Plasma Chymase Activity Reflects the Change in Hemodynamics Observed after the Surgical Treatment of Patent Ductus Arteriosus in Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgical Occlusion of PDA
2.3. Plasma Sample and Measurement of Plasma Chymase Activity
2.4. Statistical Analysis
3. Results
3.1. Animals and Echocardiography
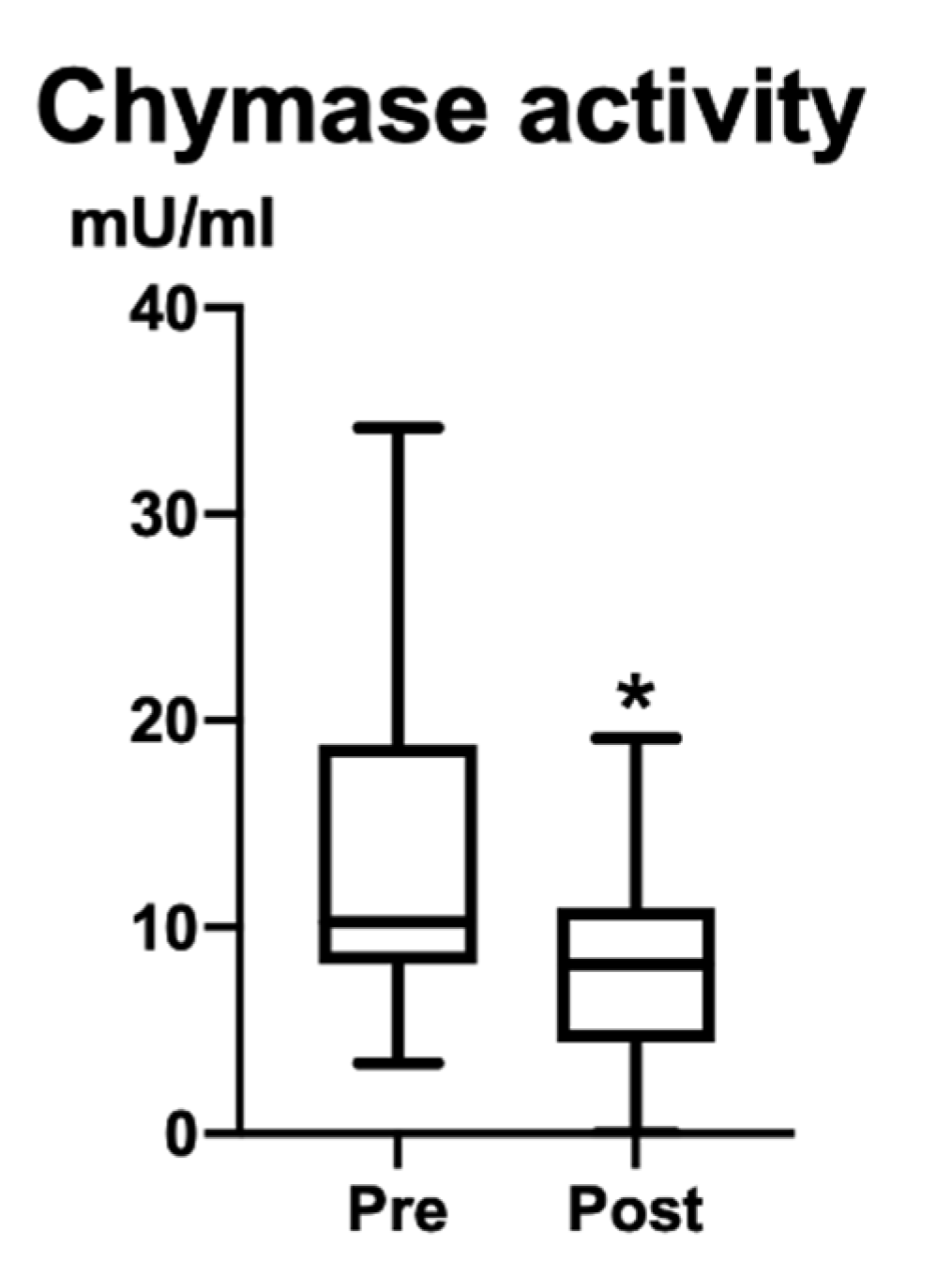
3.2. Plasma Chymase Activity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Gu, Y.; Lewis, D.F.; Alexander, J.S.; Granger, D.N. Elevated plasma chymotrypsin-like protease (chymase) activity in women with preeclampsia. Hypertens. Pregnancy 2010, 29, 253–261. [Google Scholar] [CrossRef]
- Ahmad, S.; Ferrario, C.M. Chymase inhibitors for the treatment of cardiac diseases: A patent review (2010–2018). Expert Opin. Ther. Pat. 2018, 28, 755–764. [Google Scholar] [CrossRef]
- Ahmad, S.; Varagic, J.; VonCannon, J.L.; Groban, L.; Collawn, J.F.; Dell’Italia, L.J.; Ferrario, C.M. Primacy of cardiac chymase over angiotensin converting enzyme as an angiotensin-(1-12) metabolizing enzyme. Biochem. Biophys. Res. Commun. 2016, 478, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Dell’Italia, L.J.; Collawn, J.F.; Ferrario, C.M. Multifunctional Role of Chymase in Acute and Chronic Tissue Injury and Remodeling. Circ. Res. 2018, 122, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.W.; Lin, T.Y.; Liu, Y.C.; Yeh, J.L.; Hsu, J.H. Molecular Mechanisms Underlying Remodeling of Ductus Arteriosus: Looking beyond the Prostaglandin Pathway. Int. J. Mol. Sci. 2021, 22, 3238. [Google Scholar] [CrossRef] [PubMed]
- Gillam-Krakauer, M.; Mahajan, K. Patent Ductus Arteriosus; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Crockett, S.L.; Berger, C.D.; Shelton, E.L.; Reese, J. Molecular and mechanical factors contributing to ductus arteriosus patency and closure. Congenit. Heart Dis. 2019, 14, 15–20. [Google Scholar] [CrossRef]
- Ferguson, J.M. Pharmacotherapy for patent ductus arteriosus closure. Congenit. Heart Dis. 2019, 14, 52–56. [Google Scholar] [CrossRef]
- Philip, R.; Lamba, V.; Talati, A.; Sathanandam, S. Pulmonary Hypertension with Prolonged Patency of the Ductus Arteriosus in Preterm Infants. Children 2020, 7, 139. [Google Scholar] [CrossRef]
- Zaidi, M.; Sorathia, N.; Abbasi, H.; Khashkhusha, A.; Harky, A. Interventions on patent ductus arteriosus and its impact on congenital heart disease. Cardiol. Young 2020, 30, 1566–1571. [Google Scholar] [CrossRef]
- Bonagura, J.D.; Lehmkuhl, L.B. Congenital Heart Disease. In Textbook of Canine and Feline Cardiology: Principles and Clinical Practice, 2nd ed.; Fox, P.R., Sisson, D., Moise, N.S., Eds.; W.B. Saunders: Philadelphia, PA, USA, 1999; pp. 471–535. [Google Scholar]
- Broaddus, K.; Tillson, M. Patent ductus arteriosus in dogs. Compend. Contin. Educ. Vet. 2010, 32, E3. [Google Scholar]
- Buchanan, J.W. Patent ductus arteriousus morphology, pathogenesis, types and treatment. J. Vet. Cardiol. 2001, 3, 7–16. [Google Scholar] [CrossRef]
- Strickland, K. Congenital Heart Disease. In Manual of Canine and Feline Cardiology, 4th ed.; Tilley, L.P., Smith, F.W.K., Jr., Oyama, M.A., Sleeper, M.M., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2008; pp. 223–247. [Google Scholar]
- Boon, J. The M-mode and Doppler examination. In Veterinary Echocardiography, 2nd ed.; Boon, J., Ed.; Wiley-Blackwell: Chichester, England, 2011; pp. 101–112. [Google Scholar]
- Hamabe, L.; Kim, S.; Yoshiyuki, R.; Fukayama, T.; Nakata, T.M.; Fukushima, R.; Tanaka, R. Echocardiographic evaluation of myocardial changes observed after closure of patent ductus arteriosus in dogs. J. Vet. Intern. Med. 2015, 29, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, R.; Fujii, Y.; Takano, H.; Sunahara, H.; Aoki, T.; Wakao, Y. Left ventricular reverse remodeling after ductal closure in dogs with hemodynamically significant patent ductus arteriosus. J. Appl. Res. Vet. Med. 2013, 11, 66–69. [Google Scholar]
- Greet, V.; Bode, E.F.; Dukes-McEwan, J.; Oliveira, P.; Connolly, D.J.; Sargent, J. Clinical features and outcome of dogs and cats with bidirectional and continuous right-to-left shunting patent ductus arteriosus. J. Vet. Intern. Med. 2021, 35, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.B.; Gordon, S.G.; Boggess, M.M.; Miller, M.W. Long-term outcome in dogs with patent ductus arteriosus: 520 cases (1994–2009). J. Vet. Intern. Med. 2014, 28, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Kadosawa, T.; Okumura, M.; Fujinaga, T. Peri-operative changes in echocardiographic measurements and plasma atrial and brain natriuretic peptide concentrations in 3 dogs with patent ductus arteriosus. J. Vet. Med. Sci. 1999, 61, 89–91. [Google Scholar] [CrossRef]
- Weisz, D.E.; McNamara, P.J.; El-Khuffash, A. Cardiac biomarkers and haemodynamically significant patent ductus arteriosus in preterm infants. Early Hum. Dev. 2017, 105, 41–47. [Google Scholar] [CrossRef]
- Stewart, J.A., Jr.; Wei, C.C.; Brower, G.L.; Rynders, P.E.; Hankes, G.H.; Dillon, A.R.; Lucchesi, P.A.; Janicki, J.S.; Dell’Italia, L.J. Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J. Mol. Cell. Cardiol. 2003, 35, 311–319. [Google Scholar] [CrossRef]
- Ihara, M.; Urata, H.; Shirai, K.; Ideishi, M.; Hoshino, F.; Suzumiya, J.; Kikuchi, M.; Arakawa, K. High cardiac angiotensin-II-forming activity in infarcted and non-infarcted human myocardium. Cardiology 2000, 94, 247–253. [Google Scholar] [CrossRef]
- Okamura, K.; Okuda, T.; Shirai, K.; Urata, H. Positive correlation between blood pressure or heart rate and chymase-dependent angiotensin II-forming activity in circulating mononuclear leukocytes measured by new ELISA. Clin. Exp. Hypertens. 2018, 40, 112–117. [Google Scholar] [CrossRef]
- Takai, S.; Jin, D.; Muramatsu, M.; Okamoto, Y.; Miyazaki, M. Therapeutic applications of chymase inhibitors in cardiovascular diseases and fibrosis. Eur. J. Pharmacol. 2004, 501, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.H.; Raymond, W.W.; Wolters, P.J. Angiotensin II generation by mast cell alpha- and beta-chymases. Biochim. Biophys. Acta 2000, 1480, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Parkerson, S.; Philip, R.; Talati, A.; Sathanandam, S. Management of Patent Ductus Arteriosus in Premature Infants in 2020. Front. Pediatr. 2020, 8, 590578. [Google Scholar] [CrossRef] [PubMed]
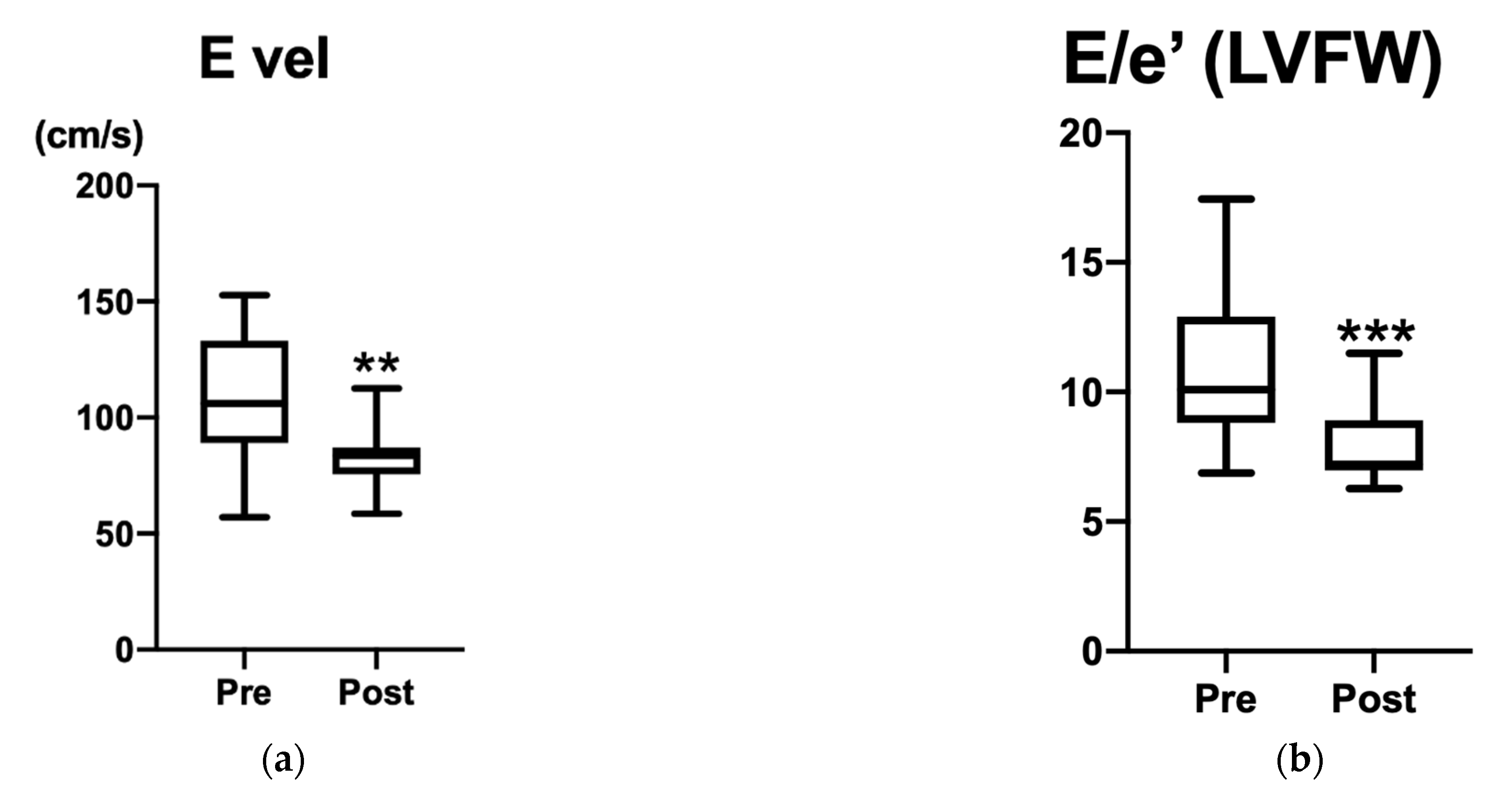
| Pre (Mean ± SD) | Post (Mean ± SD) | p-Value | CI | |
|---|---|---|---|---|
| FS% | 39.4 ± 11.8 | 40.8 ± 8.0 | 0.2522 | −2.600 to 10.10 |
| E vel (cm/s) | 108.3 ± 27.2 | 82.9 ± 12.8 | 0.0024 * | −47.20 to −10.40 |
| E/e’ IVS | 12.2 ± 3.8 | 9.7 ±2.1 | 0.0984 | −5.150 to 0.6900 |
| E/e’ LVFW | 10.9 ± 2.9 | 8.0 ± 1.6 | 0.0008 * | −4.140 to −1.470 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimada, K.; Hamabe, L.; Hirose, M.; Watanabe, M.; Yokoi, A.; Takeuchi, A.; Ozai, Y.; Yoshida, T.; Takai, S.; Jin, D.; et al. Plasma Chymase Activity Reflects the Change in Hemodynamics Observed after the Surgical Treatment of Patent Ductus Arteriosus in Dogs. Vet. Sci. 2022, 9, 682. https://doi.org/10.3390/vetsci9120682
Shimada K, Hamabe L, Hirose M, Watanabe M, Yokoi A, Takeuchi A, Ozai Y, Yoshida T, Takai S, Jin D, et al. Plasma Chymase Activity Reflects the Change in Hemodynamics Observed after the Surgical Treatment of Patent Ductus Arteriosus in Dogs. Veterinary Sciences. 2022; 9(12):682. https://doi.org/10.3390/vetsci9120682
Chicago/Turabian StyleShimada, Kazumi, Lina Hamabe, Miki Hirose, Momoko Watanabe, Aimi Yokoi, Aki Takeuchi, Yusuke Ozai, Tomohiko Yoshida, Shinji Takai, Denan Jin, and et al. 2022. "Plasma Chymase Activity Reflects the Change in Hemodynamics Observed after the Surgical Treatment of Patent Ductus Arteriosus in Dogs" Veterinary Sciences 9, no. 12: 682. https://doi.org/10.3390/vetsci9120682
APA StyleShimada, K., Hamabe, L., Hirose, M., Watanabe, M., Yokoi, A., Takeuchi, A., Ozai, Y., Yoshida, T., Takai, S., Jin, D., Kocaturk, M., Uehara, K., & Tanaka, R. (2022). Plasma Chymase Activity Reflects the Change in Hemodynamics Observed after the Surgical Treatment of Patent Ductus Arteriosus in Dogs. Veterinary Sciences, 9(12), 682. https://doi.org/10.3390/vetsci9120682







