Investigating Visual Monitoring of the Scrotum as a Supplementary Tool for Boar Semen Quality Evaluation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Animals, Semen Collection and Processing
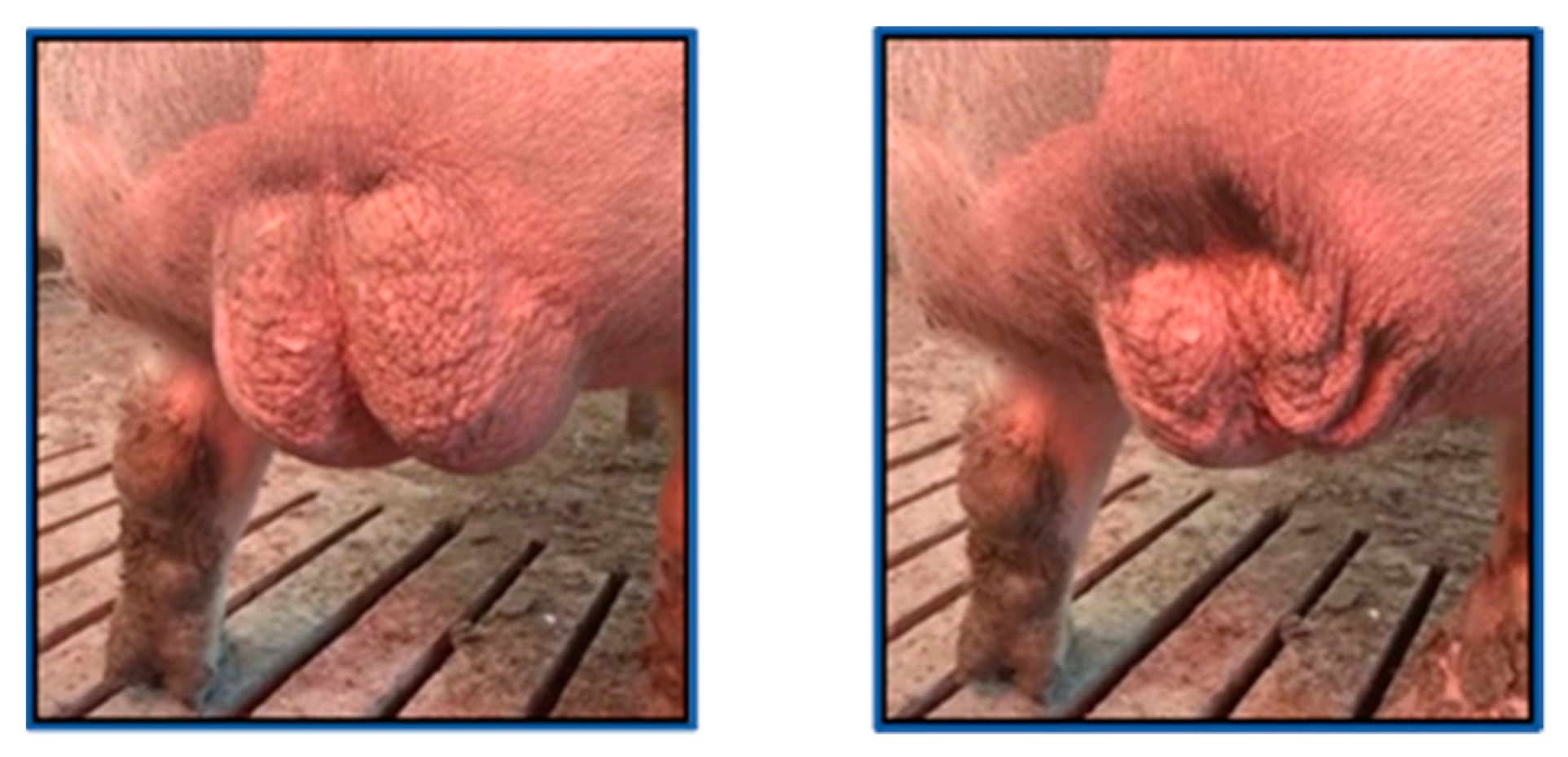
2.2. Visual Video Monitoring, Recording, and Processing
- Load and view the video of the process in Movie Player. Tag the frames of maximum contraction (e.g., Figure 2a) and maximum relaxation of the scrotum.
- For each frame of interest (contraction/relaxation), export the frame to the Image Tool and, subsequently, to Matlab’s Workspace.
- Load the image from the Workspace into the Image Segmenter and use the Graph Cut algorithm: Mark the foreground with a scribble (green) and the background with another scribble (dark red); the region of interest (scrotum) is segmented (cyan pixels, Figure 2b).
- Manipulate the region (fill holes, clear borders, erode mask) to make its boundary smoother. Create a mask (i.e., a black-and-white image) from the region (marked with a yellow color) and export it to Matlab’s Workspace.
- Load the mask (black-and-white image) from the Workspace into the Image Region Analyzer to determine the number of pixels belonging to the area of the mask (the first line in Table with the regions’ properties, Figure 3).
- Divide the number of pixels in the mask by the total number of image pixels to give a percentage. This is to be used as a proxy of the scrotum size (volume) in the video frame.
2.3. Semen Assessment
2.3.1. Computer-Assisted Semen Analysis (CASA Analysis)
2.3.2. Viability and Morphology
2.3.3. Sperm Membrane Biochemical Activity
2.3.4. Sperm DNA Fragmentation
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Druart, X.; Rickard, J.P.; Tsikis, G.; de Graaf, S.P. Seminal plasma proteins as markers of sperm fertility. Theriogenology 2019, 137, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, H. Semen evaluation techniques and their relationship with fertility. Anim. Reprod. 2018, 10, 148–159. [Google Scholar]
- Selvaraju, S.; Parthipan, S.; Somashekar, L.; Binsila, B.K.; Kolte, A.P.; Arangasamy, A.; Parameshwaraiah Ravindra, J.; Krawetz, S.A. Current status of sperm functional genomics and its diagnostic potential of fertility in bovine (Bos taurus). Syst. Biol. Reprod. Med. 2018, 64, 484–501. [Google Scholar] [CrossRef] [PubMed]
- Stravogianni, V.; Samaras, T.; Boscos, C.M.; Markakis, J.; Krystallidou, E.; Basioura, A.; Tsakmakidis, I.A. The Use of Animal’s Body, Scrotal Temperature and Motion Monitoring in Evaluating Boar Semen Production Capacity. Animals 2022, 12, 829. [Google Scholar] [CrossRef] [PubMed]
- Waters, B.E.; McDonagh, J.; Tzimiropoulos, G.; Slinger, K.R.; Huggett, Z.J.; Bell, M.J. Changes in Sheep Behavior before Lambing. Agriculture 2021, 11, 715. [Google Scholar] [CrossRef]
- Habineza, E.; Reza, M.N.; Chowdhury, M.; Kiraga, S.; Chung, S.O.; Hong, S.J. Pig diseases and crush monitoring visual symptoms detection using engineering approaches: A review. Precis. Agric. 2021, 3, 160. [Google Scholar]
- Quddus, R.A.; Ahmad, N.; Khalique, A.; Bhatti, J.A. Validation of NEDAP Monitoring Technology for Measurements of Feeding, Rumination, Lying, and Standing Behaviors, and Comparison with Visual Observation and Video Recording in Buffaloes. Animals 2022, 12, 578. [Google Scholar] [CrossRef]
- Cook, R.B.; Coulter, G.H.; Kastelic, J.P. The testicular vascular cone, scrotal thermoregulation, and their relationship to sperm production and seminal quality in beef bulls. Theriogenology 1994, 41, 653–671. [Google Scholar] [CrossRef]
- Bernardino, T.; Carvalho, C.P.T.; Batissaco, L.; Celeghini, E.C.C.; Zanella, A.J. Poor welfare compromises testicle physiology in breeding boars. PLoS ONE 2022, 17, e0268944. [Google Scholar] [CrossRef]
- Peña, S.T.; Stone, F.; Gummow, B.; Parker, A.J.; Paris, D.B. Susceptibility of boar spermatozoa to heat stress using in vivo and in vitro experimental models. Trop. Anim. Health Prod. 2021, 53, 97. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO Press: Geneva, Switzerland, 2021. [Google Scholar]
- Tsakmakidis, I.A.; Lymberopoulos, A.G.; Khalifa, T.A.A.; Boscos, C.M.; Saratsi, A.; Alexopoulos, C. Evaluation of zearalenone and α-zearalenol toxicity on boar sperm DNA integrity. J. Appl. Toxicol. 2008, 28, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zheng, H.; Zheng, Z.; Yan, W. Proteomic analyses reveal a role of cytoplasmic droplets as an energy source during epididymal sperm maturation. PLoS ONE 2013, 8, e77466. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Shibukawa, T.; Harayama, H.; Kanna, Y. Timing of shedding and disintegration of cytoplasmic droplets from boar and goat spermatozoa. J. Reprod. Dev. 1996, 42, 237–241. [Google Scholar] [CrossRef]
- Althouse, G.C. Cytoplasmic droplets on boar sperm cells. Swine Health Prod. 1998, 6, 128. [Google Scholar]
- Waberski, D.; Meding, S.; Dirksen, G.; Weitze, K.F.; Leiding, C.; Hahn, R. Fertility of long-term-stored boar semen: Influence of extender (Androhep and Kiev), storage time and plasma droplets in the semen. Anim. Reprod. Sci. 1994, 36, 145–151. [Google Scholar] [CrossRef]
- Lovercamp, K.W.; Safranski, T.J.; Fischer, K.A.; Manandhar, G.; Sutovsky, M.; Herring, W.; Sutovsky, P. High resolution light microscopic evaluation of boar semen quality sperm cytoplasmic droplet retention in relationship with boar fertility parameters. Arch. Androl. 2007, 53, 219–228. [Google Scholar] [CrossRef]
- Fetic, S.; Yeung, C.H.; Sonntag, B.; Nieschlag, E.; Cooper, T.G. Relationship of cytoplasmic droplets to motility, migration in mucus, and volume regulation of human spermatozoa. J. Androl. 2006, 27, 294–301. [Google Scholar] [CrossRef]
- Michos, I.A.; Basioura, A.G.; Boscos, C.M.; Tsakmakidis, I.A. Proper use and impact of ‘Computer Assisted Semen Analysis’ technique on semen evaluation of farm animals. J. Hell. Vet. Med. Soc. 2013, 64, 267–274. [Google Scholar] [CrossRef][Green Version]
- Ayad, B.M.; Oyeyipo, I.P.; Van der Horst, G.; Du Plessis, S.S. Cementing the relationship between conventional and advanced semen parameters. Middle East Fertil. Soc. J. 2021, 26, 39. [Google Scholar] [CrossRef]
- Broekhuijse, M.L.W.J.; Šoštarić, E.; Feitsma, H.; Gadella, B.M. Application of computer-assisted semen analysis to explain vari-531 ations in pig fertility. J. Anim. Sci. 2012, 90, 779–789. [Google Scholar] [CrossRef]
- Holt, C.; Holt, W.V.; Moore, H.D.M.; Reed, H.C.B.; Curnock, R.M. Objectivily measured boar sperm motility parameters corre-515 late with the outcomes of on-farm inseminations: Results of two fertility trials. J. Androl. 1997, 18, 312–323. [Google Scholar] [PubMed]
- Barquero, V.; Roldan, E.R.; Soler, C.; Vargas-Leitón, B.; Sevilla, F.; Camacho, M.; Valverde, A. Relationship between Fertility Traits and Kinematics in Clusters of Boar Ejaculates. Biology 2021, 10, 595. [Google Scholar] [CrossRef] [PubMed]



| Variables | Linear Regression Coefficient | 95% Confidence Interval | p Value (n = 10) | R2 | ||
|---|---|---|---|---|---|---|
| Lower Coefficient | Upper Coefficient | |||||
| Total motility (%) | Change in the scrotum volume (%) | −0.157 | −0.509 | 0.195 | 0.333 | 0.017 |
| Progressive motility (%) | Change in the scrotum volume (%) | −0.671 | −2.132 | 0.789 | 0.320 | 0.109 |
| Non-progressive motility (%) | Change in the scrotum volume (%) | 0.112 | −1.272 | 1.049 | 0.829 | 0.643 |
| Immotile spermatozoa (%) | Change in the scrotum volume (%) | 0.157 | −0.195 | 0.509 | 0.333 | 0.017 |
| Rapid spermatozoa (%) | Change in the scrotum volume (%) | −0.279 | −2.090 | 1.531 | 0.731 | 0.539 |
| Medium spermatozoa (%) | Change in the scrotum volume (%) | −0.105 | −0.950 | 0.741 | 0.783 | 0.328 |
| Slow spermatozoa (%) | Change in the scrotum volume (%) | 0.822 | −0.241 | 1.886 | 0.112 | 0.147 |
| VCL (μm/s) | Change in the scrotum volume (%) | −0.202 | −1.287 | 0.883 | 0.679 | 0.784 |
| VSL (μm/s) | Change in the scrotum volume (%) | 0.154 | −0.715 | 1.124 | 0.693 | 0.807 |
| VAP (μm/s) | Change in the scrotum volume (%) | 0.574 | 0.024 | 1.024 | 0.043 | 0.591 |
| LIN (%) | Change in the scrotum volume (%) | 0.939 | 0.175 | 1.702 | 0.022 | 0.337 |
| STR (%) | Change in the scrotum volume (%) | 1.111 | 0.315 | 1.906 | 0.012 | 0.448 |
| WOB (%) | Change in the scrotum volume (%) | 0.538 | 0.068 | 1.009 | 0.030 | 0.337 |
| ALH (μm) | Change in the scrotum volume (%) | −0.029 | −0.089 | 0.031 | 0.269 | 0.565 |
| BCF (Hz) | Change in the scrotum volume (%) | 0.055 | −0.025 | 1.134 | 0.150 | 0.983 |
| Normal morphology (%) | Change in the scrotum volume (%) | −0.004 | 0.007 | −0.001 | 0.029 | 0.342 |
| Abnormal morphology (%) | Change in the scrotum volume (%) | 0.004 | 0.001 | 0.007 | 0.029 | 0.342 |
| Head abnormalities (%) | Change in the scrotum volume (%) | 0.002 | −0.001 | 0.004 | 0.135 | 0.221 |
| Midpiece abnormalities (%) | Change in the scrotum volume (%) | 0.001 | −0.001 | 0.001 | 0.184 | 0.071 |
| Tail abnormalities (%) | Change in the scrotum volume (%) | 0.001 | −0.001 | 0.002 | 0.123 | 0.642 |
| Cytoplasmic droplets (%) | Change in the scrotum volume (%) | 0.001 | 0.001 | 0.002 | 0.036 | 0.509 |
| Viability (%) | Change in the scrotum volume (%) | −0.004 | 0.009 | 0.001 | 0.078 | 0.201 |
| Hyperactivated spermatozoa (%) | Change in the scrotum volume (%) | −0.125 | 0.269 | 0.017 | 0.077 | 0.353 |
| Host (+) spermatozoa (%) | Change in the scrotum volume (%) | −0.003 | −0.006 | −0.001 | 0.133 | 0.121 |
| Volume (mL) | Change in the scrotum volume (%) | −1.179 | −10.115 | 7.756 | 0.769 | 0.912 |
| Total ejaculation time (min) | Change in the scrotum volume (%) | 4.404 | 0.801 | 8.007 | 0.022 | 0.373 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stravogianni, V.; Samaras, T.; Boscos, C.M.; Basioura, A.; Markakis, I.; Tsakmakidis, I.A. Investigating Visual Monitoring of the Scrotum as a Supplementary Tool for Boar Semen Quality Evaluation. Vet. Sci. 2023, 10, 9. https://doi.org/10.3390/vetsci10010009
Stravogianni V, Samaras T, Boscos CM, Basioura A, Markakis I, Tsakmakidis IA. Investigating Visual Monitoring of the Scrotum as a Supplementary Tool for Boar Semen Quality Evaluation. Veterinary Sciences. 2023; 10(1):9. https://doi.org/10.3390/vetsci10010009
Chicago/Turabian StyleStravogianni, Vasiliki, Theodoros Samaras, Constantin M. Boscos, Athina Basioura, Ioannis Markakis, and Ioannis A. Tsakmakidis. 2023. "Investigating Visual Monitoring of the Scrotum as a Supplementary Tool for Boar Semen Quality Evaluation" Veterinary Sciences 10, no. 1: 9. https://doi.org/10.3390/vetsci10010009
APA StyleStravogianni, V., Samaras, T., Boscos, C. M., Basioura, A., Markakis, I., & Tsakmakidis, I. A. (2023). Investigating Visual Monitoring of the Scrotum as a Supplementary Tool for Boar Semen Quality Evaluation. Veterinary Sciences, 10(1), 9. https://doi.org/10.3390/vetsci10010009










