlyn, M.; Minna, J.; Nicholson, A.; et al. BRAF and RAS Mutations in Human Lung Cancer and Melanoma. Cancer Res. 2002, 62, 6997–7000. [Google Scholar] [PubMed]
- Tsao, H.; Goel, V.; Wu, H.; Yang, G.; Haluska, F.G. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J. Investig. Dermatol. 2004, 122, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.K.; Lazar, A.J.F.; Warneke, C.L.; Redston, M.S.; Haluska, F.G. Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneous melanoma. J. Investig. Dermatol. 2006, 126, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Savoia, P.; Fava, P.; Casoni, F.; Cremona, O. Targeting the ERK Signaling Pathway in Melanoma. Int. J. Mol. Sci. 2019, 20, 1483. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.L. ras oncogenes in human cancer: A review. Cancer Res. 1989, 49, 4682–4689. [Google Scholar] [PubMed]
- Cicenas, J.; Tamosaitis, L.; Kvederaviciute, K.; Tarvydas, R.; Staniute, G.; Kalyan, K.; Meskinyte-Kausiliene, E.; Stankevicius, V.; Valius, M. KRAS, NRAS and BRAF mutations in colorectal cancer and melanoma. Med. Oncol. 2017, 34, 26. [Google Scholar] [CrossRef]
- Yeh, I.; Jorgenson, E.; Shen, L.; Xu, M.; North, J.P.; Shain, A.H.; Reuss, D.; Wu, H.; Robinson, W.A.; Olshen, A.; et al. Targeted Genomic Profiling of Acral Melanoma. JNCI J. Natl. Cancer Inst. 2019, 111, 1068–1077. [Google Scholar] [CrossRef]
- Mayr, B.; Schaffner, G.; Reifinger, M.; Zwetkoff, S.; Prodinger, B. N-ras Mutations in Canine Malignant Melanomas. Vet. J. 2003, 165, 169–171. [Google Scholar] [CrossRef]
- Escobar, H.M.; Günther, K.; Richter, A.; Soller, J.T.; Winkler, S.; Nolte, I.; Bullerdiek, J. Absence of Ras-gene Hot-spot Mutations in Canine Fibrosarcomas and Melanomas. Anticancer Res. 2004, 24, 3027–3028. [Google Scholar]
- Hendricks, W.P.D.; Zismann, V.; Sivaprakasam, K.; Legendre, C.; Poorman, K.; Tembe, W.; Perdigones, N.; Kiefer, J.; Liang, W.; DeLuca, V.; et al. Somatic inactivating PTPRJ mutations and dysregulated pathways identified in canine malignant melanoma by integrated comparative genomic analysis. PLoS Genet. 2018, 14, e1007589. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.J.; Jankovsky, J.M.; Rohrbach, B.W.; LeBlanc, A.K. C-kit expression in canine mucosal melanomas. Vet. Pathol. 2012, 49, 760–765. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, H.; Ma, P.; Zhao, S.; Kong, Q.; Bian, T.; Gong, C.; Zhao, Q.; Liu, Y.; Qi, X.; et al. Darwinian Positive Selection on the Pleiotropic Effects of KITLG Explain Skin Pigmentation and Winter Temperature Adaptation in Eurasians. Mol. Biol. Evol. 2018, 35, 2272–2283. [Google Scholar] [CrossRef] [PubMed]
- Alexeev, V.; Yoon, K. Distinctive role of the cKit receptor tyrosine kinase signaling in mammalian melanocytes. J. Investig. Dermatol. 2006, 126, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Beadling, C.; Jacobson-Dunlop, E.; Hodi, F.S.; Le, C.; Warrick, A.; Patterson, J.; Town, A.; Harlow, A.; Cruz, F.; Azar, S.; et al. KIT gene mutations and copy number in melanoma subtypes. Clin. Cancer Res. 2008, 14, 6821–6828. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.R.; Busam, K.J.; Francone, T.D.; Wong, G.C.; Guo, T.; Agaram, N.P.; Besmer, P.; Jungbluth, A.; Gimbel, M.; Chen, C.-T.; et al. L576P KIT mutation in anal melanomas correlates with KIT protein expression and is sensitive to specific kinase inhibition. Int. J. Cancer 2007, 121, 257–264. [Google Scholar] [CrossRef]
- Curtin, J.A.; Busam, K.; Pinkel, D.; Bastian, B.C. Somatic activation of KIT in distinct subtypes of melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 4340–4346. [Google Scholar] [CrossRef]
- Chu, P.-Y.; Pan, S.-L.; Liu, C.-H.; Lee, J.; Yeh, L.-S.; Liao, A.T. KIT gene exon 11 mutations in canine malignant melanoma. Vet. J. 2013, 196, 226–230. [Google Scholar] [CrossRef]
- Smedley, R.C.; Thaiwong, T.; Deeth, L.E.; Kiupel, M. Correlation between KIT Expression and c-Kit Mutations in 2 Subtypes of Canine Oral Melanocytic Neoplasms. Vet. Pathol. 2021, 58, 683–691. [Google Scholar] [CrossRef]
- Tani, H.; Miyamoto, R.; Noguchi, S.; Kurita, S.; Nagashima, T.; Michishita, M.; Yayoshi, N.; Tamura, K.; Bonkobara, M. A canine case of malignant melanoma carrying a KIT c.1725_1733del mutation treated with toceranib: A case report and in vitro analysis. BMC Vet. Res. 2021, 17, 147. [Google Scholar] [CrossRef] [PubMed]
- Bannasch, D.; Affolter, V.; York, D.; Rebhun, R.; Grahn, R.; Weich, K.; Kallenberg, A. Pigment Intensity in Dogs is Associated with a Copy Number Variant Upstream of KITLG. Genes 2020, 11, 75. [Google Scholar] [CrossRef]
- Karyadi, D.M.; Karlins, E.; Decker, B.; vonHoldt, B.M.; Carpintero-Ramirez, G.; Parker, H.G.; Wayne, R.K.; Ostrander, E.A. A copy number variant at the KITLG locus likely confers risk for canine squamous cell carcinoma of the digit. PLoS Genet. 2013, 9, e1003409. [Google Scholar] [CrossRef] [PubMed]
- Cerezo-Echevarria, A.; Grassinger, J.M.; Beitzinger, C.; Klopfleisch, R.; Aupperle-Lellbach, H. Evaluating the Histologic Grade of Digital Squamous Cell Carcinomas in Dogs with Dark and Light Haircoat-A Comparative Study of the Invasive Front and Tumor Cell Budding Systems. Vet. Sci. 2020, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Wobeser, B.K.; Kidney, B.A.; Powers, B.E.; Withrow, S.J.; Mayer, M.N.; Spinato, M.T.; Allen, A.L. Diagnoses and clinical outcomes associated with surgically amputated canine digits submitted to multiple veterinary diagnostic laboratories. Vet. Pathol. 2007, 44, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.J.; Matthiesen, D.T.; Stefanacci, J.D.; Moroff, S.D. Evaluation of dogs with digit masses: 117 cases (1981–1991). J. Am. Vet. Med. Assoc. 1995, 207, 726–728. [Google Scholar] [PubMed]
- Schultheiss, P.C. Histologic features and clinical outcomes of melanomas of lip, haired skin, and nail bed locations of dogs. J. Vet. Diagn. Investig. 2006, 18, 422–425. [Google Scholar] [CrossRef]
- Kamstock, D.A.; Ehrhart, E.J.; Getzy, D.M.; Bacon, N.J.; Rassnick, K.M.; Moroff, S.D.; Liu, S.M.; Straw, R.C.; McKnight, C.A.; Amorim, R.L.; et al. Recommended guidelines for submission, trimming, margin evaluation, and reporting of tumor biopsy specimens in veterinary surgical pathology. Vet. Pathol. 2011, 48, 19–31. [Google Scholar] [CrossRef]
- Mochizuki, H.; Kennedy, K.; Shapiro, S.G.; Breen, M. BRAF Mutations in Canine Cancers. PLoS ONE 2015, 10, e0129534. [Google Scholar] [CrossRef]
- Davies, M.A.; Gershenwald, J.E. Targeted therapy for melanoma: A primer. Surg. Oncol. Clin. N. Am. 2011, 20, 165–180. [Google Scholar] [CrossRef][Green Version]
- Portelinha, A.; Thompson, S.; Smith, R.A.; Da Silva Ferreira, M.; Asgari, Z.; Knezevic, A.; Seshan, V.; de Stanchina, E.; Gupta, S.; Denis, L.; et al. ASN007 is a selective ERK1/2 inhibitor with preferential activity against RAS-and RAF-mutant tumors. Cell Rep. Med. 2021, 2, 100350. [Google Scholar] [CrossRef] [PubMed]
- Prouteau, A.; André, C. Canine Melanomas as Models for Human Melanomas: Clinical, Histological, and Genetic Comparison. Genes 2019, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, B.; Adissu, H.; Wei, B.-R.; Michael, H.; Merlino, G.; Simpson, R. Naturally Occurring Canine Melanoma as a Predictive Comparative Oncology Model for Human Mucosal and Other Triple Wild-Type Melanomas. Int. J. Mol. Sci. 2018, 19, 394. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.D.; Breen, M. Comparative oncology: What dogs and other species can teach us about humans with cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140231. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; van der Weyden, L.; Schott, C.R.; Foote, A.; Constantino-Casas, F.; Smith, S.; Dobson, J.M.; Murchison, E.P.; Wu, H.; Yeh, I.; et al. Cross-species genomic landscape comparison of human mucosal melanoma with canine oral and equine melanoma. Nat. Commun. 2019, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Shelly, S.; Chien, M.B.; Yip, B.; Kent, M.S.; Theon, A.P.; McCallan, J.L.; London, C.A. Exon 15 BRAF mutations are uncommon in canine oral malignant melanomas. Mamm. Genome 2005, 16, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Brocca, G.; Poncina, B.; Sammarco, A.; Cavicchioli, L.; Castagnaro, M. KIT Somatic Mutations and Immunohistochemical Expression in Canine Oral Melanoma. Animals 2020, 10, 2370. [Google Scholar] [CrossRef]
- Murakami, A.; Mori, T.; Sakai, H.; Murakami, M.; Yanai, T.; Hoshino, Y.; Maruo, K. Analysis of KIT expression and KIT exon 11 mutations in canine oral malignant melanomas. Vet. Comp. Oncol. 2011, 9, 219–224. [Google Scholar] [CrossRef]
- Pho, L.N.; Leachman, S.A. Genetics of pigmentation and melanoma predisposition. G. Ital. Dermatol. Venereol. Organo Uff. Soc. Ital. Dermatol. Sifilogr. 2010, 145, 37–45. [Google Scholar]
- Goldschmidt, M.; Goldschmidt, K.H. Epithelial and Melanocytic Tumors of the Skin. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; John Wiley & Sons Inc.: Ames, IA, USA, 2017; pp. 88–141. [Google Scholar]
- Wei, B.-R.; Michael, H.T.; Halsey, C.H.C.; Peer, C.J.; Adhikari, A.; Dwyer, J.E.; Hoover, S.B.; El Meskini, R.; Kozlov, S.; Weaver Ohler, Z.; et al. Synergistic targeted inhibition of MEK and dual PI3K/mTOR diminishes viability and inhibits tumor growth of canine melanoma underscoring its utility as a preclinical model for human mucosal melanoma. Pigment Cell Melanoma Res. 2016, 29, 643–655. [Google Scholar] [CrossRef]
- Fowles, J.S.; Denton, C.L.; Gustafson, D.L. Comparative analysis of MAPK and PI3K/AKT pathway activation and inhibition in human and canine melanoma. Vet. Comp. Oncol. 2015, 13, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Smedley, R.C.; Spangler, W.L.; Esplin, D.G.; Kitchell, B.E.; Bergman, P.J.; Ho, H.-Y.; Bergin, I.L.; Kiupel, M. Prognostic markers for canine melanocytic neoplasms: A comparative review of the literature and goals for future investigation. Vet. Pathol. 2011, 48, 54–72. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer/Probe | Sequence |
|---|---|---|
| NRAS exon 2 | Forward Reverse | 5′-CGCCCATTAAACCTAATTGC-3′ 5′-ACCAAAAGCCAGAGGTAGGG-3′ |
| NRAS exon 3 | Forward Reverse | 5′-ATCTCCTACCCTCCACACCC-3′ 5′-GGCAAATACACAGAGGAAGCC-3′ |
| KRAS exon 2 | Forward Reverse | 5′-AAAAGGTGTTGATAGAGTGGGTTATAC-3′ 5′-AAATGGGCCTGCACAAATC-3′ |
| KRAS exon 3 | Forward Reverse | 5′-ACTGTGTTTCTCCCTTCTCAGG-3′ 5′-GCCCTCGATGTCATTTTATTATATTC-3′ |
| c-kit exon 11 | Forward Reverse | 5’-CCCATGTATGAAGTACAGTGGAAG-3′ 5′-GTTCCCTAAAGTCATTGTTACACG-3′ |
| CNV KITLG | Forward Reverse Probe | 5′-TGCACAAGGGAGAAGGGTTG-3′ 5′-AGATGGTCCTGGGGAAACCA-3′ 5′-FAM-TGGCTGGGGACAGAAGCAATG-BBQ650-3′ |
| Gene | Mutation | Wild Type | Not Done |
|---|---|---|---|
| KRAS exon 2 codon 12 | 17 | 64 | 0 |
| KRAS exon 2 codon 13 | 5 | ||
| KRAS exon 3 codon 61 | 2 | 53 | 31 |
| NRAS exon 2 codon 12 | 1 | 81 | 3 |
| NRAS exon 2 codon 13 | 1 | ||
| NRAS exon 3 codon 61 | 9 | 77 | 0 |
| Dog No. | Codon | Breed (Coat Colour) | Age (y) | Sex | Toe | Morphology | Pigment (Degree) | Mitoses/ 10 HPF | Survival Time (d) |
|---|---|---|---|---|---|---|---|---|---|
| 40 | 12 | GS | 11 | MC | RF | mixed | 2 | 7 | 157 |
| 39 | 12 | GS | 8 | M | LF | epithelioid | 2 | 3 | 72 |
| 25 | 12 | LR | 12 | M | RH | epithelioid | 1 | 13 | 338 |
| 26 | 12 | LR (U) | 12 | F | LF | spindle cell | 1 | 4 | 274 |
| 27 | 12 | LR (black) | 14 | M | RF | epithelioid | 1 | 5 | A |
| 24 | 12 | LR (yellow) | 10 | FS | RF | epithelioid | 1 | 5 | 1304 |
| 49 | 12 | RW | 8 | F | RF | epithelioid | 1 | 24 | U |
| 77 | 12 | DM | U | F | LF | mixed | 2 | 5 | U |
| 75 | 12 | CT | 11 | MC | LF | epithelioid | V | 7 | 1016 |
| 73 | 12 | BS | 9 | M | LH | epithelioid | 1 | 13 | A |
| 1 | 12 | MG (black) | 13 | M | LF | mixed | 1 | 58 | U |
| 2 | 12 | MG (U) | 8 | MC | RF | epithelioid | 1 | 25 | 40 |
| 3 | 12 | MG (U) | 10 | F | LF | round | 1 | 15 | 142 |
| 4 | 12 | MG (U) | 9 | FS | RF | mixed | 2 | 22 | U |
| 5 | 12 | MG (U) | 9 | M | RH | epithelioid | 3 | 3 | 1079 |
| 6 | 12 | MG (U) | 13 | M | U | round | 1 | 22 | A |
| 7 | 12 | MG (b&t) | 9 | F | LF | epithelioid | 3 | 4 | 189 |
| 8 | 13 | MG (U) | 10 | MC | RH | epithelioid | V | 9 | A |
| 50 | 13 | RW | 5 | F | U | epithelioid | 1 | 65 | 194 |
| 51 | 13 | RW | 11 | M | LH | epithelioid | 1 | 15 | 18 |
| 28 | 13 | LR (black) | 12 | M | LH | epithelioid | 1 | 9 | 504 |
| 82 | 13 | Havanese | 15 | M | LH | round | 1 | 23 | U |
| Dog No. | Breed (Coat Colour) | Age (y) | Sex | Toe | Morphology | Pigment (Degree) | Mitoses/ 10 HPF | Survival Time (d) |
|---|---|---|---|---|---|---|---|---|
| 41 | GS | 9 | M | RH | epithelioid | 1 | 34 | 83 |
| 42 | GS | 10 | MC | LF | epithelioid | 3 | 6 | 52 |
| 43 | GS | 14 | M | U | epithelioid | V | 21 | U |
| 66 | BMD | 8 | M | LH | spindle cell | 2 | 4 | U |
| 63 | Sheepdog | 9 | M | LF | round | V | 9 | 48 |
| 70 | Poodle | 11 | M | RF | mixed | 3 | 4 | U |
| 68 | CS | 11 | FS | U | spindle cell | 2 | 5 | U |
| 10 | MG (b&t) | 14 | M | LF | mixed | 2 | 3 | 840 |
| 11 | MG (U) | 8 | MC | RF | epithelioid | 3 | 3 | A |
| Dog No. | Copy Number | RAS Mutation | Breed (Coat Colour) | Age (y) | Sex | Toe | Morphology | Pigment (Degree) | Mitoses/ 10 HPF | Survival Time (d) |
|---|---|---|---|---|---|---|---|---|---|---|
| 43 | 6 | NRAS exon 3 codon 61 | GS | 14 | M | U | epithelioid | V | 21 | U |
| 48 | 5 | - | GS | 10 | M | RH | epithelioid | 2 | 2 | A |
| 73 | 5 | KRAS exon 2 codon 12 | BS | 9 | M | LH | epithelioid | 1 | 13 | A |
| 57 | 4 | NRAS exon 2 codon 13 | GR | 6 | FS | RF | mixed | 1 | 5 | A |
| 27 | 6 | KRAS exon 2 codon 12 | LR (black) | 14 | M | RF | epithelioid | 1 | 5 | A |
| 61 | 5 | - | IT | 13 | M | LF | epithelioid | 0 | 3 | A |
| 11 | 4 | NRAS exon 3 codon 61 | MG (U) | 8 | MC | RF | epithelioid | 3 | 3 | A |
| 22 | 5 | - | MG (b&t) | 15 | M | LH | spindle cell | 1 | 15 | A |
| 21 | 4 | - | MG (black) | 8 | F | RF | round | 1 | 15 | A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conrad, D.; Kehl, A.; Beitzinger, C.; Metzler, T.; Steiger, K.; Pfarr, N.; Fischer, K.; Klopfleisch, R.; Aupperle-Lellbach, H. Molecular Genetic Investigation of Digital Melanoma in Dogs. Vet. Sci. 2022, 9, 56. https://doi.org/10.3390/vetsci9020056
Conrad D, Kehl A, Beitzinger C, Metzler T, Steiger K, Pfarr N, Fischer K, Klopfleisch R, Aupperle-Lellbach H. Molecular Genetic Investigation of Digital Melanoma in Dogs. Veterinary Sciences. 2022; 9(2):56. https://doi.org/10.3390/vetsci9020056
Chicago/Turabian StyleConrad, David, Alexandra Kehl, Christoph Beitzinger, Thomas Metzler, Katja Steiger, Nicole Pfarr, Konrad Fischer, Robert Klopfleisch, and Heike Aupperle-Lellbach. 2022. "Molecular Genetic Investigation of Digital Melanoma in Dogs" Veterinary Sciences 9, no. 2: 56. https://doi.org/10.3390/vetsci9020056
APA StyleConrad, D., Kehl, A., Beitzinger, C., Metzler, T., Steiger, K., Pfarr, N., Fischer, K., Klopfleisch, R., & Aupperle-Lellbach, H. (2022). Molecular Genetic Investigation of Digital Melanoma in Dogs. Veterinary Sciences, 9(2), 56. https://doi.org/10.3390/vetsci9020056








