Proteomics Evaluation of Semen of Clinically Healthy Beagle-Breed Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Sample Collection, Seminological Examination
2.2. Sample Preparation for Proteomics Evaluation
2.3. Peptide Generation and 1-D nanoLC-MS/MS Analysis
2.4. LC-MS/MS Analysis
2.5. Protein Clustering
2.6. Data Management and Analysis
3. Results
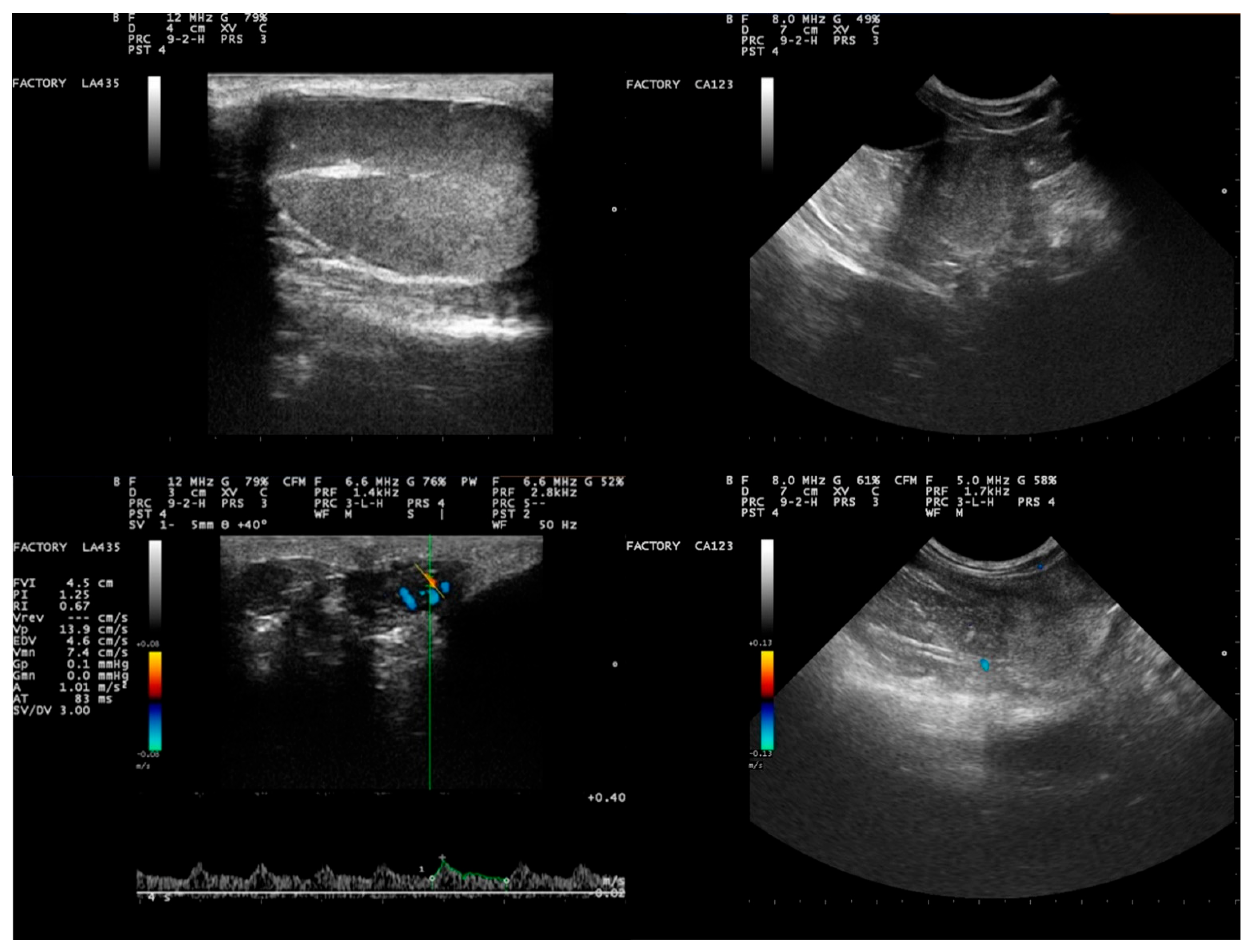
3.1. Clinical and Seminological Findings
3.2. Proteomics Findings
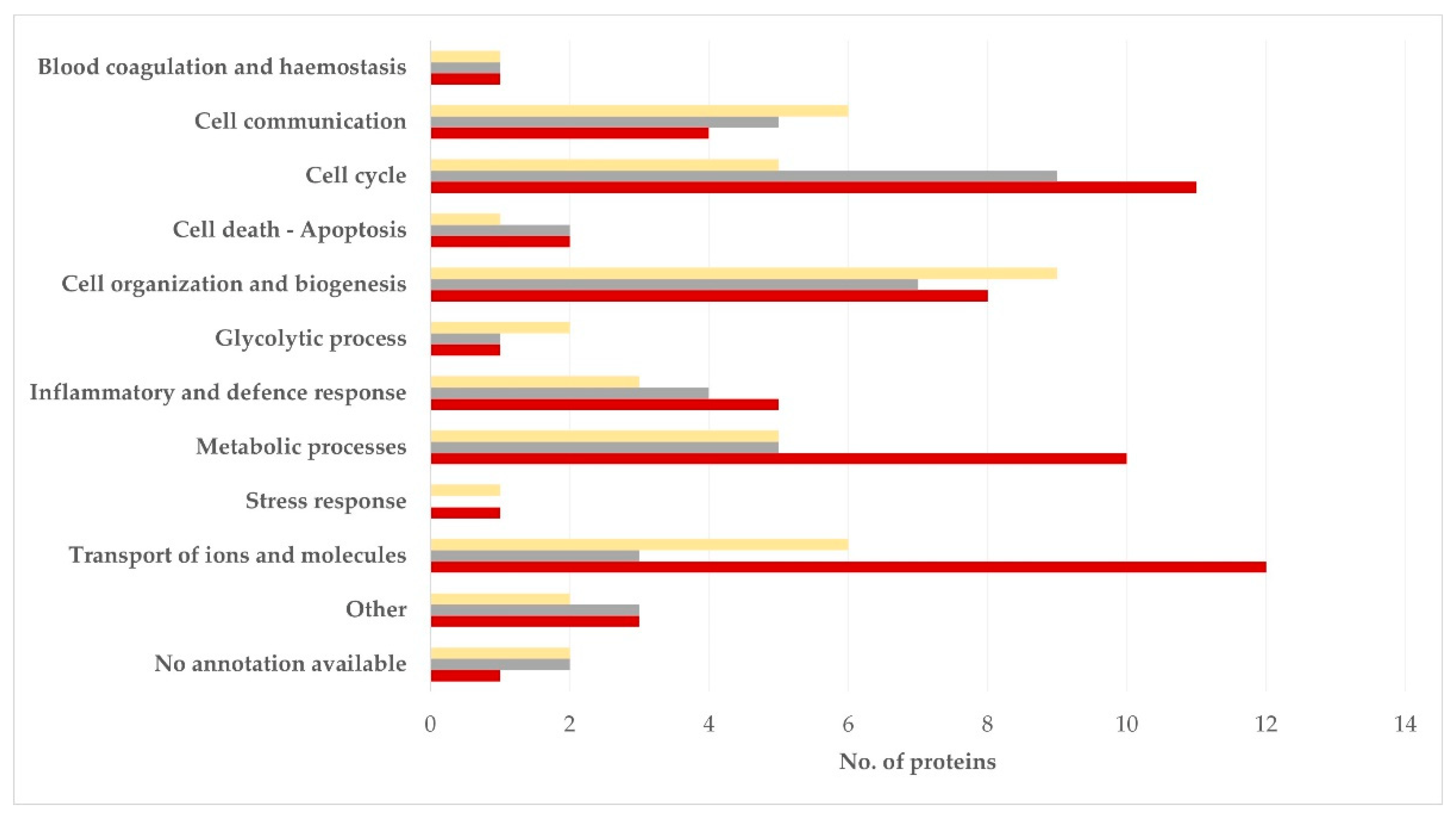
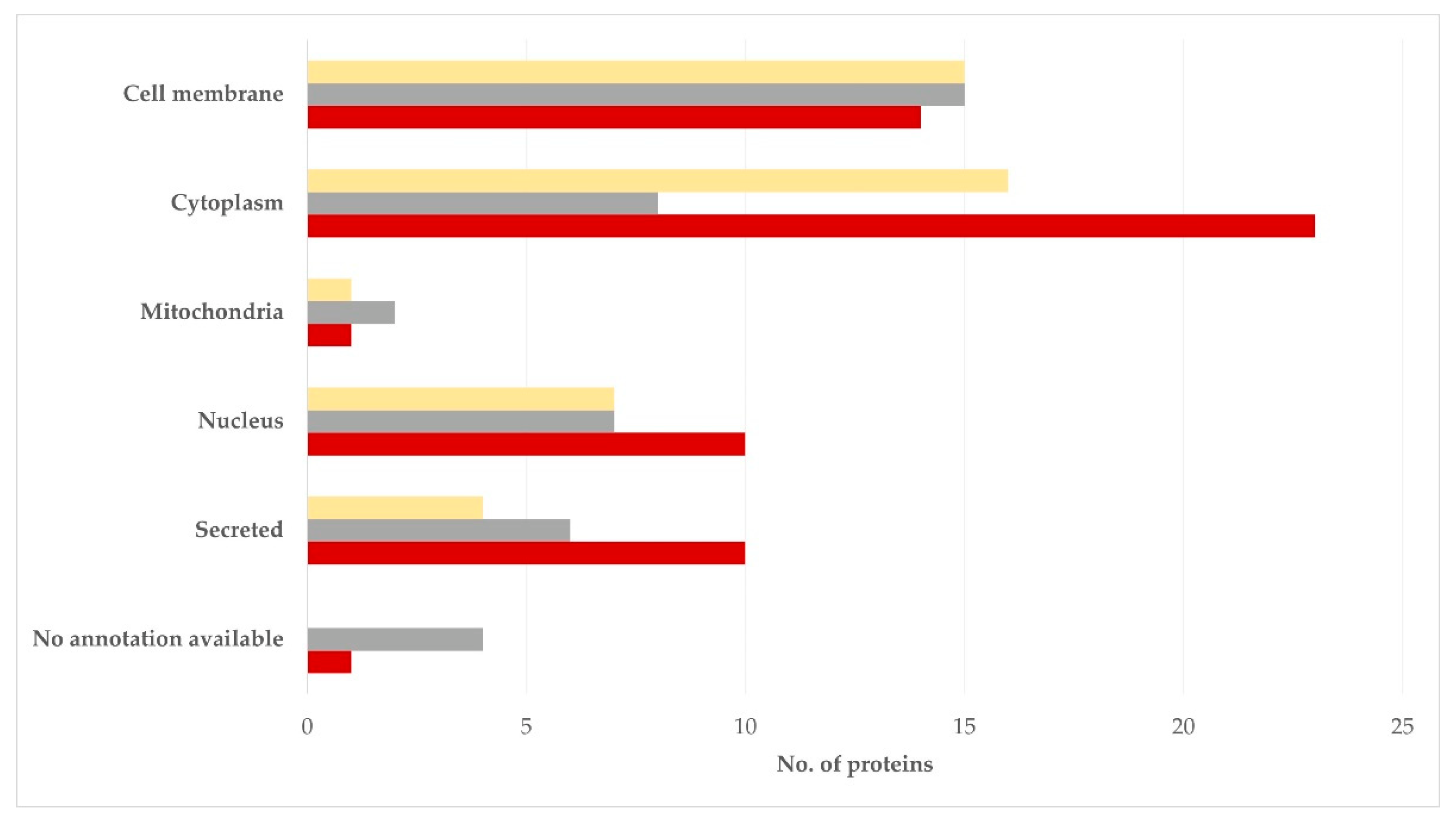
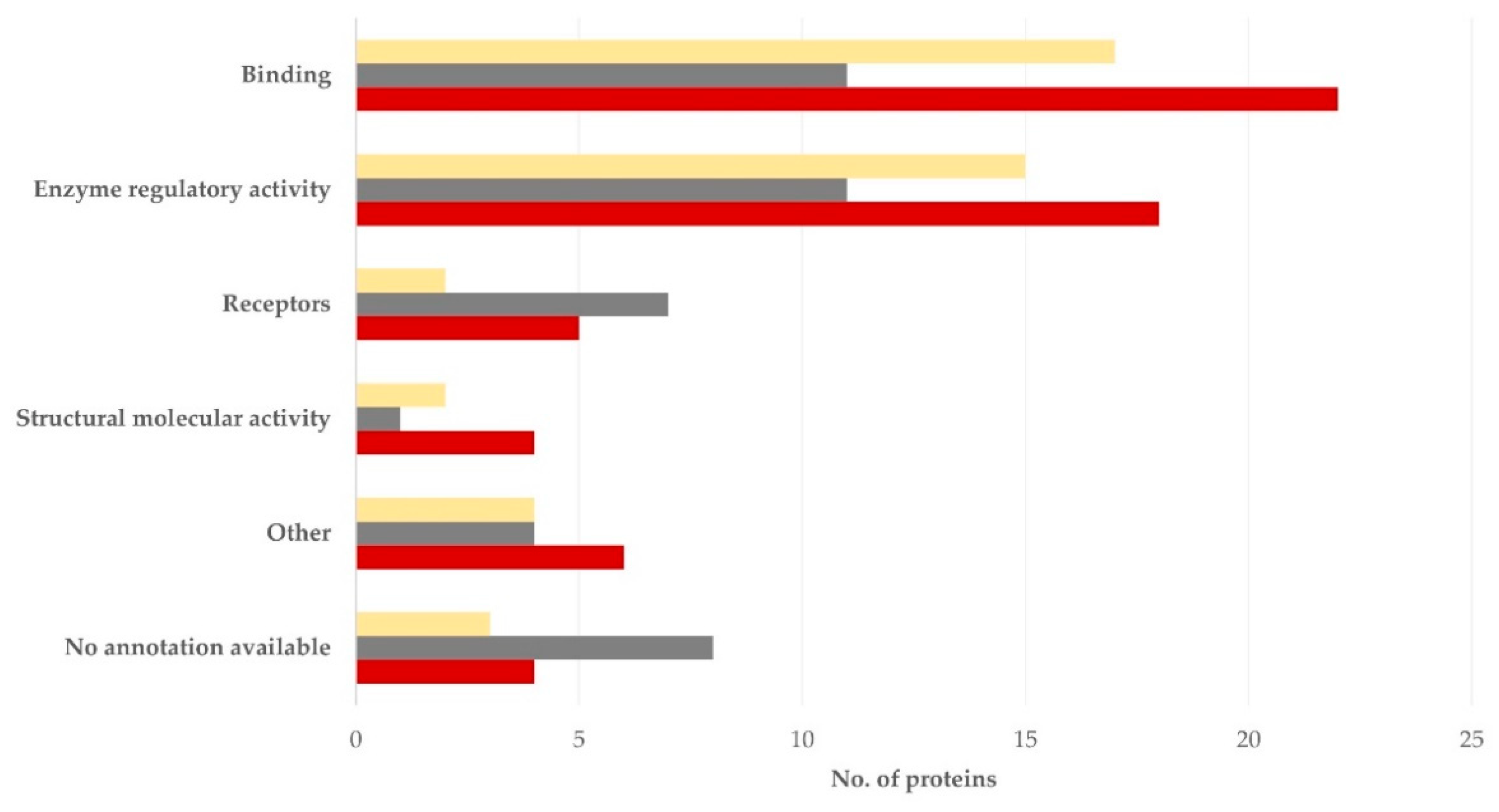
3.3. Clustering of Proteins
4. Discussion
4.1. Preamble
4.2. Significance of Proteins Detected in Semen Samples
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gingras, A.C.; Gstaiger, M.; Raught, B.; Aebersold, R. Analysis of protein complexes using mass spectrometry. Nat. Rev. Mol. Cell Biol. 2007, 8, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Witze, E.S.; Old, W.M.; Resing, K.A.; Ahn, N.G. Mapping protein post-translational modifications with mass spectrometry. Nat. Methods 2007, 4, 798–806. [Google Scholar] [CrossRef] [PubMed]
- González-Cadavid, V.; Martins, J.A.M.; Moreno, F.B.; Andrade, T.S.; Santos, A.C.L.; Monteiro-Moreira, A.C.O.; Moreira, R.A.; Moura, A.A. Seminal plasma proteins of adult boars and correlations with sperm parameters. Theriogenology 2014, 82, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Premrov Bajuk, B.; Zrimšek, P.; Zakošek Pipan, M.; Tilocca, B.; Soggiu, A.; Bonizzi, L.; Roncada, R. Proteomic analysis of fresh and liquid-stored boar spermatozoa. Animals 2020, 10, 553. [Google Scholar] [CrossRef]
- La Falci, V.S.N.; Tortorella, H.; Rodrigues, J.L.; Brandelli, A. Seasonal variation of goat seminal plasma proteins. Theriogenology 2002, 57, 1035–1048. [Google Scholar] [CrossRef]
- Kelly, V.C.; Kuy, S.; Palmer, D.J.; Xu, Z.; Davis, S.R.; Cooper, G.J. Characterization of bovine seminal plasma by proteomics. Proteomics 2006, 6, 5826–5833. [Google Scholar] [CrossRef]
- Rego, J.P.A.; Crisp, J.M.; Moura, A.A.; Nouwens, A.S.; Li, Y.; Venus, B.; Corbet, N.J.; Corbet, D.H.; Burns, B.M.; Boe-Hansen, G.B.; et al. Seminal plasma proteome of electroejaculated Bos indicus bulls. Anim. Reprod. Sci. 2014, 148, 1–17. [Google Scholar] [CrossRef]
- Souza, C.E.A.; Rego, J.P.A.; Lobo, C.H.; Oliveira, J.T.A.; Nogueira, F.C.S.; Domont, G.B.; Fioramonte, M.; Gozzo, F.C.; Moreno, F.B.; Monteino-Moreira, A.C.O.; et al. Proteomic analysis of the reproductive tract fluids from tropically-adapted Santa Ines rams. J. Proteom. 2012, 75, 4436–4456. [Google Scholar] [CrossRef]
- Druart, X.; Rickard, J.P.; Mactier, S.; Kohnke, P.L.; Kershaw-Young, C.M.; Bathgate, R.; Gibb, Z.; Crossett, B.; Tsikis, G.; Labas, V.; et al. Proteomic characterization and cross species comparison of mammalian seminal plasma. J. Proteom. 2013, 91, 13–22. [Google Scholar] [CrossRef]
- Jobim, M.I.M.; Trein, C.; Zirkler, H.; Gregory, R.M.; Sieme, H.; Mattos, R.C. Two dimensional polyacrylamide gel electrophoresis of equine seminal plasma proteins and their relation with semen freezability. Theriogenology 2011, 76, 765–771. [Google Scholar] [CrossRef]
- Guasti, P.N.; Souza, F.F.; Scott, C.; Papa, P.M.; Camargo, L.S.; Schmith, R.A.; Monteiro, G.A.; Hartwig, F.P.; Papa, F.O. Equine seminal plasma and sperm membrane: Functional proteomic assessment. Theriogenology 2020, 156, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Mogielnicka-Brzozowska, M.; Prochowska, S.; Niżański, W.; Bromke, M.A.; Wiśniewski, J.; Olejnik, B.; Kuzborska, A.; Fraser, L.; Młynarz, P.; Kordan, W. Proteome of cat semen obtained after urethral catheterization. Theriogenology 2020, 141, 68–81. [Google Scholar] [CrossRef] [PubMed]
- De Souza, F.F.; Barreto, C.S.; Lopes, M.D. Characteristics of seminal plasma proteins and their correlation with canine semen analysis. Theriogenology 2007, 68, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.S.; Henriques Paulo, O.L.O.; Paranzini, C.S.; Scott, C.; Codognoto, V.M.; Freitas Dell’Aqua, C.P.; Papa, F.O.; Ferreira de Souza, F. Proteomic data of seminal plasma and spermatozoa of four purebred dogs. Data Brief 2020, 30, 105498. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.S.; Henriques Paulo, O.L.O.; Scott, C.; Paranzini, C.S.; Codognoto, V.M.; Freitas Dell’Aqua, C.P.; Papa, F.O.; Ferreira de Souza, F. Insights into the influence of canine breed on proteomics of the spermatozoa and seminal plasma. J. Proteom. 2022, 257, 104508. [Google Scholar] [CrossRef]
- Zmudzinska, A.; Wisniewski, J.; Mlynarz, P.; Olejnik, B.; Mogielnicka-Brzozowska, M. Age-dependent variations in functional quality and proteomic characteristics of canine (Canis lupus familiaris) epididymal spermatozoa. Int. J. Mol. Sci. 2022, 23, 9143. [Google Scholar] [CrossRef]
- Gouletsou, P.G.; Galatos, A.D.; Leontides, L.S.; Sideri, A.I. Impact of fine- or large-needle aspiration on canine testes: Clinical, in vivo ultrasonographic and seminological assessment. Reprod. Domest. Anim. 2011, 46, 712–719. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Anagnostopoulos, A.K.; Katsafadou, A.I.; Pierros, V.; Kontopodis, E.; Fthenakis, G.C.; Arsenos, G.; Karkabounas, S.C.; Tzora, A.; Skoufos, I.; Tsangaris, G.T. Milk of Greek sheep and goat breeds; characterization by means of proteomics. J. Proteom. 2016, 147, 76–84. [Google Scholar] [CrossRef]
- Proikakis, S.C.; Bouroutzika, E.V.; Anagnostopoulos, A.K.; Tsangaris, G.T. Proteomic data of donkey’s milk. Data Brief 2021, 39, 107507. [Google Scholar] [CrossRef]
- Anagnostopoulos, A.K.; Stravopodis, D.J.; Tsangaris, G.T. Yield of 6,000 proteins by 1D nLC–MS/MS without pre-fractionation. J. Chromatogr. B 2017, 1047, 92–96. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Presslmayer-Hartler, A.; Wait, R.; Hummel, K.; Sensi, C.; Eberini, I.; Razzazi-Fazeli, E.; Gianazza, E. In between—Proteomics of dog biological fluids. J. Proteom. 2014, 106, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Cortez, A.; Queiroz Pinheiro, B.; Cruvinel Lima, D.B.; Rodrigues Silva, H.V.; Mota-Filho, A.C.; Matias Martins, J.A.; Rodriguez-Villamil, P.; Moura, A.A.; Machado Silva, L.D. Proteomic characterization of canine seminal plasma. Theriogenology 2017, 95, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Greco, V.; Piras, C.; Pieroni, L.; Urbani, A. Direct assessment of plasma/serum sample quality for proteomics biomarker investigation. In Serum/Plasma Proteomics; Methods in Molecular Biology; Greening, D., Simpson, R., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1619. [Google Scholar]
- Flesch, F.M.; Gadella, B.M. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim. Biophys. Acta 2000, 1469, 197–235. [Google Scholar] [CrossRef]
- Whited, A.M.; Johs, A. The interactions of peripheral membrane proteins with biological membranes. Chem. Phys. Lipids 2015, 192, 51–59. [Google Scholar] [CrossRef]
- Law, G.L.; McGuinness, M.P.; Linder, C.C.; Griswold, M.D. Expression of apolipoprotein E mRNA in the epithelium and interstitium of the testis and the epididymis. J. Androl. 1997, 18, 32–42. [Google Scholar]
- Cao, X.; Cui, Y.; Zhang, X.; Lou, J.; Zhou, J.; Bei, H.; Wei, R. Proteomic profile of human spermatozoa in healthy and asthenozoospermic individuals. Reprod. Biol. Endocrinol. 2018, 16, 16. [Google Scholar] [CrossRef]
- Calvin, X. Selective incorporation of selenium-75 into a polypeptide of the rat sperm tail. J. Exp. Zool. 1978, 204, 445–452. [Google Scholar] [CrossRef]
- Hinsch, E.; Boehm, J.G.; Groeger, S.; Mueller-Schoesser, F.; Hinsch, K.D. Identification of cytokeratins in bovine sperm outer dense fibre fractions. Reprod. Domest. Anim. 2003, 38, 155–160. [Google Scholar] [CrossRef]
- Lindemann, C.B.; Kanous, K.S. Regulation of mammalian sperm motility. Arch. Androl. 1989, 23, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Costur, P.; Filiz, S.; Gonca, S.; Çulha, M.; Gülecen, T.; Solakoğlu, S.; Canberk, Y.; Çalışkan, E. Êxpression of inducible nitric oxide synthase (iNOS) in the azoospermic human testis. Andrologia 2012, 44, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Lue, Y.; Sinha Hikim, A.P.; Wang, C.; Leung, A.; Swerdloff, R.S. Functional role of inducible nitric oxide synthase in the induction of male germ cell apoptosis, regulation of sperm number, and determination of testes size: Evidence from null mutant mice. Endocrinology 2003, 144, 3092–3100. [Google Scholar] [CrossRef] [PubMed]
- Auharek, S.A.; Avelar, G.F.; Lara, N.L.M.; Sharpe, R.M.; França, L.R. Sertoli cell numbers and spermatogenic efficiency are increased in inducible nitric oxide synthase mutant mice. Int. J. Androl. 2011, 34, e621–e629. [Google Scholar] [CrossRef] [PubMed]
- Pilch, B.; Mann, M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006, 7, 40. [Google Scholar] [CrossRef]
- Davalieva, K.; Kiprijanovska, S.; Noveski, P.; Plaseski, T.; Kocevska, B.; Broussard, C.; Plaseska-Karanfilska, D. Proteomic analysis of seminal plasma in men with different spermatogenic impairment. Andrologia 2012, 44, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Primakoff, P.; Myles, D.G. Penetration, adhesion, and fusion in mammalian sperm-egg interaction. Science 2002, 296, 2183–2185. [Google Scholar] [CrossRef]
- Varilova, T.; Seménková, H.; Horák, P.; Madera, M.; Pacáková, V.; Tichá, M.; Stulík, K. Affinity liquid chromatography and capillary electrophoresis of seminal plasma proteins. J. Sep. Sci. 2006, 29, 1110–1115. [Google Scholar] [CrossRef]
- Visconti, P.E.; Westbrook, V.A.; Chertihin, O.; Demarco, I.; Sleight, S.; Diekman, A.B. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J. Reprod. Immunol. 2002, 53, 133–150. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kwon, W.S.; Pang, M.G. Calcium influx and male fertility in the context of the sperm proteome: An update. BioMed Res. Int. 2014, 2014, 841615. [Google Scholar] [CrossRef]
- Yanez, M.; Gil-Longo, J.; Campos-Toimil, M. Calcium binding proteins. Adv. Exp. Med. Biol. 2012, 740, 461–482. [Google Scholar] [PubMed]
- Shawki, H.H.; Ishikawa-Yamauchi, Y.; Kawashima, A.; Katoh, Y.; Matsuda, M.; Al-Soudy, A.S.; Minisy, F.M.; Kuno, A.; Gulibaikelamu, X.; Hirokawa, T.; et al. EFCAB2 is a novel calcium-binding protein in mouse testis and sperm. PLoS ONE 2019, 14, e0214687. [Google Scholar] [CrossRef] [PubMed]
- Piprek, R.P.; Kloc, M.; Mizia, P.; Kubiak, J.Z. The Central Role of Cadherins in Gonad Development, Reproduction, and Fertility. Int. J. Mol. Sci. 2020, 21, 8264. [Google Scholar] [CrossRef] [PubMed]
- Schaller, J.; Glander, H.J. Flow cytometric analysis of enzymes in live spermatozoa before and after cryostorage. Andrologia 2000, 32, 357–364. [Google Scholar] [CrossRef]
- Togo, T.; Morisawa, M. GPI-anchored aminopeptidase is involved in the acrosome reaction in sperm of the mussel Mytilus edulis. Mol. Reprod. Dev. 2004, 67, 465–471. [Google Scholar] [CrossRef]
- Pompella, A.; Visvikis, A.; Paolicchi, A.; De Tata, V.; Casini, A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 2003, 66, 1499–1503. [Google Scholar] [CrossRef]
- Van Tilburg, M.F.; Salles, M.G.F.; Silva, M.M.; Moreira, R.A.; Moreno, F.B.; Monteiro-Moreira, A.C.O.; Martins, J.A.M.; Cândido, M.J.D.; Araújo, A.A.; Moura, A.A.A. Semen variables and sperm membrane protein profile of Saanen bucks (Capra hircus) in dry and rainy seasons of the northeastern Brazil (3°S). Int. J. Biometeorol. 2015, 59, 561–573. [Google Scholar] [CrossRef]
- Jiang, S.T.; Chiou, Y.Y.; Wang, E.; Lin, H.K.; Lee, S.P.; Lu, H.Y.; Wang, C.K.; Tang, M.J.; Li, H. Targeted disruption of Nphp1 causes male infertility due to defects in the later steps of sperm morphogenesis in mice. Hum. Mol. Genet. 2008, 17, 3368–3379. [Google Scholar] [CrossRef]
- Alazami, A.M.; Alshammari, M.J.; Baig, M.; Salih, M.A.; Hassan, H.H.; Alkuraya, F.S. NPHP4 mutation is linked to cerebello-oculo-renal syndrome and male infertility. Clin. Genet. 2014, 85, 371–375. [Google Scholar] [CrossRef]
- Inaba, K. Molecular Architecture of the Sperm Flagella: Molecules for Motility and Signaling. Zool. Sci. 2003, 20, 1043–1056. [Google Scholar] [CrossRef]
- Tanaka, H.; Iguchi, N.; Toyama, Y.; Kitamura, K.; Takahashi, T.; Kaseda, K.; Maekawa, M.; Nishimune, Y. Mice deficient in the axonemal protein tektin-t exhibit male infertility and immotile-cilium syndrome due to impaired inner arm dynein function. Mol. Cell. Biol. 2004, 24, 7958–7964. [Google Scholar] [CrossRef] [PubMed]
- Hermo, L.; Jacks, D. Nature’s ingenuity: Bypassing the classical secretory route via apocrine secretion. Mol. Reprod. Dev. 2002, 63, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.J.; Manandhar, G.; Sutovsky, M.; Li, R.; Jonáková, V.; Oko, R.; Park, C.; Prather, R.S.; Sutovsky, P. Ubiquitin C-terminal hydrolase-activity is involved in sperm acrosomal function and anti-polyspermy defense during porcine fertilization. Biol. Reprod. 2007, 77, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Sutovsky, P. Ubiquitin-dependent proteolysis in mammalian spermatogenesis, fertilization, and sperm quality control: Killing three birds with one stone. Microsc. Res. Tech. 2003, 61, 88–102. [Google Scholar] [CrossRef]
- Sutovsky, P.; Moreno, R.; Ramalho-Santos, J.; Dominko, T.; Thompson, W.E.; Schatten, G. A putative, ubiquitin-dependent mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis. J. Cell Sci. 2001, 114, 1665–1675. [Google Scholar] [CrossRef]
- Muratori, M.; Marchiani, S.; Forti, G.; Baldi, E. Sperm ubiquitination positively correlates to normal morphology in human semen. Hum. Reprod. 2005, 20, 1035–1043. [Google Scholar] [CrossRef]
- Haraguchi, C.M.; Mabuchi, T.; Hirata, S.; Shoda, T.; Tokumoto, T.; Hoshi, K.; Yokota, S. Possible function of caudal nuclear pocket: Degradation of nucleoproteins by ubiquitin-proteasome system in rat spermatids and human sperm. J. Histochem. Cytochem. 2007, 55, 585–595. [Google Scholar] [CrossRef]
- Kouprina, N.; Pavlicek, A.; Collins, N.K.; Nakano, M.; Noskov, V.N.; Ohzeki, J.-I.; Mochida, G.H.; Risinger, J.I.; Goldsmith, P.; Gunsior, M.; et al. The microcephaly ASPM gene is expressed in proliferating tissues and encodes for a mitotic spindle protein. Hum. Mol. Genet. 2005, 14, 2155–2165. [Google Scholar] [CrossRef]
- Lüers, G.H.; Michels, M.; Schwaab, U.; Franz, T. Murine calmodulin-binding protein 1 (Calmbp1): Tissue-specific expression durin development and in adult tissues. Mech. Dev. 2002, 118, 229–232. [Google Scholar] [CrossRef]
- Drake, R.R.; White, K.Y.; Fuller, T.W.; Igwe, E.; Clements, M.A.; Nyalwidhe, J.O.; Given, R.W.; Lance, R.S.; Semmes, O.J. Clinical Collection and Protein Properties of Expressed Prostatic Secretions as a Source for Biomarkers of Prostatic Disease. J. Proteom. 2009, 72, 907–917. [Google Scholar] [CrossRef]
- Doherty, G.J.; McMahon, H.T. Mediation, modulation, and consequences of membrane-cytoskeleton interactions. Annu. Rev. Biophys. 2008, 37, 65–95. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Hess, R.; Aitken, J.R. Induction of sperm maturation in vitro in epididymal cell cultures of the tammar wallaby (Macropus eugenii): Disruption of motility initiation and sperm morphogenesis by inhibition of actin polymerization. Reproduction 2002, 124, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.N.; Bozzola, J.J.; Hunt, W.P.; Darabi, A. Characterization of membrane-associated actin in boar spermatozoa. J. Exp. Zool. 1990, 253, 202–214. [Google Scholar] [CrossRef] [PubMed]
- De Las Heras, M.A.; Valcarcel, A.; Pérez, L.J.; Moses, D.F. Actin localization in ram spermatozoa: Effect of freezing/thawing, capacitation and calcium ionophore-induced acrosomal exocytosis. Tissue Cell 1997, 29, 47–53. [Google Scholar] [CrossRef]
- McEntee, M.; Isaacs, W.; Smith, C. Adenocarcinoma of the canine prostate: Immunohistochemical examination for secretory antigens. Prostate 1987, 11, 163–170. [Google Scholar] [CrossRef]
- Dube, J.Y.; Lazure, C.; Tremblay, R.R. Dog prostate arginine esterase is related to human prostate specific antigen. Clin. Investig. Med. 1986, 9, 51–54. [Google Scholar]
- Calvete, J.; Sanz, L.; Reinert, M.; Dostalova, Z.; Topfer-Petersen, E. Heparin-binding proteins on bull, boar, stallion, and human spermatozoa. Mem. Mus. Nat. Hist. Nat. 1995, 166, 515–524. [Google Scholar]
- Madding, C.I.; Jacob, M.; Ramsay, V.P.; Sokol, R.Z. Serum and semen zinc levels in normozoospermic and oligozoospermic men. Ann. Nutr. Metab. 1986, 30, 213–218. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chang, T.C.; Tseng, Y.J.; Lin, Y.L.; Huang, F.T.; Chang, S.Y. Seminal plasma zinc levels and sperm motion characteristics in infertile samples. Chang. Gung. Med. J. 2000, 23, 260–266. [Google Scholar]
- Foresta, C.; Garolla, A.; Cosci, I.; Menegazzo, M.; Ferigo, M.; Gandin, V.; De Toni, L. Role of zinc trafficking in male fertility: From germ to sperm. Hum. Reprod. 2014, 29, 1134–1145. [Google Scholar] [CrossRef]
- Inayat, S.; Larsson, A.; Ronquist, G.K.; Ronquist, G.; Egberg, N.; Eliasson, R.; Carlsson, L. High levels of cathepsins B, L and S in human seminal plasma and their association with prostasomes. Andrologia 2012, 44, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.A.; Chapman, D.A.; Koc, H.; Killian, G.J. A comprehensive proteomic analysis of the accessory sex gland fluid from mature Holstein bulls. Anim. Reprod. Sci. 2007, 98, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Troedsson, M.H.T.; Foster, D.N.; Loseth, K.J.; Farris, J.A.; Blaschuk, O.; Crabo, B.G. Reproductive tract secretions and bull spermatozoa contain different clusterin isoforms that cluster cells and inhibit complement-induced cytolysis. J. Androl. 1999, 20, 230–240. [Google Scholar] [PubMed]
- Moura, A.A.; Souza, C.E.; Stanley, B.A.; Chapman, D.A.; Killian, G.J. Proteomics of cauda epididymal fluid from mature Holstein bulls. J. Proteom. 2010, 73, 2006–2020. [Google Scholar] [CrossRef]
- Bailey, R.; Griswold, M.D. Clusterin in the male reproductive system: Localization and possible function. Mol. Cell. Endocrinol. 1999, 151, 17–23. [Google Scholar] [CrossRef]
- Fouchecourt, S.; Metayer, S.; Locatelli, A.; Dacheux, F.; Dacheux, J.L. Stallion epididymal fluid proteome: Qualitative and quantitative characterization; secretion and dynamic changes of major Proteins1. Biol. Reprod. 2000, 62, 1790–1803. [Google Scholar] [CrossRef]
- Bailey, R.W.; Aronow, B.; Harmony, J.A.; Griswold, M.D. Heatshock-initiated apoptosis is accelerated and removal of damaged cells is delayed in the testis of clusterin/ApoJ knock-out mice. Biol. Reprod. 2002, 66, 1042–1053. [Google Scholar] [CrossRef]
- Zalata, A.; El-Samanoudy, A.Z.; Shaalan, D.; El-Baiomy, Y.; Taymour, M.; Mostafa, T. Seminal clusterin gene expression associated withseminal variables in fertile and infertile men. J. Urol. 2012, 188, 1260–1264. [Google Scholar] [CrossRef]
- Merveille, A.C.; Davis, E.E.; Becker-Heck, A.; Legendre, M.; Amirav, I.; Bataille, G.; Belmont, J.; Beydon, N.; Billen, F.; Clement, A.; et al. CCDC39 is required for assembly of inner dynein arms and the dynein regulatory complex and for normal ciliary motility in humans and dogs. Nat. Genet. 2011, 43, 72–78. [Google Scholar] [CrossRef]
- Miller, D.; Brough, S.; Al-Harbi, O. Characterization and cellular distribution of human spermatozoal heat shock proteins. Hum. Reprod. 1992, 7, 637–645. [Google Scholar] [CrossRef]
- Volpe, S.; Galeati, G.; Bernardini, C.; Tamanini, C.; Mari, G.; Zambelli, D.; Seren, E.; Spinaci, M. Comparative immunolocalization of heat shock proteins (HSP)-60, -70, -90 in boar, stallion, dog and cat spermatozoa. Reprod. Dom. Anim. 2008, 43, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Spinaci, M.; Volpe, S.; Bernardini, C.; De Ambrogi, M.; Tamanini, C.; Seren, E.; Galeati, G. Immunolocalization of heat shock protein 70 (HSP 70) in boar spermatozoa and its role during fertilization. Mol. Reprod. Dev. 2005, 72, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Van Tilburg, M.F.; Rodrigues, M.A.M.; Moreira, R.A.; Moreno, F.B.; Monteiro-Moreira, A.C.O.; Cândido, M.J.D.; Moura, A.A. Membrane associated proteins of ejaculated sperm from Morada Nova rams. Theriogenology 2013, 79, 1247–1261. [Google Scholar] [CrossRef] [PubMed]
- Gulum, M.; Gumus, K.; Yeni, E.; Dogantekin, E.; Ciftci, H.; Akin, Y.; Savas, M.; Altunkol, A. Blood and semen paraoxonase-arylesterase activities in normozoospermic and azoospermic men. Andrologia 2017, 49, e12752. [Google Scholar] [CrossRef]
- Kothari, S.; Thompson, A.; Agarwal, A.; du Plessis, S.S. Free radicals: Their beneficial and detrimental effects on sperm function. Rev. Indian J. Exp. Biol. 2010, 48, 425–435. [Google Scholar]
- Michael, L.; Sweeney, D.E.; Davies, J.A. A role for microfilament-based contraction in branching morphogenesis of the ureteric bud. Kidney Int. 2005, 68, 2010–2018. [Google Scholar] [CrossRef]
- Batruch, I.; Lecker, I.; Kagedan, D.; Smith, R.C.; Mullen, B.J.; Grober, E.; Lo, K.C.; Diamandis, E.P.; Jarvi, K.A. Proteomic Analysis of Seminal Plasma from Normal Volunteers and Post-Vasectomy Patients Identifies over 2000 Proteins and Candidate Biomarkers of the Urogenital System. J. Proteome Res. 2011, 10, 941–953. [Google Scholar] [CrossRef]
- Samanta, L.; Parida, R.; Dias, T.R.; Agarwal, A. The enigmatic seminal plasma: Proteomics insight from ejaculation to fertilization. Reprod. Biol. Endocrinol. 2018, 16, 41. [Google Scholar] [CrossRef]
- Milardi, D.; Grande, G.; Vincenzoni, F.; Castagnola, M.; Marana, R. Proteomics of human seminal plasma: Identification of biomarker candidates for fertility and infertility and the evolution of technology. Mol. Reprod. Dev. 2013, 80, 350–357. [Google Scholar] [CrossRef]
- Camargo, M.; Intasqui, P.; Bertolla, R.P. Understanding the seminal plasma proteome and its role in male fertility. Basic Clin. Androl. 2018, 28, 6. [Google Scholar] [CrossRef]

| Parameter | Median (Min.–Max.) |
|---|---|
| Volume of the sperm-rich fraction (mL) | 1.80 (0.90–2.10) |
| Spermatozoal motility (%) | 87.0 (80.0–95.0) |
| Total number of spermatozoa (mL−1) | 363,000 (210,000–490,000) |
| Proportion of dead spermatozoa (%) | 6.7 (5.0–11.0) |
| Proportion of abnormal spermatozoa (%) | 9.0 (8.0–12.0) |
| Accession No. | Description | Simultaneous Detection of Proteins | ||
|---|---|---|---|---|
| BP | SF | PF | ||
| P62286 | Abnormal spindle-like microcephaly associated protein homologue | Yes | Yes | No |
| O18840 | Actin, cytoplasmic 1 | Yes | No | Yes |
| P18649 | Apolipoprotein E | No | Yes | Yes |
| P09582 | Arginine esterase | Yes | No | Yes |
| P25473 | Clusterin | Yes | No | Yes |
| E2R1I5 | Coiled-coil domain-containing protein 39 | Yes | No | Yes |
| Q8WN22 | DNA-dependent protein kinase catalytic subunit | Yes | No | Yes |
| Q28259 | Glyceraldehyde-3-phosphate dehydrogenase | Yes | No | Yes |
| Q659K0 | G2/mitotic-specific cyclin-B3 | Yes | No | Yes |
| Q7YQC6 | Heat shock 70 kDa protein 1 | Yes | No | Yes |
| Q6EIY9 | Keratin, type II cytoskeletal 1 | No | Yes | Yes |
| Q9TU19 | Nephrocystin-1(Fragment) | Yes | Yes | No |
| O62699 | Nitric oxide synthase, inducible | No | Yes | Yes |
| P54832 | Serum paraoxonase/arylesterase 2 | Yes | No | Yes |
| O62683 | Tight junction protein ZO-3 | Yes | No | Yes |
| P63050 | Ubiquitin-60S ribosomal protein L40 | Yes | No | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouletsou, P.G.; Tsangaris, G.T.; Katsarou, E.I.; Bourganou, M.V.; Barbagianni, M.S.; Venianaki, A.P.; Bouroutzika, E.; Anagnostopoulos, A.K.; Fthenakis, G.C.; Katsafadou, A.I. Proteomics Evaluation of Semen of Clinically Healthy Beagle-Breed Dogs. Vet. Sci. 2022, 9, 697. https://doi.org/10.3390/vetsci9120697
Gouletsou PG, Tsangaris GT, Katsarou EI, Bourganou MV, Barbagianni MS, Venianaki AP, Bouroutzika E, Anagnostopoulos AK, Fthenakis GC, Katsafadou AI. Proteomics Evaluation of Semen of Clinically Healthy Beagle-Breed Dogs. Veterinary Sciences. 2022; 9(12):697. https://doi.org/10.3390/vetsci9120697
Chicago/Turabian StyleGouletsou, Pagona G., George Th. Tsangaris, Eleni I. Katsarou, Maria V. Bourganou, Mariana S. Barbagianni, Athina P. Venianaki, Efterpi Bouroutzika, Athanasios K. Anagnostopoulos, George C. Fthenakis, and Angeliki I. Katsafadou. 2022. "Proteomics Evaluation of Semen of Clinically Healthy Beagle-Breed Dogs" Veterinary Sciences 9, no. 12: 697. https://doi.org/10.3390/vetsci9120697
APA StyleGouletsou, P. G., Tsangaris, G. T., Katsarou, E. I., Bourganou, M. V., Barbagianni, M. S., Venianaki, A. P., Bouroutzika, E., Anagnostopoulos, A. K., Fthenakis, G. C., & Katsafadou, A. I. (2022). Proteomics Evaluation of Semen of Clinically Healthy Beagle-Breed Dogs. Veterinary Sciences, 9(12), 697. https://doi.org/10.3390/vetsci9120697








