Particulate Cell Wall Materials of Lactobacillus acidophilus as Vaccine Adjuvant
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Lactobacillus acidophilus (LA) Particles
2.2. High-Pressured Homogenization Treatment
2.3. Dynamic Light Scattering (DLS)
2.4. Mice Immunization Test
2.5. OVA-Specific IgG Measurement
2.6. Cytokine Determination in the Cultured Supernatants of Splenocytes by ELISA
2.7. Inactivated NDV Antigen and Chicken Vaccination Test
2.8. Mice Acute Toxicity Experiments
2.9. Statistical Analysis
3. Result
3.1. Particle Size
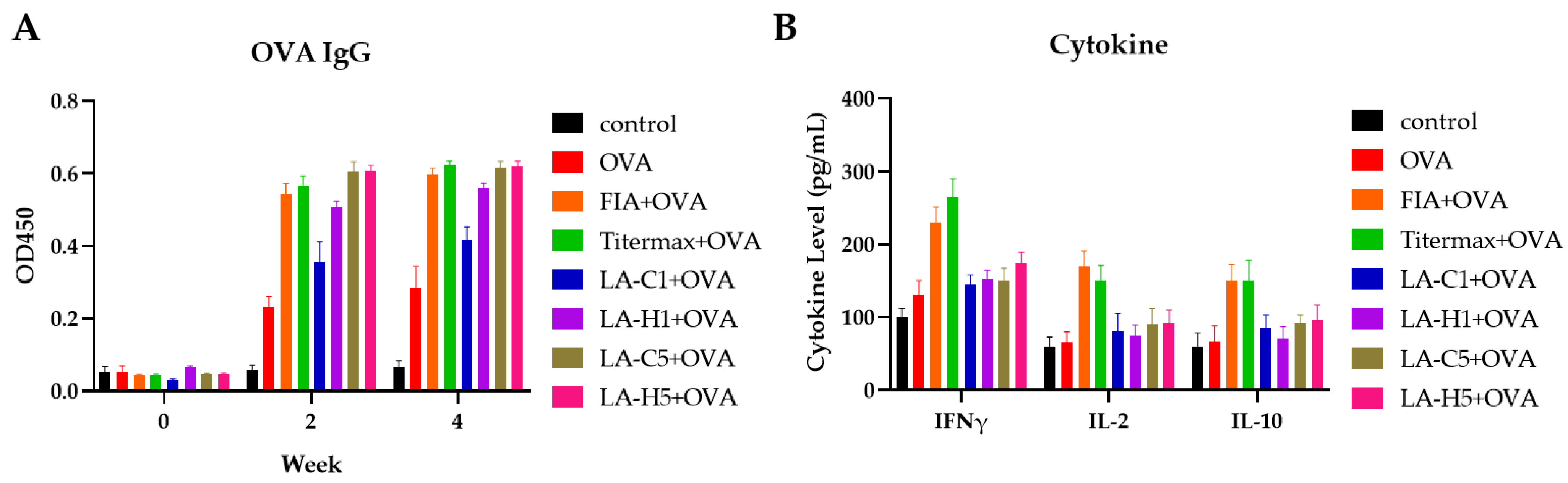
3.2. Murine Immunization
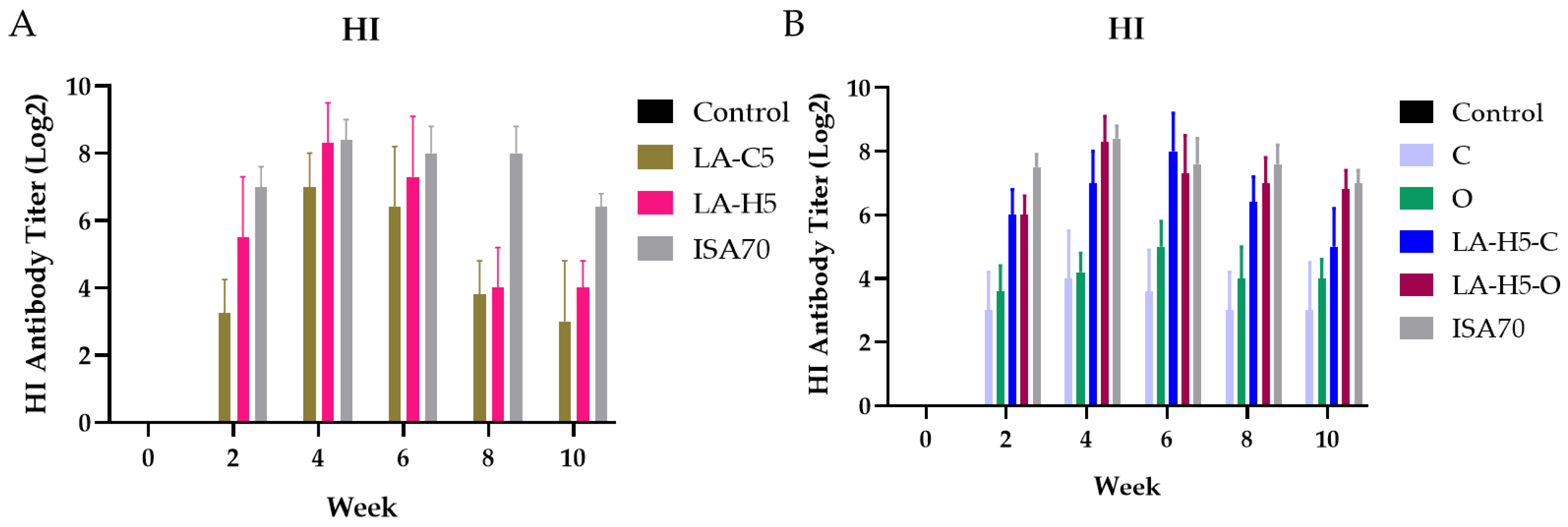
3.3. Primary Immunization
3.4. Secondary Immunization
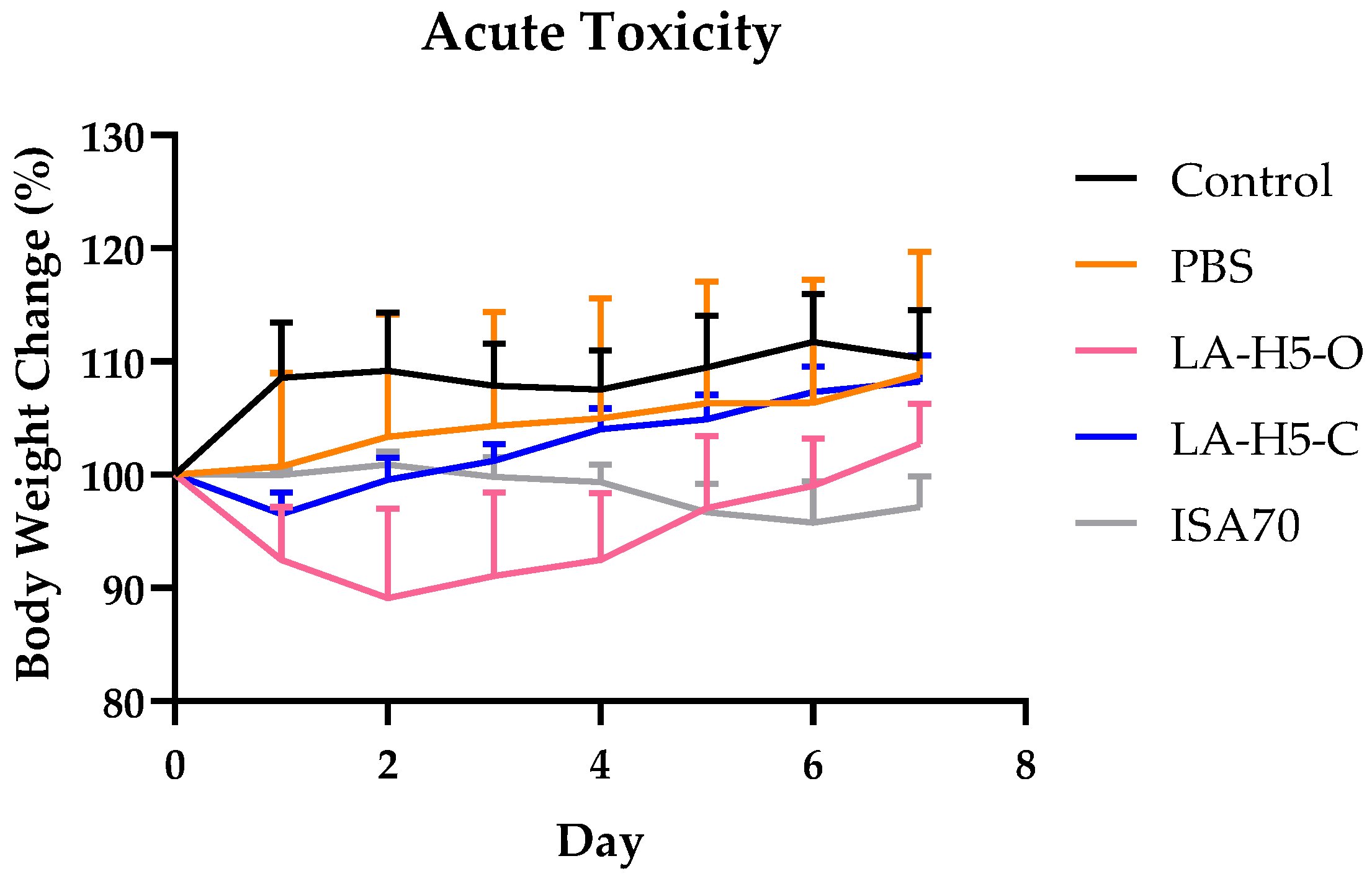
3.5. Acute Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, C.; Yue, H.; Zhu, L.; Ma, G.-h.; Wang, H.-l. Prophylactic vaccine delivery systems against epidemic infectious diseases. Adv. Drug Deliv. Rev. 2021, 176, 113867. [Google Scholar] [CrossRef] [PubMed]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef] [PubMed]
- Minor, P.D. Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 479–480, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Renukaradhya, G.J.; Meng, X.-J.; Calvert, J.G.; Roof, M.; Lager, K.M. Inactivated and subunit vaccines against porcine reproductive and respiratory syndrome: Current status and future direction. Vaccine 2015, 33, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Marciani, D.J. Vaccine adjuvants: Role and mechanisms of action in vaccine immunogenicity. Drug Discov. Today 2003, 8, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.; Kim, M.; Ahn, J.; Oh, H.; Lim, J.; Chae, W.; Yang, S.W.; Kim, M.S.; Yu, J.E.; Byun, S.; et al. Epigallocatechin-3-Gallate as a Novel Vaccine Adjuvant. Front. Immunol. 2021, 12, 769088. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef]
- Erf, G.F. Cell-mediated immunity in poultry. Poult. Sci. 2004, 83, 580–590. [Google Scholar] [CrossRef]
- Lefebvre, J.S.; Lorenzo, E.C.; Masters, A.R.; Hopkins, J.W.; Eaton, S.M.; Smiley, S.T.; Haynes, L. Vaccine efficacy and T helper cell differentiation change with aging. Oncotarget 2016, 7, 33581–33594. [Google Scholar] [CrossRef]
- Sugai, T.; Mori, M.; Nakazawa, M.; Ichino, M.; Naruto, T.; Kobayashi, N.; Kobayashi, Y.; Minami, M.; Yokota, S. A CpG-containing oligodeoxynucleotide as an efficient adjuvant counterbalancing the Th1/Th2 immune response in diphtheria–tetanus–pertussis vaccine. Vaccine 2005, 23, 5450–5456. [Google Scholar] [CrossRef]
- Antonialli, R.; Sulczewski, F.B.; Amorim, K.N.d.S.; Almeida, B.d.S.; Ferreira, N.S.; Yamamoto, M.M.; Soares, I.S.; Ferreira, L.C.d.S.; Rosa, D.S.; Boscardin, S.B. CpG Oligodeoxinucleotides and Flagellin Modulate the Immune Response to Antigens Targeted to CD8α+ and CD8α− Conventional Dendritic Cell Subsets. Front. Immunol. 2017, 8, 1727. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Klaenhammer, T.R. Invited Review: The Scientific Basis of Lactobacillus acidophilus NCFM Functionality as a Probiotic. J. Dairy Sci. 2001, 84, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Pochart, P.; Flourié, B.; Pellier, P.; Santos, L.; Desjeux, J.F.; Rambaud, J.C. Effect of chronic ingestion of a fermented dairy product containing Lactobacillus acidophilus and Bifidobacterium bifidum on metabolic activities of the colonic flora in humans. Am. J. Clin. Nutr. 1990, 52, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, S.E.; Nelson, C.R.; Maxwell, C. Assimilation of cholesterol by Lactobacillus acidophilus. Appl. Environ. Microbiol. 1985, 49, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Factories 2020, 19, 203. [Google Scholar] [CrossRef]
- Khanna, V.; Alam, S.; Malik, A.; Malik, A. Efficacy of tyndalizedLactobacillus acidophilus in acute diarrhea. Indian J. Pediatr. 2005, 72, 935–938. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Hou, L.; Chen, J.; Li, P.; Qiao, X.; Zheng, Q.; Hou, J. CTA1: Purified and display onto gram-positive enhancer matrix (GEM) particles as mucosal adjuvant. Protein Expr. Purif. 2018, 141, 19–24. [Google Scholar] [CrossRef]
- Saluja, V.; Visser, M.R.; ter Veer, W.; van Roosmalen, M.L.; Leenhouts, K.; Hinrichs, W.L.J.; Huckriede, A.; Frijlink, H.W. Influenza antigen-sparing by immune stimulation with Gram-positive enhancer matrix (GEM) particles. Vaccine 2010, 28, 7963–7969. [Google Scholar] [CrossRef]
- Thayer, S.G.; Beard, C.W. Serologic Procedures. In Laboratory Manual for the Isolation and Identification of Avian Pathogens; International Book: Dulles, VA, USA, 2006; Volume 46. [Google Scholar]
- Slütter, B.; Jiskoot, W. Sizing the optimal dimensions of a vaccine delivery system: A particulate matter. Expert Opin. Drug Deliv. 2016, 13, 167–170. [Google Scholar] [CrossRef]
- Adler-Moore, J.; Munoz, M.; Kim, H.; Romero, J.; Tumpey, T.; Zeng, H.; Petro, C.; Ernst, W.; Kosina, S.; Jimenez, G.; et al. Characterization of the murine Th2 response to immunization with liposomal M2e influenza vaccine. Vaccine 2011, 29, 4460–4468. [Google Scholar] [CrossRef]
- Khabazzadeh Tehrani, N.; Mahdavi, M.; Maleki, F.; Zarrati, S.; Tabatabaie, F. The role of Montanide ISA 70 as an adjuvant in immune responses against Leishmania major induced by thiol-specific antioxidant-based protein vaccine. J. Parasit. Dis. 2016, 40, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Bosma, T.; Kanninga, R.; Neef, J.; Audouy, S.A.L.; Roosmalen, M.L.v.; Steen, A.; Buist, G.; Kok, J.; Kuipers, O.P.; Robillard, G.; et al. Novel Surface Display System for Proteins on Non-Genetically Modified Gram-Positive Bacteria. Appl. Environ. Microbiol. 2006, 72, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wu, R.; Han, N.; Fu, J.; Luo, Z.; Guo, L.; Su, Y.; Du, J.; Liu, Y. Porphyromonas gingivalis and Lactobacillus rhamnosus GG regulate the Th17/Treg balance in colitis via TLR4 and TLR2. Clin. Transl. Immunol. 2020, 9, e1213. [Google Scholar] [CrossRef] [PubMed]
- Castillo, N.A.; Perdigón, G.; de Moreno de LeBlanc, A. Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol. 2011, 11, 177. [Google Scholar] [CrossRef]
- Petrovsky, N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef] [PubMed]

| Name | Z-Average Diameter (nm) | PDI |
|---|---|---|
| LA | 914.27 | 0.56 |
| LA-C | 510.03 | 0.28 |
| LA-H | 179.43 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-C.; Chang, P.-C.; Lin, C.-H.; Liang, H.-J.; Huang, C.-H. Particulate Cell Wall Materials of Lactobacillus acidophilus as Vaccine Adjuvant. Vet. Sci. 2022, 9, 698. https://doi.org/10.3390/vetsci9120698
Lin S-C, Chang P-C, Lin C-H, Liang H-J, Huang C-H. Particulate Cell Wall Materials of Lactobacillus acidophilus as Vaccine Adjuvant. Veterinary Sciences. 2022; 9(12):698. https://doi.org/10.3390/vetsci9120698
Chicago/Turabian StyleLin, Shu-Ching, Pu-Chieh Chang, Chien-Hung Lin, Hong-Jen Liang, and Chih-Hung Huang. 2022. "Particulate Cell Wall Materials of Lactobacillus acidophilus as Vaccine Adjuvant" Veterinary Sciences 9, no. 12: 698. https://doi.org/10.3390/vetsci9120698
APA StyleLin, S.-C., Chang, P.-C., Lin, C.-H., Liang, H.-J., & Huang, C.-H. (2022). Particulate Cell Wall Materials of Lactobacillus acidophilus as Vaccine Adjuvant. Veterinary Sciences, 9(12), 698. https://doi.org/10.3390/vetsci9120698





