The Effectiveness of Commercial Vaccination against Lawsonia intracellularis in Mitigating the Reduction in ADWG, the Increased Mortality and Fecal Shedding of the Vaccinated Pigs: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Selection Criteria
2.2. Study Selection Process
2.3. Data Extraction
2.4. Quality Assessment
2.5. Outcome Measures
2.6. Data Analysis
3. Results
3.1. Characteristics of the Included Studies
3.2. Data Synthesis
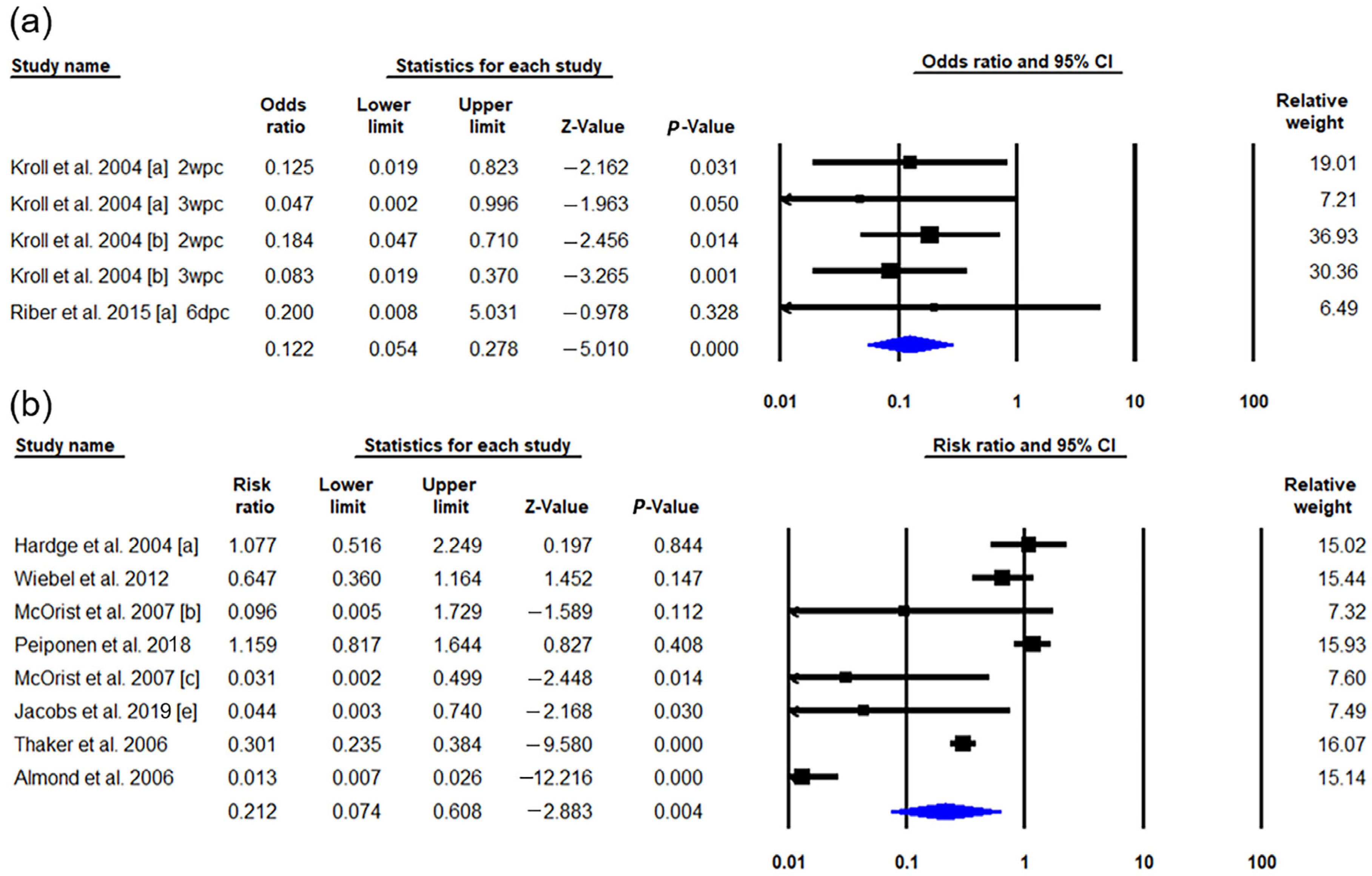
3.2.1. ADWG
3.2.2. Fecal Shedding Rate
3.2.3. Mortality Rate
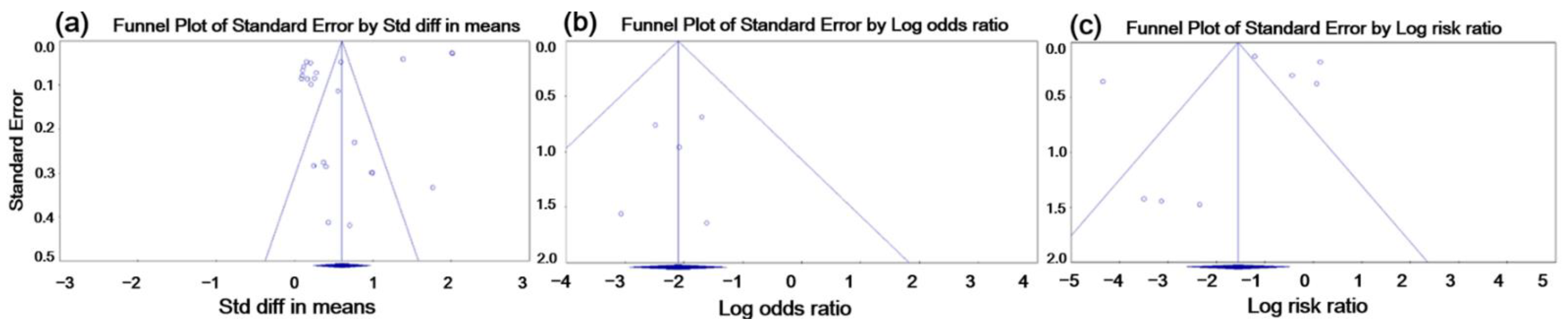
3.3. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Crienen, A.; Swam, H.; von Berg, S.; Jolie, R.; Nathues, H. Prevalence of Lawsonia intracellularis in Pig Herds in Different European Countries. Porc. Health Manag. 2019, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Paradis, M.; McKay, R.I.; Wilson, J.B.; Vessie, G.H.; Winkelman, N.L.; Gebhart, C.; Dick, C.P. Subclinical Ileitis Produced by Sequential Dilutions of Lawsonia intracellularis in a Mucosal Homogenate Challenge Model. Proc. 36th Ann. Meeting Am. Ass. Swine Vet. 2005, 189–191. [Google Scholar]
- Almond, P.K.; Bilkei, G. Effects of Oral Vaccination against Lawsonia intracellularis on Growing-Finishing Pig’s Performance in a Pig Production Unit with Endemic Porcine Proliferative Enteropathy (PPE). Dtsch. Tierarztl. Wochenschr. 2006, 113, 232–235. [Google Scholar] [PubMed]
- Roerink, F.; Morgan, C.L.; Knetter, S.M.; Passat, M.-H.; Archibald, A.L.; Ait-Ali, T.; Strait, E.L. A Novel Inactivated Vaccine against Lawsonia intracellularis Induces Rapid Induction of Humoral Immunity, Reduction of Bacterial Shedding and Provides Robust Gut Barrier Function. Vaccine 2018, 36, 1500–1508. [Google Scholar] [CrossRef]
- Karuppannan, A.K.; Opriessnig, T. Lawsonia intracellularis: Revisiting the Disease Ecology and Control of This Fastidious Pathogen in Pigs. Front. Vet. Sci. 2018, 5, 181. [Google Scholar] [CrossRef]
- Caspari, K.; Kummerlen, D.; Voets, H.; Eichin, E.; Zeeh, H.; Zimmermann, W. Field study about the use of Enterisol Ileitis in a swine herd in Switzerland. Schweiz. Arch. Tierheilkd. 2009, 151, 31–32. [Google Scholar] [CrossRef]
- Weibel, H.; Sydler, T.; Brugnera, E.; Voets, H.; Grosse Liesner, B.; Sidler, X. Efficacy of Simultaneous Vaccination with Enterisol(R) Ileitis and Ingelvac(R) CircoFLEXTM in a Swiss Breeding Farm. Schweiz. Arch. Tierheilkd. 2012, 154, 445–450. [Google Scholar] [CrossRef]
- Haidich, A.-B. Meta-Analysis in Medical Research. Hippokratia 2010, 14, 29. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. J. Cereb. Blood Flow Metab. 2020, 40, 1769–1777. [Google Scholar] [CrossRef]
- Furukawa, T.A.; Barbui, C.; Cipriani, A.; Brambilla, P.; Watanabe, N. Imputing Missing Standard Deviations in Meta-Analyses Can Provide Accurate Results. J. Clin. Epidemiol. 2006, 59, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 1119536618. [Google Scholar] [CrossRef]
- Lipsey, M.W.; Wilson, D.B. Practical Meta-Analysis; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2001; ISBN 0761921672. [Google Scholar]
- Riber, U.; Cordes, H.; Boutrup, T.S.; Jensen, T.K.; Heegaard, P.M.H.; Jungersen, G. Primary Infection Protects Pigs against Re-Infection with Lawsonia intracellularis in Experimental Challenge Studies. Vet. Microbiol. 2011, 149, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.G.; Collins, A.M.; Donahoo, M.; Emery, D. Immunological Responses to Vaccination Following Experimental Lawsonia intracellularis Virulent Challenge in Pigs. Vet. Microbiol. 2013, 164, 131–138. [Google Scholar] [CrossRef]
- Bak, H.; Rathkjen, P.H. Reduced Use of Antimicrobials after Vaccination of Pigs against Porcine Proliferative Enteropathy in a Danish SPF Herd. Acta Vet. Scand. 2009, 51, 1. [Google Scholar] [CrossRef]
- Guedes, R.M.C.; Gebhart, C.J. Onset and Duration of Fecal Shedding, Cell-Mediated and Humoral Immune Responses in Pigs after Challenge with a Pathogenic Isolate or Attenuated Vaccine Strain of Lawsonia intracellularis. Vet. Microbiol. 2003, 91, 135–145. [Google Scholar] [CrossRef]
- Jansen, T.; Weersink, A.; von Massow, M.; Poljak, Z. Assessing the Value of Antibiotics on Farms: Modeling the Impact of Antibiotics and Vaccines for Managing Lawsonia intracellularis in Hog Production. Front. Vet. Sci. 2019, 6, 364. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.G.; Collins, A.M.; Dunlop, R.H.; Emery, D. Effect of the Route of Administration on the Mucosal and Systemic Immune Responses to Lawsonia intracellularis Vaccine in Pigs. Aust. Vet. J. 2015, 93, 124–126. [Google Scholar] [CrossRef]
- Riber, U.; Boesen, H.T.; Jakobsen, J.T.; Nguyen, L.T.M.; Jungersen, G. Co-Incubation with IL-18 Potentiates Antigen-Specific IFN-Gamma Response in a Whole-Blood Stimulation Assay for Measurement of Cell-Mediated Immune Responses in Pigs Experimentally Infected with Lawsonia intracellularis. Vet. Immunol. Immunopathol. 2011, 139, 257–263. [Google Scholar] [CrossRef]
- Cordes, H.; Riber, U.; Jensen, T.K.; Jungersen, G. Cell-Mediated and Humoral Immune Responses in Pigs Following Primary and Challenge-Exposure to Lawsonia Intracellularis. Vet. Res. 2012, 43, 9. [Google Scholar] [CrossRef]
- Watson, E.; Clark, E.M.; Alberdi, M.P.; Inglis, N.F.; Porter, M.; Imrie, L.; McLean, K.; Manson, E.; Lainson, A.; Smith, D.G.E. A Novel Lawsonia intracellularis Autotransporter Protein Is a Prominent Antigen. Clin. Vaccine Immunol. 2011, 18, 1282–1287. [Google Scholar] [CrossRef]
- Watson, E.; Alberdi, M.P.; Inglis, N.F.; Lainson, A.; Porter, M.E.; Manson, E.; Imrie, L.; Mclean, K.; Smith, D.G.E. Proteomic Analysis of Lawsonia intracellularis Reveals Expression of Outer Membrane Proteins during Infection. Vet. Microbiol. 2014, 174, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Won, G.; Park, S.; Lee, J.H. Identification of Lawsonia intracellularis Putative Hemolysin Protein A and Characterization of Its Immunoreactivity. Vet. Microbiol. 2017, 205, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Won, G.; Lee, J.H. An Attenuated Salmonella Vaccine Secreting Lawsonia intracellularis Immunogenic Antigens Confers Dual Protection against Porcine Proliferative Enteropathy and Salmonellosis in a Murine Model. J. Vet. Sci. 2019, 20, e24. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Won, G.; Kim, J.; Kim, H.B.; Lee, J.H. Potent O-Antigen-Deficient (Rough) Mutants of Salmonella Typhimurium Secreting Lawsonia intracellularis Antigens Enhance Immunogenicity and Provide Single-Immunization Protection against Proliferative Enteropathy and Salmonellosis in a Murine Model. Vet. Res. 2018, 49, 57. [Google Scholar] [CrossRef]
- Won, G.; Lee, J.H. Antigenic and Functional Profiles of a Lawsonia intracellularis Protein That Shows a Flagellin-like Trait and Its Immuno-Stimulatory Assessment. Vet. Res. 2018, 49, 17. [Google Scholar] [CrossRef]
- Obradovic, M.; Pasternak, J.A.; Hon Ng, S.; Allan, B.; Brownlie, R.; Wilson, H.L. Immunoproteomic Analysis of Lawsonia intracellularis Identifies Candidate Neutralizing Antibody Targets for Use in Subunit Vaccine Development. Vet. Microbiol. 2019, 235, 270–279. [Google Scholar] [CrossRef]
- Rathkjen, P.H.; Trela, T.; Voets, H.; Adam, M. Experience with Enterisol® Ileitis under Pre-Wean Administration. In Proceedings of the 2007 Allen D. Leman Swine Conference, Minneapolis, MN, USA, 15 September 2007. [Google Scholar]
- Gaumann, H.; Wesselmann, S.; Gottschalk, F. Global Efficacy and Economics of Enterisol® Ileitis: A Meta-Analysis. In Proceedings of the European Enterisol® Ileitis Symposium, Barcelona, Spain, 13–15 October 2005; Boehringer Ingelheim: Ingelheim am Rhein, Germany, 2005; pp. 2–4. [Google Scholar]
- Henke, N.; Gaumann, H.; Gottschalk, F. Experiences with Enterisol Ileitis under Field Circumstances in Germany. In Proceedings of the 19th IPVS Congress, Copenhagen, Denmark, 6–19 July 2006; Volume 2, p. 203. [Google Scholar]
- Obradovic, M.R.; Wilson, H.L. Immune Response and Protection against Lawsonia intracellularis Infections in Pigs. Vet. Immunol. Immunopathol. 2020, 219, 109959. [Google Scholar] [CrossRef]
- Jacobson, M.; Fellström, C.; Jensen-Waern, M. Porcine Proliferative Enteropathy: An Important Disease with Questions Remaining to Be Solved. Vet. J. 2010, 184, 264–268. [Google Scholar] [CrossRef]
- Kroll, J.J.; Roof, M.B.; Hoffman, L.J.; Dickson, J.S.; Harris, D.L.H. Proliferative Enteropathy: A Global Enteric Disease of Pigs Caused by Lawsonia Intracellularis. Anim. Health Res. Rev. 2005, 6, 173–197. [Google Scholar] [CrossRef]
- Okones, J.; Dutler, D.; Walter, D.; Holck, J.T. Use of Enterisol® Ileitis in Suckling Pigs. In Proceedings of the 2005 Allen D. Leman Swine Conference, Minneapolis, MN, USA, 17 September 2005. [Google Scholar]
- Klien, K.; Adam, M. Effekte Des Ausstiegs Aus Der Ileitisimpfung Auf Leistungsparameter Sowie Antibiotikaverbrauch. Prakt. Tierarzt 2011, 6, 510–515. [Google Scholar]
- Dominique, K.D.; Aline, L.; Delphine, P., Jr. Vaccination with Enterisol® Ileitis In 23 French Pig Farms Improves Technical Parameters While Reducing the Total Amount of Antibiotics Used. In Proceedings of the 6th European Symposium of Porcine, Sorrento, Italy, 7–9 May 2014; p. 127. [Google Scholar]
- Park, S.; Lee, J.-B.; Kim, K.-J.; Oh, Y.-S.; Kim, M.-O.; Oh, Y.-R.; Hwang, M.-A.; Lee, J.-A.; Lee, S.-W. Efficacy of a Commercial Live Attenuated Lawsonia intracellularis Vaccine in a Large Scale Field Trial in Korea. Clin. Exp. Vaccine Res. 2013, 2, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Deitmer, R.; Bubikat, A.; Keller, C.; Adam, M.; Voets, H. Embedded Ileitis Vaccination or Antibiotic Treatment-a Comparison of Efficacy and Economic Impact during the Nursery Period. Prakt. Tierarzt-Hann. 2008, 1, 142–151. [Google Scholar]
- Peiponen, K.S.; Tirkkonen, B.T.; Junnila, J.J.T.; Heinonen, M.L. Effect of a Live Attenuated Vaccine against Lawsonia intracellularis in Weaned and Finishing Pig Settings in Finland. Acta Vet. Scand. 2018, 60, 18. [Google Scholar] [CrossRef] [PubMed]
- Bornhorn, R. Efficacy and Economical Impact of Oral Vaccination of Partially Infected Piglets with Enterisol (R) Ileitis. Prakt. Tierarzt 2007, 88, 172–178. [Google Scholar]
- Thaker, M.Y.C.; Bilkei, G. Comparison of the Effects of Oral Vaccination and Different Dietary Antibiotic Prophylactic Treatment against Lawsonia intracellularis Associated Losses in a Fattening Pig Production Unit with High Prevalence of Porcine Proliferative Enteropathy (PPE). Tierarztl. Umsch. 2006, 61, 372–376. [Google Scholar]
- Visscher, C.; Kruse, A.; Sander, S.; Keller, C.; Mischok, J.; Tabeling, R.; Henne, H.; Deitmer, R.; Kamphues, J. Experimental Studies on Effects of Diet on Lawsonia intracellularis Infections in Fattening Boars in a Natural Infection Model. Acta Vet. Scand. 2018, 60, 22. [Google Scholar] [CrossRef]
- Kroll, J.J.; Roof, M.B.; McOrist, S. Evaluation of Protective Immunity in Pigs Following Oral Administration of an Avirulent Live Vaccine of Lawsonia intracellularis. Am. J. Vet. Res. 2004, 65, 559–565. [Google Scholar] [CrossRef]
- Corbeil, L.B.; Blau, K.; Inzana, T.J.; Nielsen, K.H.; Jacobson, R.H.; Corbeil, R.R.; Winter, A.J. Killing of Brucella Abortus by Bovine Serum. Infect. Immun. 1988, 56, 3251–3261. [Google Scholar] [CrossRef]
- Jacobs, A.A.C.; Harks, F.; Pauwels, R.; Cao, Q.; Holtslag, H.; Pel, S.; Segers, R. Efficacy of a Novel Intradermal Lawsonia intracellularis Vaccine in Pigs against Experimental Infection and under Field Conditions. Porc. Health Manag. 2020, 6, 25. [Google Scholar] [CrossRef]
- Nathues, H.; Grosse Beilage, E. Diagnosis of Lawsonia intracellularis Infection in Pigs after Vaccination or Antimicrobial Treatment. Dtsch. Tierarztl. Wochenschr. 2008, 115, 404–409. [Google Scholar]
- McOrist, S.; Smits, R.J. Field Evaluation of an Oral Attenuated Lawsonia intracellularis Vaccine for Porcine Proliferative Enteropathy (Ileitis). Vet. Rec. 2007, 161, 26–28. [Google Scholar] [CrossRef]
- Hardge, T.; Nickoll, E.; Grunert, H.; Elbers, K.; Langbein, U.; Keller, C.; Bleier, T.; Pohlenz, J.; Ohlinger, V.F.; Schroeder, B. Prevention of Porcine Proliferative Enteropathy (PPE) by Vaccination—Efficacy and Economics in European Farms. Pig J. 2004, 54, 17–34. [Google Scholar]
- Jacobs, A.A.C.; Harks, F.; Hazenberg, L.; Hoeijmakers, M.J.H.; Nell, T.; Pel, S.; Segers, R.P.A.M. Efficacy of a Novel Inactivated Lawsonia intracellularis Vaccine in Pigs against Experimental Infection and under Field Conditions. Vaccine 2019, 37, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Riber, U.; Heegaard, P.M.H.; Cordes, H.; Ståhl, M.; Jensen, T.K.; Jungersen, G. Vaccination of Pigs with Attenuated Lawsonia intracellularis Induced Acute Phase Protein Responses and Primed Cell-Mediated Immunity without Reduction in Bacterial Shedding after Challenge. Vaccine 2015, 33, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Nathues, H.; Holthaus, K.; Grosse Beilage, E. Quantification of Lawsonia intracellularis in Porcine Faeces by Real-time PCR. J. Appl. Microbiol. 2009, 107, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, D. Economic Losses Associated with Ileitis; MSD Animal Health: Kenilworth, NJ, USA, 2019. [Google Scholar]
- Regina-Silva, S.; Feres, A.M.L.T.; França-Silva, J.C.; Dias, E.S.; Michalsky, É.M.; de Andrade, H.M.; Coelho, E.A.F.; Ribeiro, G.M.; Fernandes, A.P.; Machado-Coelho, G.L.L. Field Randomized Trial to Evaluate the Efficacy of the Leish-Tec® Vaccine against Canine Visceral Leishmaniasis in an Endemic Area of Brazil. Vaccine 2016, 34, 2233–2239. [Google Scholar] [CrossRef]
- Tizard, I.R. Porcine Vaccines. Vaccines Vet. 2021, 225–242.e1. [Google Scholar] [CrossRef]
- Slack, M.K.; Draugalis, J.R., Jr. Establishing the Internal and External Validity of Experimental Studies. Am. J. Health Pharm. 2001, 58, 2173–2181. [Google Scholar] [CrossRef]
- Weinberg, G.A.; Szilagyi, P.G. Vaccine Epidemiology: Efficacy, Effectiveness, and the Translational Research Roadmap. J. Infect. Dis. 2010, 201, 1607–1610. [Google Scholar] [CrossRef]
- Montesino, R.; Gutierrez, N.; Camacho, F.; Farnos, O.; Andrades, S.; Gonzalez, A.; Acosta, J.; Cortez-San Martin, M.; Sanchez, O.; Ruiz, A.; et al. Multi-Antigenic Recombinant Subunit Vaccine against Lawsonia intracellularis: The Etiological Agent of Porcine Proliferative Enteropathy. Vaccine 2019, 37, 1340–1349. [Google Scholar] [CrossRef]
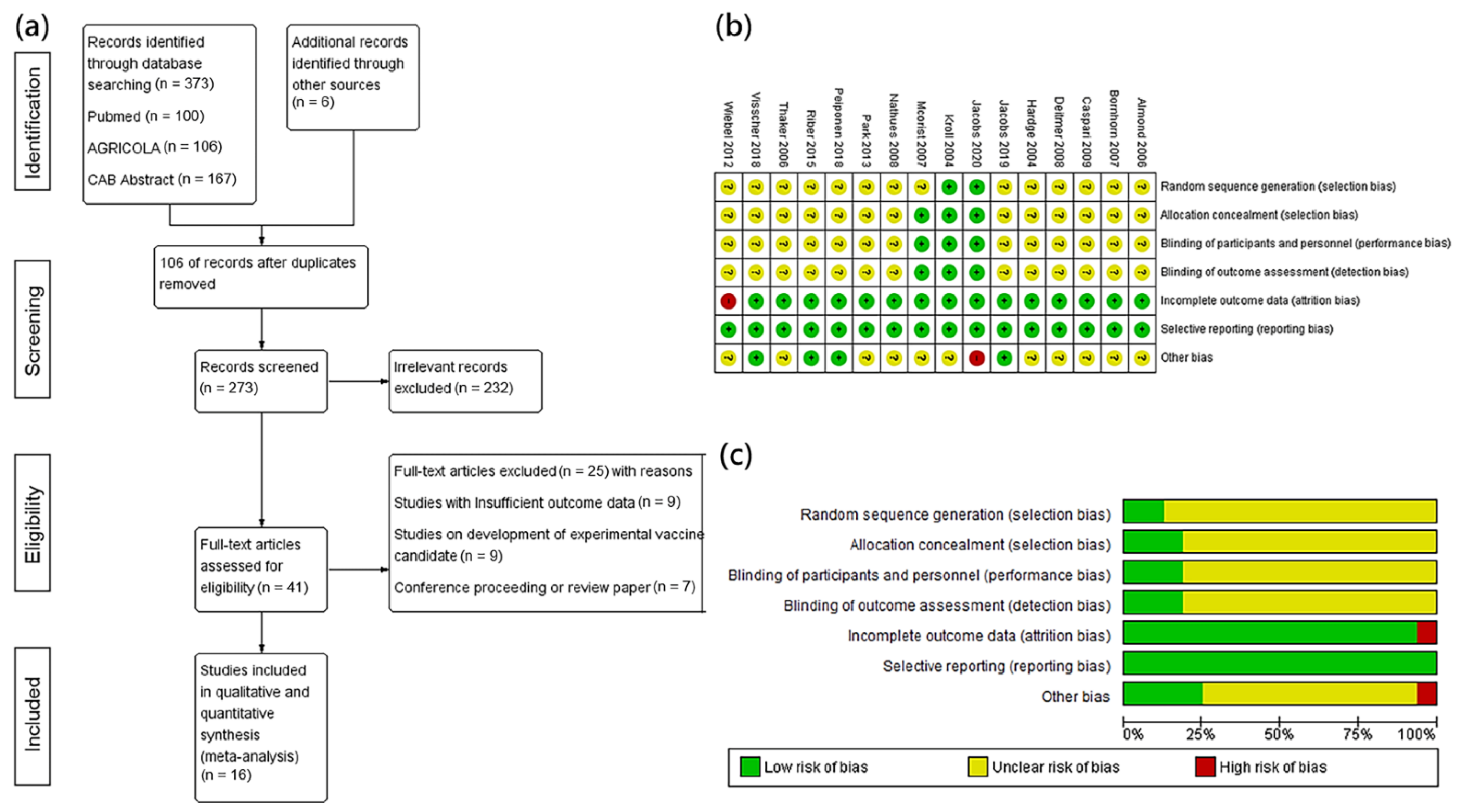

| Reason | References |
|---|---|
| Insufficient outcome data included | Riber et al., 2011a [14], Nogueira et al., 2013 [15], Bak et al., 2009 [16], Guedes et al., 2003 [17], Jansen et al., 2019 [18], Nogueira et al., 2015 [19], Riber et al., 2011b [20], Cordes et al., 2012 [21], Roerink et al., 2018 [4] |
| Studies involved with development of experimental vaccine candidates | Watson et al., 2011 [22], Watson et al., 2014 [23], Kim et al., 2017 [24], Park et al., 2019 [25], Park et al., 2018 [26], Won et al., 2018 [27], Obradovic et al., 2019 [28] |
| Conference proceeding or review paper | Rathkjen et al., 2007 [29], Gaumann et al., 2005 [30], Henke et al., 2006 [31], Obradovic et al., 2020 [32], Jacobson et al., 2010 [33], Kroll et al., 2005 [34], Okones et al., 2005 [35], Klien et al., 2011 [36], Dominique et al., 2014 [37]. |
| Sub-Group | No. of Trials | Point Estimate | Standard Error | Z-Value | p-Value | Heterogeneity (Total between) | |||
|---|---|---|---|---|---|---|---|---|---|
| Q-Value | Df (Q) | p-Value | |||||||
| Production phase | Nursery | 10 | 0.256 | 0.005 | 3.580 | 0.000 | |||
| Growing- finishing | 15 | 0.720 | 0.230 | 3.131 | 0.002 | ||||
| 3.711 | 1 | 0.054 * | |||||||
| Study type | Field trial | 16 | 0.567 | 0.052 | 2.478 | 0.013 | |||
| Controlled trial | 9 | 0.675 | 0.027 | 4.074 | 0.000 | ||||
| 0.145 | 1 | 0.703 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, G.; Chi, N.-K.; Park, Y. The Effectiveness of Commercial Vaccination against Lawsonia intracellularis in Mitigating the Reduction in ADWG, the Increased Mortality and Fecal Shedding of the Vaccinated Pigs: A Systematic Review and Meta-Analysis. Vet. Sci. 2022, 9, 536. https://doi.org/10.3390/vetsci9100536
Won G, Chi N-K, Park Y. The Effectiveness of Commercial Vaccination against Lawsonia intracellularis in Mitigating the Reduction in ADWG, the Increased Mortality and Fecal Shedding of the Vaccinated Pigs: A Systematic Review and Meta-Analysis. Veterinary Sciences. 2022; 9(10):536. https://doi.org/10.3390/vetsci9100536
Chicago/Turabian StyleWon, Gayeon, Na-Kyoung Chi, and Yebin Park. 2022. "The Effectiveness of Commercial Vaccination against Lawsonia intracellularis in Mitigating the Reduction in ADWG, the Increased Mortality and Fecal Shedding of the Vaccinated Pigs: A Systematic Review and Meta-Analysis" Veterinary Sciences 9, no. 10: 536. https://doi.org/10.3390/vetsci9100536
APA StyleWon, G., Chi, N.-K., & Park, Y. (2022). The Effectiveness of Commercial Vaccination against Lawsonia intracellularis in Mitigating the Reduction in ADWG, the Increased Mortality and Fecal Shedding of the Vaccinated Pigs: A Systematic Review and Meta-Analysis. Veterinary Sciences, 9(10), 536. https://doi.org/10.3390/vetsci9100536






