Abstract
(1) Background: Anaplasmosis is an infectious disease in camels caused by an obligate intracellular bacterium that is transmitted by ticks. (2) Methods: A cross-sectional study was conducted during 2020 to study the seroprevalence of Anaplasma spp. among Camelus dromedarius in three governorates in Egypt and assess the associated risk factors. Serum samples from 365 camels were examined by a competitive enzyme-linked immunosorbent assay (cELISA) test. (3) Results: Overall, the seroprevalence of anaplasmosis among camels was 18.6%. Multivariable logistic regression was performed, and it was discovered that tick infestation, application of acaricides, grooming practice and body condition were potential risk factors for Anaplasma spp. infection (odds ratio > 1) in dromedary camels. In contrast, the locality in which the camels lived and their age were not significant effects with regard to the occurrence of anaplasmosis. (4) Conclusions: The current findings suggest that improvement of protective measures to limit the effects of the identified risk factors can help to reduce the spread of anaplasmosis among camels in Egypt.
1. Introduction
The camel is a multipurpose animal that lives in arid and semi-arid areas. In many countries, including Egypt, the dromedary (Camelus dromedarius), often known as the one-humped camel or Arabian camel, is a very valuable species. Camels are of socioeconomic importance in Egypt since they can be utilized as sources of meat and milk and as a mode of transportation and tourist rides. Moreover, Camel milk is a healthy food for people since it contains more vitamin C and has less cholesterol [1]. Camels outperform farmed cattle due to their unique physiological characteristics that allow them to survive lengthy periods of time without access to water [2]. Despite their vast resources, camels can get various infectious diseases that affect their health and productivity [3,4,5,6,7].
Many animals, including camels, are affected by haemoparasites. The primary vectors for the transmission of these pathogens are ticks. Many studies have been conducted on tick-borne pathogens in camels, including trypanosomiasis, theileriosis, babesiosis and anaplasmosis [8,9,10].
Anaplasmosis is a vector-borne disease of ruminants [11,12,13]. Several varieties of Anaplasma species can infect camels, such as Anaplasma centrale (A. centrale), Anaplasma marginale (A. marginale), Anaplasma phagocytophilum (A. phagocytophilum) and Anaplasma platys (A. platys) [14]. Anaplasma is an obligate intracellular bacterium that belongs to the order Rickettsiales, Anaplasmataceae family, and is transmitted biologically and mechanically by hard ticks such as Ixodes ricinus, Dermacentor spp., Rhipicephalus spp. and Boophilus spp. [15,16]. Anaplasma spp. are transmitted both biologically and mechanically [16]. In Egypt, most of the hard tick species infesting camels belong to Hyalomma, Haemaphysalis, Amblyomma and Rhipicephalus [17]. At the same time, Hyalomma anatolicum excavatum and Boophilus annulatus were found on cows [18].
In camels, anaplasmosis usually appears as a subclinical infection or as a co-infection. However, it can manifest clinically as fever, anaemia, emaciation, slight ataxia, anorexia, jaundice or enlargement of the lymph nodes [3,19].
Clinical diagnosis of these organisms is difficult due to the nonspecific clinical indications [20]. As a result, in disease-endemic areas, care is important, as well as appropriate diagnostic tests to aid in infection confirmation.
Routine diagnosis in the laboratory for direct detection of anaplasmosis in camels depends mainly on microscopic examination. Light microscopy is the cheapest and fastest laboratory test, but it is a low sensitive technique, and it is heavily reliant on examiner skill [21]. Moreover, the efficacy of this method is affected by the time interval that passes between the onset of the clinical signs of disease and the collection of the sample. This delay leads to the unreliability of this method in carrier animals [3].
In both laboratory and field research, nucleic acid-based techniques such as loop-mediated isothermal amplification (LAMP), polymerase chain reaction (PCR) and quantitative real-time PCR (qPCR) have been used to detect Anaplasma infection [22]. However, the sensitivity of these techniques is limited, especially in persistently infected animals characterized by low-level bacteremia [23].
On the other hand, serological assays have advantages for the investigation of antibodies in infected animals at all stages of the Anaplasma infection [24]. In addition, serological tests are preferred to identify previous exposure to the pathogens as well as carrier animals. The most common of these tests is the indirect fluorescent antibody technique (IFAT); however, the enzyme-linked immunosorbent assay (ELISA) is reliable and convenient and offers more advantages than IFAT [25,26,27].
Anaplasmosis has been reported in dogs, cattle, water buffalo, camels and humans in several localities of Egypt [28,29,30,31,32,33]. Nonetheless, there is a lack of frequent monitoring and control procedures in the field. Moreover, A. marginale is most commonly seen in cattle, camels, and arthropods that live on a variety of host animals [34]. In camels, anaplasmosis has been reported in several parts of Egypt, such as Assuit, South Sinai, Matrouh and Luxor. These findings were based on microscopic examination and use of cELISA, IFAT and polymerase chain reaction (PCR) tests [25,35]. Recently, a study detected antibodies against Anaplasma spp. in camels from Egypt based on commercial cELISA kits that showed 100% sensitivity and specificity [4]. However, few studies have focused on the risk factors that are associated with Anaplasma spp. infection in camels.
Therefore, the present study aimed to determine the seroprevalence of Anaplasma spp. in camels and evaluate the associated risk factors for Anaplasma spp. infection.
2. Materials and Methods
2.1. Ethics Statement
The ethical committee for Animal Experiment of the faculty of veterinary medicine, Benha University, approved all procedures involving the handling and collection of samples from camels used in this study. The camel’s owners gave their verbal approval for the samples to be collected.
2.2. Study Area
A cross-sectional study was conducted during 2020 in three governorates of Egypt that had high camel populations. The study areas were the governorates of: Qalyubia (30°25 N to 31°13 E), Kafr ElSheikh (31.1107° N, 30.9388° E) and the Red Sea (25°32′1″ N 33°26′18″ E) (Figure 1). The climatic conditions of Qalyubia and Kafr ElSheikh governorates are wet winters with moderate rainfall and dry summers, while the Red Sea area has a desert climate during the whole year with virtually no rainfall. These warm climatic conditions are suitable for tick propagation. Ticks are the principal vector for transmission of Anaplasma spp. Moreover, the types of observed ticks in examined camels in the study areas were mainly Rhipicephalus annulatus, Hyalomma dromedarii and Rhipicephalus turanicus [17].
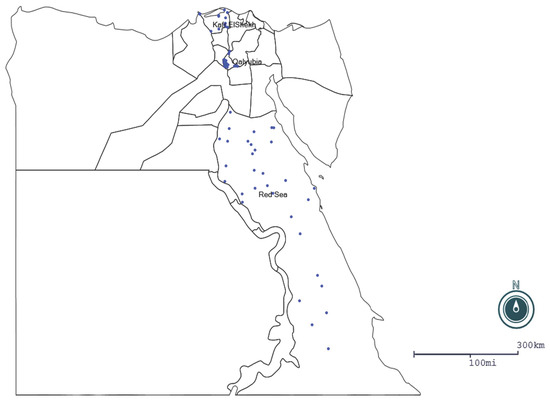
Figure 1.
Geographical areas that were visited in the study and number of positive samples represented by blue dots. MAP generated by EPI MAP (CDC).
2.3. Sample Collection and Preparation
The required sample size of the study was calculated according to a formula devised by Thrusfield [36] as follows:
In which n is the sample size, Pexp is the expected prevalence rate and d is precision. The expected prevalence rate that was used in this study was 34.1%, as previously reported by Parvizi et al. [4], with a 95% confidence interval and 5% precision. The majority of the study animals were chosen at random from small-scale farmers that keep camels as working animals. A total of 365 blood samples were collected from the jugular vein of camels using a vacuum tube without EDTA. The collected blood samples were transferred in iceboxes to the Veterinary Diagnostic Laboratory, Faculty of Veterinary Medicine, Benha University. The sera were collected using clean, sterile vacuum tubes and were separated by centrifugation at 3500× g for 10 min. The examined camels were categorized according to their locality (Qalyubia, Kafr Elsheikh or the Red Sea), sex (male or female) and age (≤2, >2–5 and >5 years old). Moreover, information regarding tick infestation, whether or not acaricides had been applied (trimonthly application), grooming practice (removing thick hair that has accumulated grain, grime, and mats on a regular basis) and body condition (emaciated, decrease the bodyweight than normal or healthy) was collected to investigate their association to infection.
2.4. Serological Analysis
The specific antibodies against Anaplasma spp. were investigated in the collected sera through the use of a commercial competitive ELISA (cELISA) v2 (VMRD Inc, Pullman, WA, USA), which is able to detect antibodies against the major surface protein 5 (MSP5) of A. marginale, A. centrale and A. ovis [37]. The process of the test was performed according to the guidelines of the manufacturer. This kit had previously been validated to show 100% sensitivity and specificity in the detection of Anaplasma spp. antibodies in camels [4]. The sample was considered positive if the cut-off value (Ct) was equal to 0.42.
2.5. Statistical Analysis
Data regarding the anaplasmosis surveillance were analyzed by use of the statistical program for the social sciences (SPSS) software v24 (IBM SPSS Inc., Chicago, IL, USA). The data were verified through the use of the chi-square test, and the results were considered significant if p < 0.05. The results were analyzed through the use of univariable logistic regression to evaluate the association between each variable and prevalence of Anaplasma spp. The Hosmer–Lemeshow goodness-of-fit test was applied to evaluate the fit of the multivariable logistic regression model. The variables with p ≤ 0.2 were included in the multivariable regression model, which was used to determine the risk factors, odds ratios (ORs) and confidence intervals (CIs) of each significant variable in the univariable analyses. Odds ratios of >1 suggested an increased risk of seroprevalence of anaplasma infection, whereas odds ratios of <1 suggested a decreased risk of seroprevalence of anaplasma infection.
3. Results
The present study demonstrated an overall 18.6% (68/365) seroprevalence of anaplasmosis among camels that lived in the three investigated areas. The highest seroprevalence for Anaplasma spp. in camels was estimated to be in the Red Sea governorate (21.3%, n = 32) (Table 1). In order of increasing magnitude, the seroprevalence was 13% (n = 13) and 20% (n = 23) in Kafr ElSheikh and Qalyubia governorates, respectively (Table 1).

Table 1.
Risk factors associated with Anaplasma spp. infection in camels.
According to the univariable analysis, seropositivity to Anaplasma spp. in camels was associated significantly with female sex, tick infestation, non-application of acaricides, poor grooming practice and poor body condition (p < 0.005). The highest seroprevalence rates were observed in females (21.5%), infested camels with ticks (33%) and in cases of the absence of acaricides application (23.5.%) and grooming application (25.9%), Table 1.
In addition, a strong association was found between animals in an emaciated condition and Anaplasma spp. infection. On the other hand, there was no significant interaction between age and Anaplasma spp. infection (Table 1).
Significant variables that were obtained through univariable studies were then analyzed multivariably. The animal’s age and locality were removed as factors. In this study, it was found that females were two times more likely to be infected than males (95% CI: 0.91–4.35). Furthermore, tick infestation of camels (OR = 1.12, 95% CI: 0.54–2.32), lack of acaricide application (OR = 1.02, 95% CI: 0.39–2.68), absence of grooming (OR = 1.3, 95% CI: 0.53–3.18) and an emaciated condition of the examined camels (OR = 9.36, 95% CI: 4.36–20.10) were found to be potential risk factors for Anaplasma spp. infection in camels (Table 2).

Table 2.
Multivariable analysis of the potential risk variables for camel anaplasmosis.
4. Discussion
Dromedary camels can harbor a variety of pathogens, including Anaplasma. This genus has been reported in the last few years in some studies, but the epidemiological data remains limited.
A description of the epidemiological status of anaplasmosis and evaluation of the risk factors that are potentially related to disease in camels helps to improve the understanding of the dynamics of and potential control methods for the disease [38].
In this study, the antibodies against Anaplasma spp. in camels were detected in 68 of 365 animals, and the seroprevalence rate was recorded as 18.6%. Despite the large differences in bioclimatic features between the three sites studied, the prevalence rates do not differ significantly (p > 0.05). The highest rate, 21.3%, was observed in the Red Sea, while the lowest rate (13%) was found in the Kafr ElSheikh governorate. This is likely due to the frequent movement of camels between these areas and the similarities of tick populations infesting camels in the sampling locations [39]. The high rate observed in the Red Sea governorate may be due to the nature of this area, which is a border governorate that receives camels from neighbouring countries. These camels may be carriers of haemoparasites. In addition, different humidity levels that enable the proliferation of vectors and transmission of Anaplasma can affect the prevalence rate [40].
In Egypt, recent studies revealed 47.4%, 47.4% and 67.37% prevalence rates of anaplasmosis in camels. These findings were based on tests that employed cELISA, microscopic examination and PCR techniques [3,4]. Other studies conducted in various countries have reported high prevalence rates of 26–95.5%, 34.2%, 39.6% and 61.11% in Saudi Arabia, Iran, Morocco and Niger, respectively [14,41,42,43]. However, other studies have reported low prevalence rates of anaplasmosis that ranged from 6% to 13.33% [44,45,46,47].
The differences in prevalence rates may be attributable to sample numbers, the diagnostic techniques were used, demographics of the research locations and disease endemicity in each study region [46]. Furthermore, tick control programmes, farm management, husbandry practises, wildlife reservoir hosts and/or abiotic variables may all play a role in the large disparity in prevalence rates. Several studies have found that the incidence of Anaplasma species in ruminants varies depending on geographic location, as well as tick habitat and animal care [48,49]. Moreover, different humidity levels that enable the proliferation of vectors and transmission of anaplasmosis can affect the prevalence rate [40].
From the results, it is clear that the age of the camels did not affect the prevalence of anaplasmosis and animals aged >2 to 5 years were at a higher risk than those aged >5 years. This finding is in contrast to those of Farooqi et al. [50], who reported that the age of the camels was a potential risk factor for the occurrence of anaplasmosis. In addition, Kocan et al. [51] observed that animals over the age of five are found to be less affected, which can be related to the fact that low-level infections over time lead to immunity against clinical anaplasmosis.
A further finding was that the sex of the animal was a significant variable for camel anaplasmosis, and females were more susceptible to infection than males. The findings contradict those of Azmat et al. [46], who found that male animals have higher infection rates than female animals. Our result is consistent with the findings of Maurizi et al. [52] and Belkahia et al. [53], who reported a higher prevalence rate in females in comparison with males. The differences between these findings can be explained since people in the area of the Javed study kept female camels for breeding purposes, so these animals performed few draft activities and were more exposed to tick infestation. Moreover, this is may be due to female immunosuppression, which can develop during pregnancy and lactation and has the potential to last two years [54].
Other factors that were found to be significant were the tick infestation of examined camels and whether acaricides were previously applied. Camels that were infested with ticks were at high risk of anaplasma infection compared with non-infested camels. Overall, these findings are in accordance with those reported by Atif [55], who reported that tick infestation made animals more susceptible to infection. We believe that Egyptian camels can be infested by a variety of ticks, particularly hard ticks, which are the main vector for Anaplasma spp. [56].
Furthermore, in line with the results of Azmat et al. [46], the current study found that good grooming practice significantly reduced the rate of Anaplasma spp. infection, because frequent grooming led to the early observation of ticks, which could be controlled immediately. Furthermore, grooming practises have a considerable impact on disease dynamics, which could be attributed to the fact that regular grooming practice allows for early detection of vectors and prompt control. In addition, the absence of grooming allowed the existence of hiding spots for vectors that are difficult to manage through farm manipulation. Similarly, emaciated camels were found to be more susceptible to infection, as was previously concluded by Azmat et al. [46]. This may be explained by the emaciation due to infestation with ticks and concurrent infection by other parasites or bacteria, which can increase the risk of infection [57].
The limitation of this study was the use of a commercial ELISA test for the detection antibodies against A. marginale, A. centrale and A. ovis. A specific test such as PCR is needed to determine the prevalent species among camels in Egypt. In addition, the present study is a cross-sectional study able to evaluate association only; therefore, longitudinal studies are required to prove causation.
5. Conclusions
It has been concluded that tick infestation, tick control status, grooming practice and body condition are strongly associated with Anaplasma spp. infection. In Egypt, the link between Anaplasma spp. infection and their arthropod vectors is mostly unknown, and more research is needed. However, further epidemiological and molecular studies are required to evaluate the situation of the disease across the country and to identify the genetic features of Anaplasma spp. in camels.
Author Contributions
A.S., K.A.A., R.A.A., F.M.A. and I.K.; methodology, A.S.; validation, A.S., K.A.A., R.A.A. and I.K.; formal analysis, A.S.; investigation, K.A.A., F.M.A. and R.A.A.; resources, A.S.; writing—original draft preparation, A.S. and M.Z.S.-A.; writing—review and editing, I.K. and M.Z.S.-A.; supervision, K.A.A. and R.A.A.; project administration, K.A.A., R.A.A., F.M.A. and M.Z.S.-A.; funding acquisition, K.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by King Saud University, Riyadh, Saudi Arabia, grant number RSP-2021/369.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Benha University and approved by the Ethics Committee of the Faculty of Veterinary Medicine, Benha University (BUFVTM).
Informed Consent Statement
Informed consent was obtained from the owner to collect the sample.
Data Availability Statement
All data analyzed during this study are included in this published article.
Acknowledgments
The authors extend their appreciation to Researchers Supporting Project number (RSP-2021/369), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mullaicharam, A. A review on medicinal properties of camel milk. World J. Pharm. Sci. 2014, 2, 237–242. [Google Scholar]
- Gahlot, T.; Chhabra, M. Selected Research on Camelid Parasitology; Camel Pub. House: Bikaner, India, 2009. [Google Scholar]
- El-Naga, T.R.A.; Barghash, S. Blood parasites in camels (Camelus dromedarius) in Northern West Coast of Egypt. J. Bacteriol. Parasitol. 2016, 7, 258. [Google Scholar]
- Parvizi, O.; El-Adawy, H.; Roesler, U.; Neubauer, H.; Mertens-Scholz, K. Performance analysis of Anaplasma antibody competitive ELISA using the ROC curve for screening of anaplasmosis in camel populations in Egypt. Pathogens 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.; Abdelhady, A. The first detection of anti-West Nile virus antibody in domestic ruminants in Egypt. Trop. Anim. Health Prod. 2020, 52, 3147–3151. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.; Attia, K.A.; Alsubki, R.A.; Kimiko, I.; Sayed-Ahmed, M.Z. Cross-sectional survey on Mycobacterium avium Subsp. paratuberculosis in Dromedary Camels: Seroprevalence and risk factors. Acta Trop. 2022, 226, 106261. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.; Ali, A.-F. Seroprevalence and risk factors for C. burentii infection in camels in Egypt. Comp. Immunol. Microbiol. Infect. Dis. 2020, 68, 101402. [Google Scholar] [CrossRef]
- Sazmand, A.; Eigner, B.; Mirzaei, M.; Hekmatimoghaddam, S.H.; Harl, J.; Duscher, G.G.; Fuehrer, H.-P.; Joachim, A. Molecular identification of hemoprotozoan parasites in camels (Camelus dromedarius) of Iran. Iran. J. Parasitol. 2016, 11, 568. [Google Scholar]
- Ranjbar Bahadori, S.; Afshari Moghadam, A. Study on the prevalence of blood parasites in camels of Zabol in 2008. Vet. Clin. Pathol. Q. Sci. J. 2009, 3, 503–507. [Google Scholar]
- Alanazi, A.D.; Nguyen, V.L.; Alyousif, M.S.; Manoj, R.R.; Alouffi, A.S.; Donato, R.; Sazmand, A.; Mendoza-Roldan, J.A.; Dantas-Torres, F.; Otranto, D. Ticks and associated pathogens in camels (Camelus dromedarius) from Riyadh Province, Saudi Arabia. Parasites Vectors 2020, 13, 110. [Google Scholar] [CrossRef]
- Hairgrove, T.; Schroeder, M.E.; Budke, C.M.; Rodgers, S.; Chung, C.; Ueti, M.W.; Bounpheng, M.A. Molecular and serological in-herd prevalence of Anaplasma marginale infection in Texas cattle. Prev. Vet. Med. 2015, 119, 1–9. [Google Scholar] [CrossRef]
- Silveira, J.; Rabelo, E.; Ribeiro, M. Molecular detection of tick-borne pathogens of the family Anaplasmataceae in Brazilian brown brocket deer (Mazama gouazoubira, Fischer, 1814) and marsh deer (Blastocerus dichotomus, Illiger, 1815). Transbound. Emerg. Dis. 2012, 59, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Wernery, U.; Kaaden, O.R. Infectious Diseases in Camelids; Georg Thieme Verlag: Stuttgart, Germany, 2002. [Google Scholar]
- Lorusso, V.; Wijnveld, M.; Latrofa, M.S.; Fajinmi, A.; Majekodunmi, A.O.; Dogo, A.G.; Igweh, A.C.; Otranto, D.; Jongejan, F.; Welburn, S.C. Canine and ovine tick-borne pathogens in camels, Nigeria. Vet. Parasitol. 2016, 228, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Kocan, K.M.; De La Fuente, J.; Blouin, E.; Garcia-Garcia, J. Anaplasma marginale (Rickettsiales: Anaplasmataceae): Recent advances in defining host–pathogen adaptations of a tick-borne rickettsia. Parasitology 2004, 129, S285–S300. [Google Scholar] [CrossRef] [PubMed]
- Kocan, K.M.; de la Fuente, J.; Blouin, E.F.; Coetzee, J.F.; Ewing, S. The natural history of Anaplasma marginale. Vet. Parasitol. 2010, 167, 95–107. [Google Scholar] [CrossRef]
- Abd El-Baky, S.M.M.; Allam, N.A. Anaplasmosis in ruminants and infesting ticks vectors settling Egyptian desert: Epidemiological updates regarding genetic profiles. Biosci. Res. 2018, 15, 2651–2667. [Google Scholar]
- Abdel-Shafy, S.; Allam, N.A.; Mediannikov, O.; Parola, P.; Raoult, D. Molecular detection of spotted fever group rickettsiae associated with ixodid ticks in Egypt. Vector-Borne Zoonotic Dis. 2012, 12, 346–359. [Google Scholar] [CrossRef]
- Sudan, V.; Sharma, R.; Borah, M. Subclinical anaplasmosis in camel (Camelus dromedarius) and its successful therapeutic management. J. Parasit. Dis. 2014, 38, 163–165. [Google Scholar] [CrossRef]
- Dumler, J.S.; Barbet, A.F.; Bekker, C.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and’HGE agent’as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar]
- Silaghi, C.; Santos, A.S.; Gomes, J.; Christova, I.; Matei, I.A.; Walder, G.; Domingos, A.; Bell-Sakyi, L.; Sprong, H.; Von Loewenich, F.D. Guidelines for the direct detection of Anaplasma spp. in diagnosis and epidemiological studies. Vector-Borne Zoonotic Dis. 2017, 17, 12–22. [Google Scholar] [CrossRef]
- Chi, Q.; Liu, Z.; Li, Y.; Yang, J.; Chen, Z.; Yue, C.; Luo, J.; Yin, H. Development of a Real-Time PCR Assay for Detection and Quantification of A naplasma ovis Infection. Transbound. Emerg. Dis. 2013, 60, 119–124. [Google Scholar] [CrossRef]
- Shompole, S.; Waghela, S.D.; Rurangirwa, F.R.; McGuire, T. Cloned DNA probes identify Anaplasma ovis in goats and reveal a high prevalence of infection. J. Clin. Microbiol. 1989, 27, 2730–2735. [Google Scholar] [CrossRef] [PubMed]
- McGuire, T.C.; Davis, W.; Brassfield, A.; McElwain, T.; Palmer, G. Identification of Anaplasma marginale long-term carrier cattle by detection of serum antibody to isolated MSP-3. J. Clin. Microbiol. 1991, 29, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, O. Overview of Anaplasmosis in Arab Countries in North Africa and the Middle East, and Optimizing a commercial c-ELISA for Camels. Ph.D. Thesis, Free University, Berlin, Germany, 2021. [Google Scholar]
- von Fricken, M.E.; Lkhagvatseren, S.; Boldbaatar, B.; Nymadawa, P.; Weppelmann, T.A.; Baigalmaa, B.-O.; Anderson, B.D.; Reller, M.E.; Lantos, P.M.; Gray, G.C. Estimated seroprevalence of Anaplasma spp. and spotted fever group Rickettsia exposure among herders and livestock in Mongolia. Acta Trop. 2018, 177, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Chung, J.-H.; Kim, C.-M.; Yun, N.R.; Kim, D.-M. Asymptomatic-anaplasmosis confirmation using genetic and serological tests and possible coinfection with spotted fever group Rickettsia: A case report. BMC Infect. Dis. 2020, 20, 445. [Google Scholar] [CrossRef]
- Nasreldin, N.; Ewida, R.M.; Hamdon, H.; Elnaker, Y.F. Molecular diagnosis and biochemical studies of tick-borne diseases (anaplasmosis and babesiosis) in Aberdeen Angus Cattle in New Valley, Egypt. Vet. World 2020, 13, 1884. [Google Scholar] [CrossRef]
- Amira, A.-H.; Răileanu, C.; Tauchmann, O.; Fischer, S.; Nijhof, A.M.; Silaghi, C. Epidemiology and genotyping of Anaplasma marginale and co-infection with piroplasms and other Anaplasmataceae in cattle and buffaloes from Egypt. Parasites Vectors 2020, 13, 495. [Google Scholar]
- Tumwebaze, M.A.; Lee, S.-H.; Moumouni, P.F.A.; Mohammed-Geba, K.; Sheir, S.K.; Galal-Khallaf, A.; Abd El Latif, H.M.; Morsi, D.S.; Bishr, N.M.; Galon, E.M. First detection of Anaplasma ovis in sheep and Anaplasma platys-like variants from cattle in Menoufia governorate, Egypt. Parasitol. Int. 2020, 78, 102150. [Google Scholar] [CrossRef]
- Ghafar, M.W.; Amer, S.A. Prevalence and first molecular characterization of Anaplasma phagocytophilum, the agent of human granulocytic anaplasmosis, in Rhipicephalus sanguineus ticks attached to dogs from Egypt. J. Adv. Res. 2012, 3, 189–194. [Google Scholar] [CrossRef]
- Selim, A.; Alanazi, A.D.; Sazmand, A.; Otranto, D. Seroprevalence and associated risk factors for vector-borne pathogens in dogs from Egypt. Parasites Vectors 2021, 14, 175. [Google Scholar] [CrossRef]
- Selim, A.; Almohammed, H.; Abdelhady, A.; Alouffi, A.; Alshammari, F.A. Molecular detection and risk factors for Anaplasma platys infection in dogs from Egypt. Parasites Vectors 2021, 14, 429. [Google Scholar] [CrossRef]
- Sazmand, A.; Joachim, A.; Otranto, D. Zoonotic parasites of dromedary camels: So important, so ignored. Parasites Vectors 2019, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- Barghash, S.; Hafez, A.; Darwish, A.; El-Naga, T. Molecular detection of pathogens in ticks infesting camels in Matrouh Governorate, Egypt. J. Bacteriol. Parasitol. 2016, 7, 2. [Google Scholar] [CrossRef]
- Thrusfield, M. Veterinary Epidemiology; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Dreher, U.; De La Fuente, J.; Hofmann-Lehmann, R.; Meli, M.L.; Pusterla, N.; Kocan, K.; Woldehiwet, Z.; Braun, U.; Regula, G.; Staerk, K. Serologic cross-reactivity between Anaplasma marginale and Anaplasma phagocytophilum. Clin. Vaccine Immunol. 2005, 12, 1177–1183. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mentaberre, G.; Gutiérrez, C.; Rodríguez, N.F.; Joseph, S.; González-Barrio, D.; Cabezón, O.; de la Fuente, J.; Gortazar, C.; Boadella, M. A transversal study on antibodies against selected pathogens in dromedary camels in the Canary Islands, Spain. Vet. Microbiol. 2013, 167, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Belkahia, H.; Said, M.B.; El Hamdi, S.; Yahiaoui, M.; Gharbi, M.; Daaloul-Jedidi, M.; Mhadhbi, M.; Jedidi, M.; Darghouth, M.A.; Klabi, I. First molecular identification and genetic characterization of Anaplasma ovis in sheep from Tunisia. Small Rumin. Res. 2014, 121, 404–410. [Google Scholar] [CrossRef]
- Chepkwony, R.; Castagna, C.; Heitkönig, I.; Van Bommel, S.; Van Langevelde, F. Associations between monthly rainfall and mortality in cattle due to East Coast fever, anaplasmosis and babesiosis. Parasitology 2020, 147, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Ghafar, M.W.; Shobrak, M.Y. Molecular detection and characterization of Anaplasma phagocytophilum, the causative agent of human granulocytic anaplasmosis, in some animals suspected to be competent reservoirs in Taif district, Kingdom of Saudi Arabia. Life Sci. J. 2014, 11, 63–69. [Google Scholar]
- Bahrami, S.; Hamidinejat, H.; Tafreshi, A.R.G. First molecular detection of Anaplasma phagocytophilum in dromedaries (Camelus dromedarius). J. Zoo Wildl. Med. 2018, 49, 844–848. [Google Scholar]
- Bastos, A.D.; Mohammed, O.B.; Bennett, N.C.; Petevinos, C.; Alagaili, A.N. Molecular detection of novel Anaplasmataceae closely related to Anaplasma platys and Ehrlichia canis in the dromedary camel (Camelus dromedarius). Vet. Microbiol. 2015, 179, 310–314. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Chen, Z.; Qin, G.; Li, Y.; Li, Q.; Liu, J.; Liu, Z.; Guan, G.; Yin, H. Anaplasma infection of Bactrian camels (Camelus bactrianus) and ticks in Xinjiang, China. Parasites Vectors 2015, 8, 313. [Google Scholar] [CrossRef] [PubMed]
- Sharifiyazdi, H.; Jafari, S.; Ghane, M.; Nazifi, S.; Sanati, A. Molecular investigation of Anaplasma and Ehrlichia natural infections in the dromedary camel (Camelus dromedarius) in Iran. Comp. Clin. Pathol. 2017, 26, 99–103. [Google Scholar] [CrossRef]
- Azmat, M.; Ijaz, M.; Farooqi, S.; Ghaffar, A.; Ali, A.; Masud, A.; Saleem, S.; Rehman, A.; Ali, M.; Mehmood, K. Molecular epidemiology, associated risk factors, and phylogenetic analysis of anaplasmosis in camel. Microb. Pathog. 2018, 123, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Islam, S.; Ferdous, J.; Rahman, M.K.; Uddin, M.H.; Akter, S.; Rahman, M.H.; Hassan, M.M. Diversity and prevalence of parasitic infestation with zoonotic potential in dromedary camel (Camelus dromedarius) and fat-tailed sheep (dhumba) in Bangladesh. J. Adv. Vet. Anim. Res. 2019, 6, 142. [Google Scholar] [CrossRef] [PubMed]
- Torina, A.; Alongi, A.; Naranjo, V.; Estrada-Peña, A.; Vicente, J.; Scimeca, S.; Marino, A.M.; Salina, F.; Caracappa, S.; de la Fuente, J. Prevalence and genotypes of Anaplasma species and habitat suitability for ticks in a Mediterranean ecosystem. Appl. Environ. Microbiol. 2008, 74, 7578–7584. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, M.; Wang, Z.; Wang, J.; Peng, Y.; Li, Y.; Guan, G.; Luo, J.; Yin, H. Molecular survey and genetic identification of Anaplasma species in goats from central and southern China. Appl. Environ. Microbiol. 2012, 78, 464–470. [Google Scholar] [CrossRef]
- Farooqi, S.H.; Ijaz, M.; Rashid, M.I.; Nabi, H.; Islam, S.; Aqib, A.I.; Hussain, K.; Khan, A.; Rizvi, S.N.B.; Mahmood, S. Molecular epidemiology of bovine anaplasmosis in Khyber Pakhtunkhwa, Pakistan. Trop. Anim. Health Prod. 2018, 50, 1591–1598. [Google Scholar] [CrossRef]
- Kocan, K.M.; De la Fuente, J.; Guglielmone, A.A.; Meléndez, R.D. Antigens and alternatives for control of Anaplasma marginale infection in cattle. Clin. Microbiol. Rev. 2003, 16, 698–712. [Google Scholar] [CrossRef]
- Maurizi, L.; Marié, J.-L.; Courtin, C.; Gorsane, S.; Chal, D.; Davoust, B. Seroprevalence survey of equine anaplasmosis in France and in sub-Saharan Africa. Clin. Microbiol. Infect. 2009, 15, 68–69. [Google Scholar] [CrossRef][Green Version]
- Belkahia, H.; Said, M.B.; Sayahi, L.; Alberti, A.; Messadi, L. Detection of novel strains genetically related to Anaplasma platys in Tunisian one-humped camels (Camelus dromedarius). J. Infect. Dev. Ctries. 2015, 9, 1117–1125. [Google Scholar] [CrossRef]
- ElWishy, A. A study of the genital organs of the female dromedary (Camelus dromedarius). Reproduction 1988, 82, 587–593. [Google Scholar] [CrossRef]
- Atif, F.A. Anaplasma marginale and Anaplasma phagocytophilum: Rickettsiales pathogens of veterinary and public health significance. Parasitol. Res. 2015, 114, 3941–3957. [Google Scholar] [CrossRef] [PubMed]
- Okely, M.; Anan, R.; Gad-Allah, S.; Samy, A. Hard ticks (Acari: Ixodidae) infesting domestic animals in Egypt: Diagnostic characters and a taxonomic key to the collected species. Med. Vet. Entomol. 2021, 35, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Maidala, A. A survey of cattle, sheep, and goat tick infestation in Katagum local government area of Bauchi State, Nigeria. Available online: https://iiardpub.org/get/IJAES/VOL%201/1-5.pdf (accessed on 10 December 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).