Endoparasites Infecting Domestic Animals and Spectacled Bears (Tremarctos ornatus) in the Rural High Mountains of Colombia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
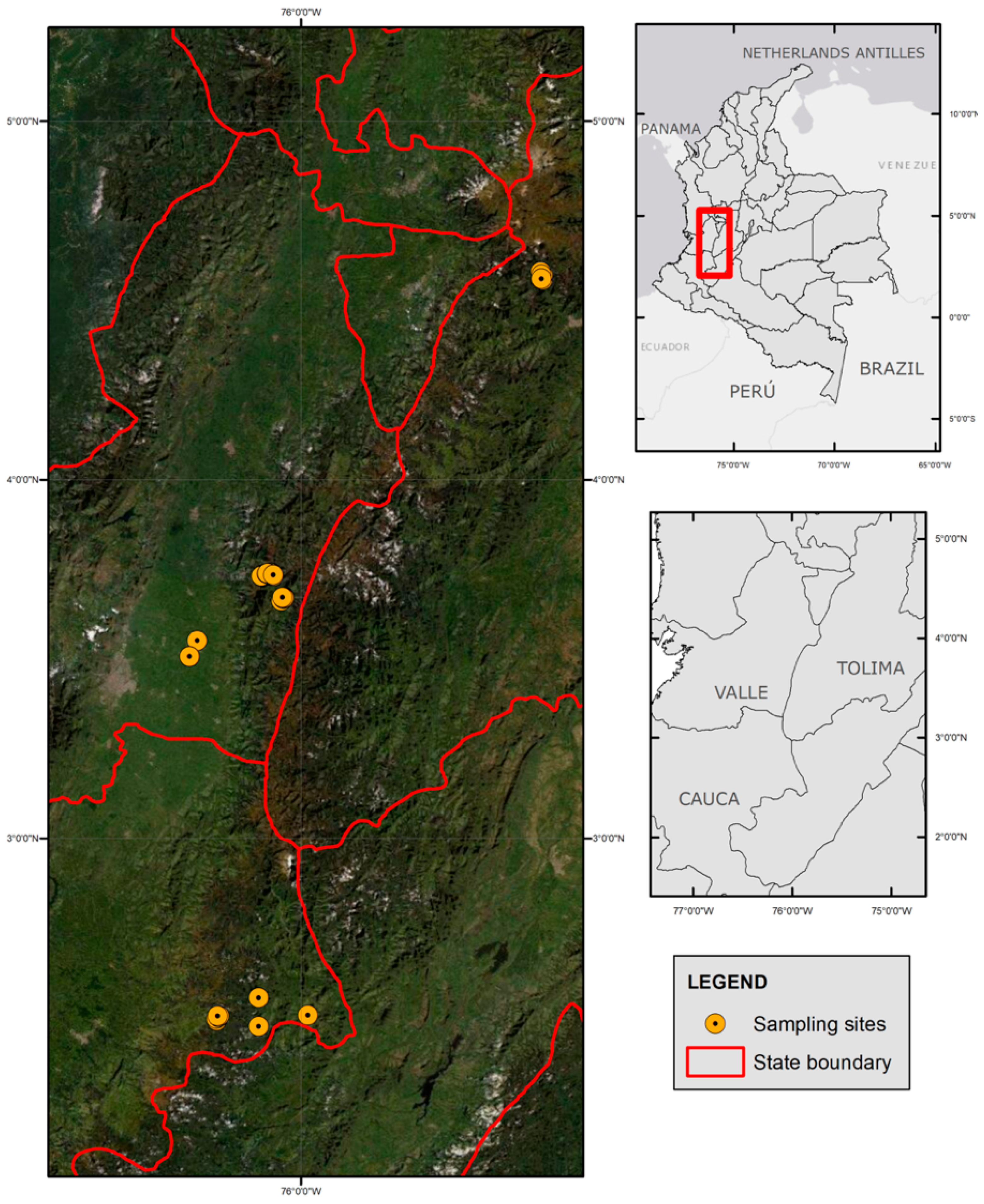
2.1. Study Area and Population
2.1.1. Rural High Mountains of Tenerife, Valle del Cauca
2.1.2. El Silencio, Cañon del Combeima (Tolima)
2.1.3. The Village of Gabriel Lopez, Municipalities of Totoró (Cauca)
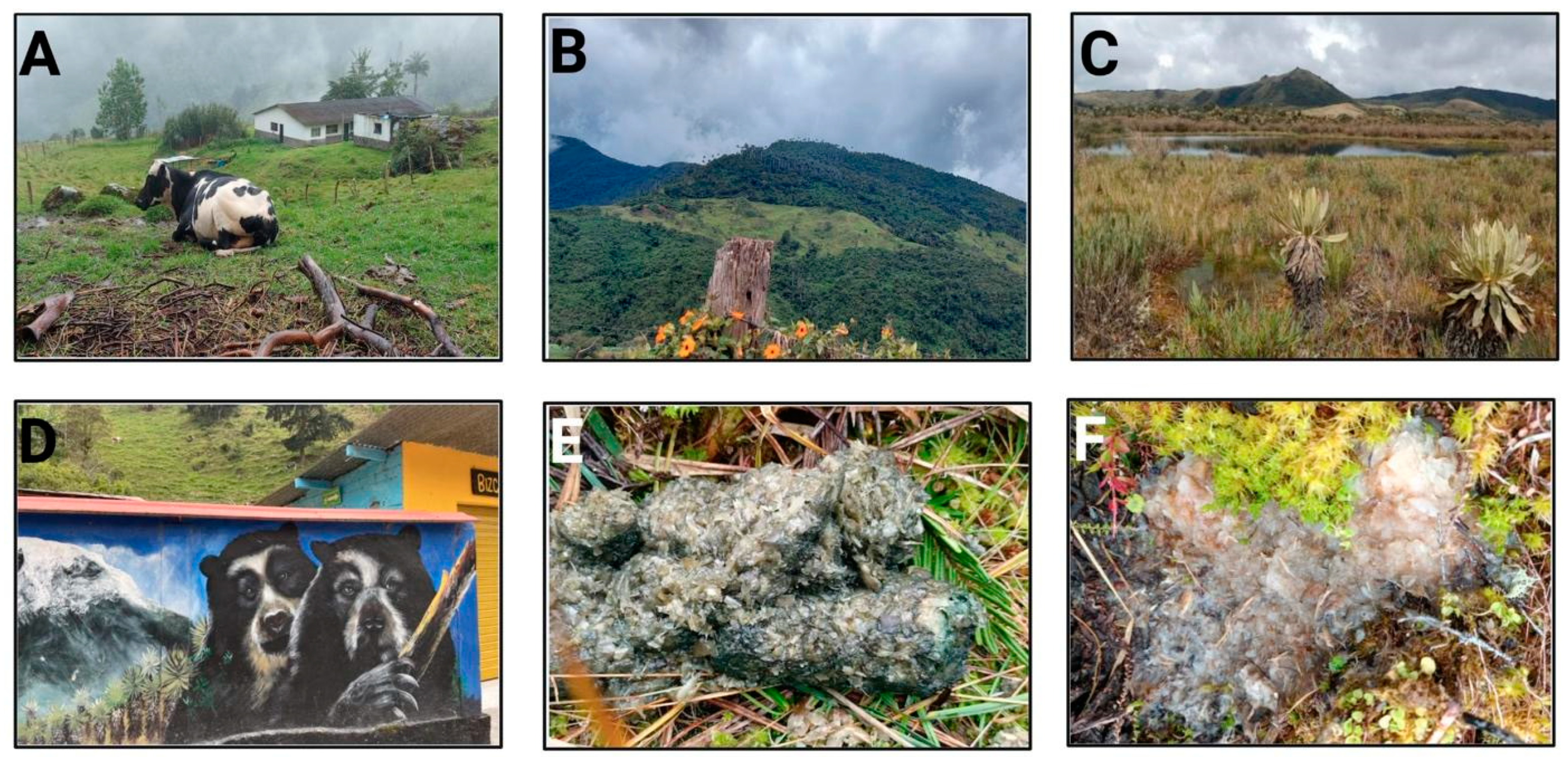
2.2. Description Area
2.3. Type of Study
2.4. Samples
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cristina, C.S.; Lenin, M.H. Sexaje Molecular a Partir de Heces en Osos de Anteojos (Tremarctos ornatus). Rev. Investig. Vet. Perú 2016, 27, 252–258. [Google Scholar] [CrossRef][Green Version]
- Juárez-Casillas, L.A.; Varas, C. Genética evolutiva y molecular de la familia Ursidae: Una revisión bibliográfica actualizada. Therya 2011, 2, 47–65. [Google Scholar] [CrossRef]
- Yu, L.; Li, Q.W.; Ryder, O.A.; Zhang, Y.P. Phylogeny of the bears (Ursidae) based on nuclear and mitochondrial genes. Mol. Phylogenet. Evol. 2004, 32, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, M.; Vásquez, J.Y.A.; Castellanos, A.; Kolter, L.; Shostell, J.M. Molecular evolution (mitochondrial and nuclear microsatellites markers) in the andean bear (Tremarctos ornatus; Ursidae, Carnivora): How many ESUs are there? In Conservation Genetics in Mammals: Integrative Research Using Novel Approaches; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 165–194. [Google Scholar] [CrossRef]
- GarcÍA-Rangel, S. Andean bear Tremarctos ornatus natural history and conservation. Mammal Rev. 2012, 42, 85–119. [Google Scholar] [CrossRef]
- Goldstein, I.; Paisley, S.; Wallace, R.; Jorgenson, J.P.; Cuesta, F.; Castellanos, A. Andean bear–livestock conflicts: A review. Ursus 2006, 17, 8–15. [Google Scholar] [CrossRef]
- Peyton, B.; Yerena, E.; Rumiz, D.I.; Jorgenson, J.; Orejuela, J. Status of wild Andean bears and policies for their management. Ursus 1998, 10, 87–100. Available online: https://www.jstor.org/stable/3873115 (accessed on 13 March 2022).
- Kattan, G.; Hernández, O.L.; Goldstein, I.; Rojas, V.; Murillo, O.; Gómez, C.; Restrepo, H.; Cuesta, F. Range fragmentation in the spectacled bear Tremarctos ornatus in the northern Andes. Oryx 2004, 38, 155–163. [Google Scholar] [CrossRef]
- Ruiz-García, M.; Arias Vásquez, J.Y.; Restrepo, H.; Cáceres-Martínez, C.H.; Shostell, J.M.; Jezkova, T. The genetic structure of the spectacled bear (Tremarctos ornatus; Ursidae, Carnivora) in Colombia by means of mitochondrial and microsatellite markers. J. Mammal. 2020, 101, 1072–1090. [Google Scholar] [CrossRef]
- Quintero Romero, L.D. Determinación de la Carga Parasitaria en Muestras Fecales de oso Andino (Tremarctos ornatus) en la Región Occidental del Parque Nacional Natural (PNN) Chingaza; Pontificia Universidad Javeriana: Bogotá, Colombia, 2019; Available online: http://purl.org/coar/version/c_ab4af688f83e57aa (accessed on 13 March 2022).
- Figueroa Pizarro, J. Interacciones humano–oso andino Tremarctos ornatus en el Perú: Consumo de cultivos y depredación de ganado. Therya 2015, 6, 251–278. [Google Scholar] [CrossRef]
- Pérez Mata, A.; García Pérez, H.; Gauta Parra, J. Caracterización Morfológica y molecular de Baylisascaris Venezuelensis, N. Sp. De una Infección Natural en el oso Andino de anteojos, Tremarctos ornatus Cuvier, 1825 en Venezuela. Neotrop. Helminthol. 2020, 10. [Google Scholar] [CrossRef]
- Wang, T.; Xie, Y.; Zheng, Y.; Wang, C.; Li, D.; Koehler, A.V.; Gasser, R.B. Parasites of the Giant Panda: A Risk Factor in the Conservation of a Species. Adv. Parasitol. 2018, 99, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Peña-Quistial, M.; Benavides-Montaño, J.A.; Duque, N.J.R.; Benavides-Montaño, G. Prevalence and associated risk factors of Intestinal parasites in rural high-mountain communities of the Valle del Cauca-Colombia. PLoS Negl. Trop. Dis. 2020, 14, e0008734. [Google Scholar] [CrossRef] [PubMed]
- Delgado Chávez, A.L.; de Méndez Acero, A. Formulación del Plan de Emergencias Para el Parque Nacional Natural Tayrona Como Herramienta Técnica Para el Fortalecimiento del Plan de Manejo del Área Protegida; Universidad de La Salle: Philadelphia, PA, USA, 2008; Available online: https://ciencia.lasalle.edu.co/ing_ambiental_sanitaria/648 (accessed on 13 March 2022).
- UAESPNN. Plan de Manejo del Parque Nacional Natural Los Nevados. 2006–2010. Available online: https://www.guao.org/sites/default/files/biblioteca/Los%20nevados.pdf (accessed on 13 March 2022).
- Caicedo Collazos, J.J.; Cortés Landázury, R. De la cuestión agropecuaria, las economías de enclave y los desequilibrios ecológicos en el Valle de Malvazá: Un análisis económico de impacto ambiental. Biotecnol. Sect. Agropecu. Agroind. 2008, 6, 105–119. [Google Scholar]
- Morales-Betancourt, J.A.; Estévez-Varón, J.V. El páramo: Ecosistema en vía de extinción? Rev. Luna Azul 2006, 22, 39–51. Available online: http://www.redalyc.org/articulo.oa?id=321727224004 (accessed on 13 March 2022).
- Diazgranados, M. A nomenclator for the frailejones (Espeletiinae Cuatrec., Asteraceae). PhytoKeys 2012, 1–52. [Google Scholar] [CrossRef]
- Rózsa, L.; Reiczigel, J.; Majoros, G. Quantifying Parasites in Samples of Hosts. J. Parasitol. 2000, 86, 228–232. [Google Scholar] [CrossRef]
- Adhikari, B.B.; Rana, H.; Sultan, K.; Devkota, B.; Nakao, T.; Kobayashi, K.; Sato, H.; Dhakal, I. Prevalence of Buxtonella sulcata in water buffaloes and cows in Chitwan Valley, southern Nepal. J. Vet. Parasitol. 2013, 11, 1–6. [Google Scholar]
- Ganai, A.; Parveen, S.; Kaur, D.; Katoch, R.; Yadav, A.; Godara, R.; Ahamed, I. Incidence of Buxtonella sulcata in bovines in R.S. Pura, Jammu. J. Parasit. Dis. 2015, 39, 446–447. [Google Scholar] [CrossRef]
- Ahmed, A.; Ijaz, M.; Ayyub, R.M.; Ghaffar, A.; Ghauri, H.N.; Aziz, M.U.; Ali, S.; Altaf, M.; Awais, M.; Naveed, M.; et al. Balantidium coli in domestic animals: An emerging protozoan pathogen of zoonotic significance. Acta Trop. 2020, 203, 105298. [Google Scholar] [CrossRef]
- Lopez-Osorio, S.; Villar, D.; Failing, K.; Taubert, A.; Hermosilla, C.; Chaparro-Gutierrez, J.J. Epidemiological survey and risk factor analysis on Eimeria infections in calves and young cattle up to 1 year old in Colombia. Parasitol. Res. 2020, 119, 255–266. [Google Scholar] [CrossRef]
- Butploy, N.; Kanarkard, W.; Maleewong Intapan, P. Deep Learning Approach for Ascaris lumbricoides Parasite Egg Classification. J. Parasitol. Res. 2021, 2021, 6648038. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, X.; Pei, J.; Su, L.; Zhang, H.; Liu, Y.; Wu, X. Investigation on the Morphology and infection situation of intestinal parasites in the wild giant pandas. Anim. J. Econ. Anim. 2018, 22, 106–111. [Google Scholar]
- Shrestha, S.; Maharjan, M. Parasitic burden in Red panda (Ailurus fulgens Cuvier, 1825) of Illam district Community forest, Nepal. Nepal. J. Zool. 2015, 3, 49–58. [Google Scholar] [CrossRef]
- Hair, J.D.; Mahrt, J.L. Eimeria albertensis n.sp. and E. borealis n.sp. (Sporozoa: Eimeriidae) in black bears Ursus americanus from Alberta. J. Protozool. 1970, 17, 663–664. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga Espinosa, M.G. Estudio Químico y Parasitológico de Muestras Fecales del oso Andino (Tremarctos ornatus) Provenientes de 2 Reservas Ecológicas, 2 Zoológicos y un Centro de Rescate en el Ecuador; USFQ: Quito, Ecuador, 2014. [Google Scholar]
- Pinilla Leon, J.C.; Delgado, N.U.; Florez, A.A. Prevalence of gastrointestinal parasites in cattle and sheep in three municipalities in the Colombian Northeastern Mountain. Vet. World 2019, 12, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Pinilla León, J.C.; Flórez, P.; Sierra, M.T.; Morales Ramírez, E.; Sierra, R.; Vásquez de Díaz, M.C.; Tobon, J.C.; Sánchez, A.; Ortiz, D. Prevalence of Gastrointestinal Parasitism in Bovines of Cesar State, Colombia. Available online: https://alicia.concytec.gob.pe/vufind/Record/1609-9117_4e1d2758c2737f0873551aea65de8136/Details (accessed on 13 March 2022).
- Johnson, K.P.; Adams, R.J.; Page, R.D.; Clayton, D.H. When do parasites fail to speciate in response to host speciation? Syst. Biol. 2003, 52, 37–47. [Google Scholar] [CrossRef]
- Kvičerová, J.; Hypša, V. Host-parasite incongruences in rodent Eimeria suggest significant role of adaptation rather than cophylogeny in maintenance of host specificity. PLoS ONE 2013, 8, e63601. [Google Scholar] [CrossRef]
- Urquhart, G.; Armour, J.; Duncan, J.; Dunn, A.; Jennings, F. Veterinary Parasitology; Blackwell Science LTD: Oxford, UK, 2003. [Google Scholar]
- Soulsby, E.J.L. Helminths, Arthropods and Protozoa of Domesticated Animals; Baillière Tindall: London, UK, 1982. [Google Scholar]
- Galván-Díaz, A.L. Cryptosporidiosis in Colombia: A Systematic Review. Curr. Trop. Med. Rep. 2018, 5, 144–153. [Google Scholar] [CrossRef]
- Laatamna, A.E.; Wagnerova, P.; Sak, B.; Kvetonova, D.; Xiao, L.; Rost, M.; McEvoy, J.; Saadi, A.R.; Aissi, M.; Kvac, M. Microsporidia and Cryptosporidium in horses and donkeys in Algeria: Detection of a novel Cryptosporidium hominis subtype family (Ik) in a horse. Vet. Parasitol. 2015, 208, 135–142. [Google Scholar] [CrossRef]
- Santín, M.; Vecino, J.A.C.; Fayer, R. A large scale molecular study of Giardia duodenalis in horses from Colombia. Vet. Parasitol. 2013, 196, 31–36. [Google Scholar] [CrossRef]
- Dixon, B.R. Giardia duodenalis in humans and animals—Transmission and disease. Res. Vet. Sci. 2021, 135, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J. New records of parasites in free-ranging Andean bears from Peru. Ursus 2015, 26, 21–27. [Google Scholar] [CrossRef]
- Appelbee, A.J.; Thompson, R.C.; Olson, M.E. Giardia and Cryptosporidium in mammalian wildlife—Current status and future needs. Trends Parasitol. 2005, 21, 370–376. [Google Scholar] [CrossRef]
- Chapman, C.A.; Schoof, V.A.; Bonnell, T.R.; Gogarten, J.F.; Calmé, S. Competing pressures on populations: Long-term dynamics of food availability, food quality, disease, stress and animal abundance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140112. [Google Scholar] [CrossRef]
- Johnston, A.R.; Gillespie, T.R.; Rwego, I.B.; McLachlan, T.L.; Kent, A.D.; Goldberg, T.L. Molecular epidemiology of cross-species Giardia duodenalis transmission in western Uganda. PLoS Negl. Trop. Dis. 2010, 4, e683. [Google Scholar] [CrossRef]
- Beck, R.; Sprong, H.; Bata, I.; Lucinger, S.; Pozio, E.; Cacciò, S.M. Prevalence and molecular typing of Giardia spp. in captive mammals at the zoo of Zagreb, Croatia. Vet. Parasitol. 2011, 175, 40–46. [Google Scholar] [CrossRef]
- Cali, A.; Owen, R.L. Microsporidiosis. In Laboratory Diagnosis of Infectious Diseases Principles and Practice; Balows, A., Hausler, J.W.J., Ohashi, M., Turano, A., Eds.; Springer: New York, NY, USA, 1988; p. 1. [Google Scholar]
- Han, B.; Takvorian, P.M.; Weiss, L.M. Invasion of Host Cells by Microsporidia. Front. Microbiol. 2020, 11, 172. [Google Scholar] [CrossRef]
- Udonsom, R.; Prasertbun, R.; Mahittikorn, A.; Chiabchalard, R.; Sutthikornchai, C.; Palasuwan, A.; Popruk, S. Identification of Enterocytozoon bieneusi in goats and cattle in Thailand. BMC Vet. Res. 2019, 15, 308. [Google Scholar] [CrossRef]
- Li, W.; Zhong, Z.; Song, Y.; Gong, C.; Deng, L.; Cao, Y.; Zhou, Z.; Cao, X.; Tian, Y.; Li, H.; et al. Human-Pathogenic Enterocytozoon bieneusi in Captive Giant Pandas (Ailuropoda melanoleuca) in China. Sci. Rep. 2018, 8, 6590. [Google Scholar] [CrossRef]
- Park, E.; Poulin, R. Two parasites in one host: Spatiotemporal dynamics and co-occurrence of Microsporidia and Rickettsia in an amphipod host. Parasitology 2021, 148, 1099–1106. [Google Scholar] [CrossRef]
- Norman Grim, J.; Jirků-Pomajbíková, K.; Ponce-Gordo, F. Light microscopic morphometrics, ultrastructure, and molecular phylogeny of the putative pycnotrichid Ciliate, Buxtonella sulcata. Eur. J. Protistol. 2015, 51, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Mughal, M.A.S.; Khan, M.K.; Abbas, Z.; Chatha, A.K.; Abbas, R.Z.; Qureshi, A.S.; Mahmood, M.S.; Ali, S.; Sindhu, Z.-U.-D.; Zafar, A.; et al. First report on the epidemiology of Buxtonella sulcata in bovines in Pakistan. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Al-Bakri, H.S.; Suliman, E.G.; Al-Saffar, T.M. Prevalence of intestinal ciliate Buxtonella sulcata in cattle in Mosul. Iraqi J. Vet. Sci. 2010, 24, 27–30. [Google Scholar] [CrossRef]
- El-Dakhly, K.M.; Arafa, W.M.; Mahrous, L.N.; Yousef, A.M. Gastrointestinal Helminthic Infections in Egyptian Domestic Camels, Camelus dromedarius, with a Special Reference to Trichostrongylids. J. Adv. Vet. Res. 2020, 10, 21–28. [Google Scholar]
- Correa, O.; Castro, O.R.A. Presence of the ciliated protozoan Buxtonella sulcata (Trichostomatia, Balantidiidae) in cattle in Uruguay. Veterinaria 2015, 51, 32–37. [Google Scholar]
- Forero, J.A.V.; Bernal, C.E.M. Prevalencia de Buxtonella sulcata en bovinos de la Sabana de Bogota; Universidad Nacional de Colombia; Facultad de Medicina Veterianria y Zootecnia: Bogotá, Colombia, 2015. [Google Scholar]
- Griffiths, I.B.; Parra, D.G.; Vizcaino, O.G.; Gallego, M.I. Prevalence of parasite eggs and cysts in faeces from dairy cows in Colombia. Trop. Anim. Health Prod. 1986, 18, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Hernández Guzmán, J.A. Presencia de Parásitos Gastrointestinales y Pulmonares en Bovinos Lecheros de dos Hatos de la Sabana de Bogotá, Colombia; Pontificia Universidad Javeriana: Bogotá, Colombia, 2021. [Google Scholar]
- Pomajbíková, K.; Oborník, M.; Horák, A.; Petrželková, K.J.; Grim, J.N.; Levecke, B.; Todd, A.; Mulama, M.; Kiyang, J.; Modrý, D. Novel insights into the genetic diversity of Balantidium and Balantidium-like cyst-forming ciliates. PLoS Negl. Trop. Dis. 2013, 7, e2140. [Google Scholar] [CrossRef]
- Schuster, R.K. Parasites of dromedaries and bactrian camels—A review Part 1: Stenoxenous parasites. J. Camel Pract. Res. 2018, 25, 1. [Google Scholar] [CrossRef]
- Schaul, J. Baylisascaris Transfuga in Captive and Free-Ranging Populations of Bears (Family: Ursidae). Doctoral Dissertation, Ohio State University, Columbus, OH, USA, 2006. [Google Scholar]
- Štrkolcová, G.; Goldová, M.; Šnábel, V.; Špakulová, M.; Orosová, T.; Halán, M.; Mojžišová, J. A frequent roundworm Baylisascaris transfuga in overpopulated brown bears (Ursus arctos) in Slovakia: A problem worthy of attention. Acta Parasitol. 2018, 63, 167–174. [Google Scholar] [CrossRef]
- Sapp, S.G.; Gupta, P.; Martin, M.K.; Murray, M.H.; Niedringhaus, K.D.; Pfaff, M.A.; Yabsley, M.J. Beyond the raccoon roundworm: The natural history of non-raccoon Baylisascaris species in the New World. Int. J. Parasitol. Parasites Wildl. 2017, 6, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-S.; Daszak, P.; Huang, H.-L.; Yang, G.-Y.; Kilpatrick, A.M.; Zhang, S. Parasite threat to panda conservation. EcoHealth 2008, 5, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.L. Parasites of black bears of the Lake Superior region. J. Wildl. Dis. 1975, 11, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Mackenstedt, U.; Jenkins, D.; Romig, T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int. J. Parasitol. Parasites Wildl. 2015, 4, 71–79. [Google Scholar] [CrossRef]
- Rojas Vera Pinto, R.A.; Butrón, R.; Martel, C. Reports of feeding incidents of cattle by andean bear (Tremarctos ornatus) in Central Peru. Rev. Mex. Mastozool. (Nueva Epoca) 2020, 10, 25–32. [Google Scholar] [CrossRef]
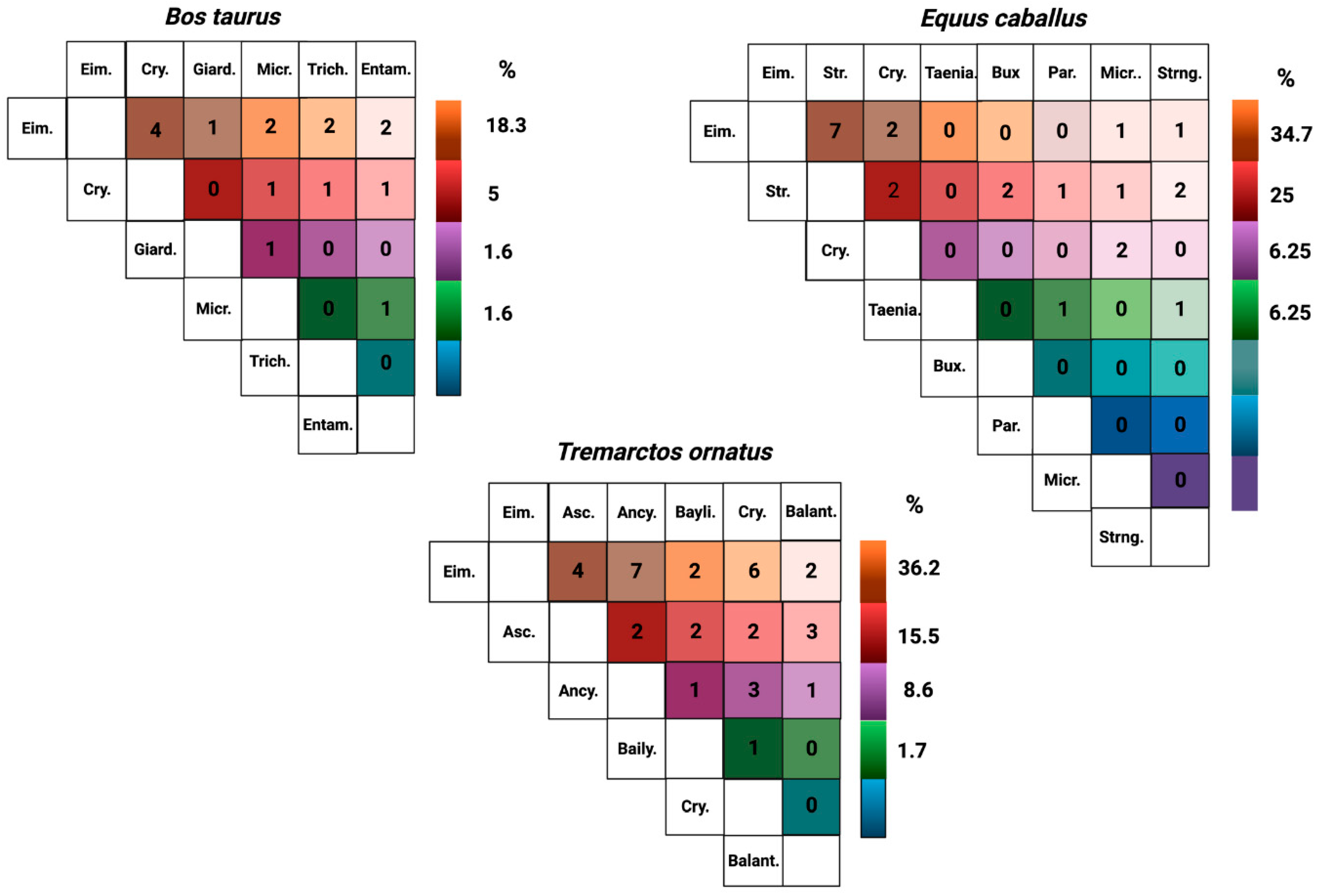
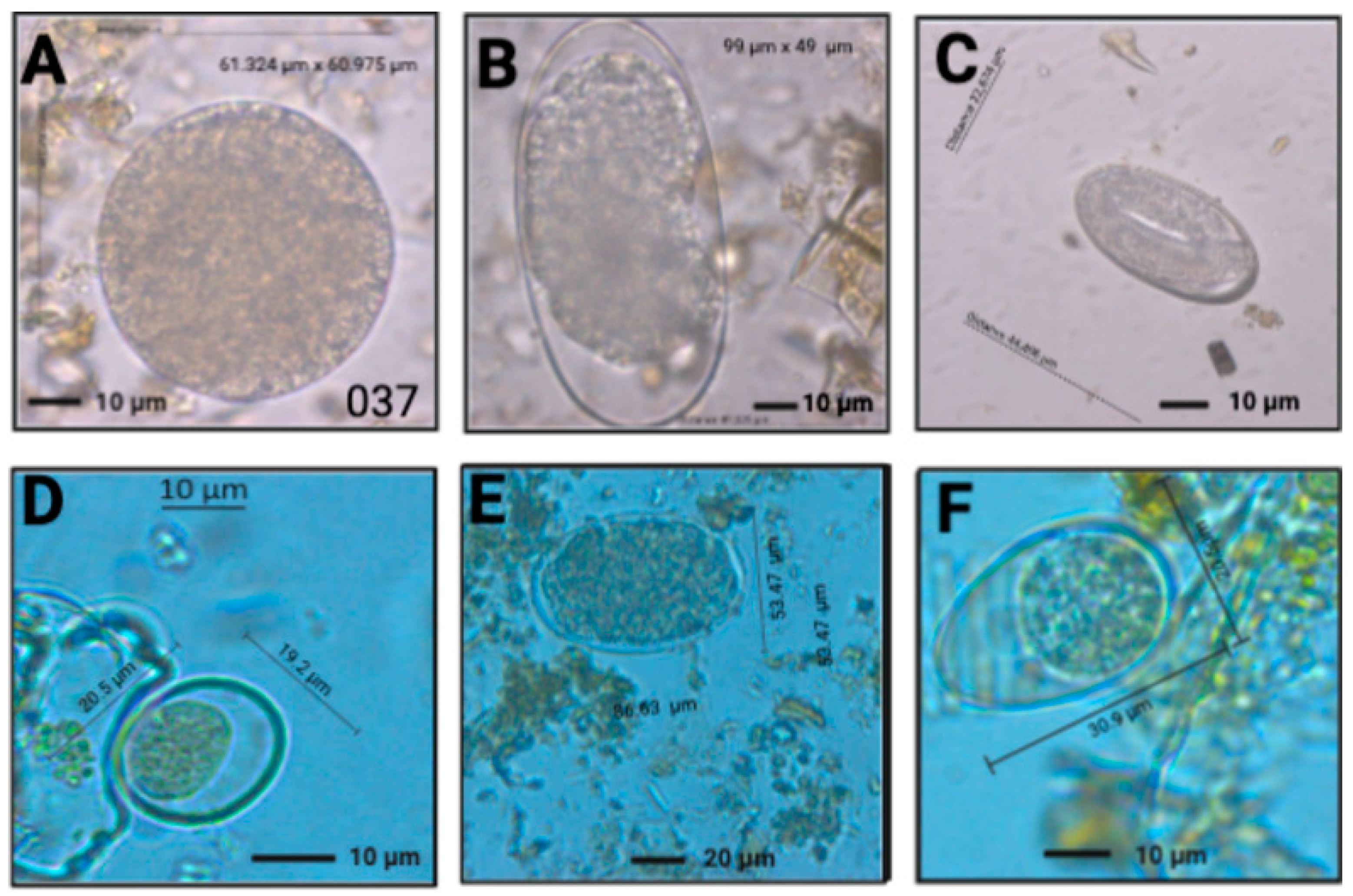
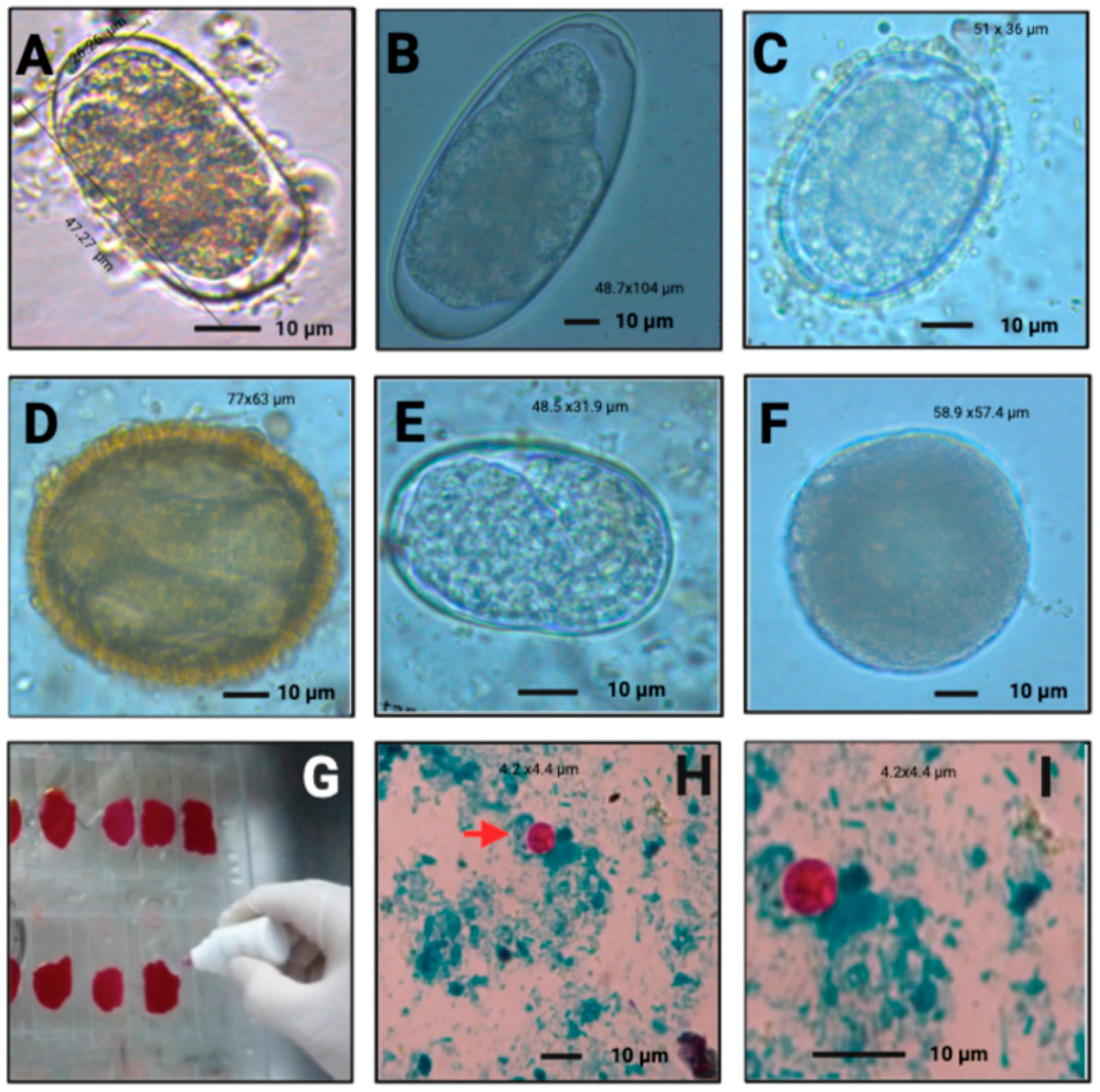
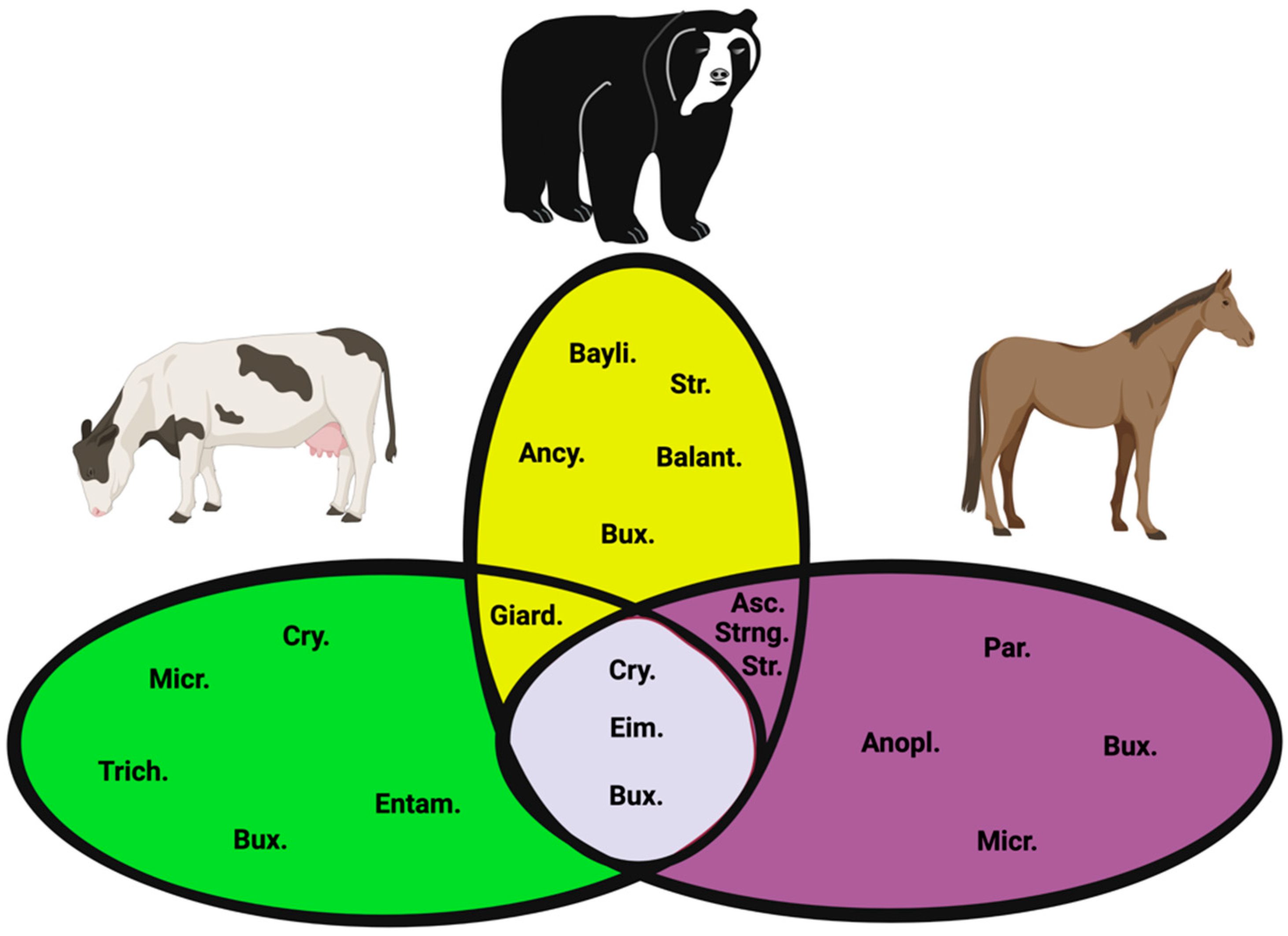
| Species | Prevalence Means | Prevalence IC 95% |
|---|---|---|
| Cattle-Bos tauros | ||
| Eimeria spp. | 53.89% (SD ± 4.6%) | 41–66% |
| Cryptosporidium spp. | 5.36% (SD ± 2.5%) | 0.3–11% |
| Giardia spp. | 3.57% (SD ± 1.7%) | 1.1–8.3% |
| Microsporidium spp. | 2.68% (SD ± 1.3%) | 1.4–6.8% |
| Trichostrongylus spp. | 2.68% (SD ± 1.3%) | 1.4–6.8% |
| Entamoeba spp. | 1.79% (SD ± 0.9%) | 1.6–5.1% |
| Fasciola spp. | 1.79% (SD ± 0.86%) | 1.6–5.1% |
| Buxtonella spp. | 0.89 % (SD ± 0.43) | −1.5–0.03% |
| Equus caballus | ||
| Eimeria spp. | 33.08% (SD ± 2.08%) | 16.8–49.4% |
| Strongylus spp. | 18.08% (SD ± 2.50%) | 4.7–31.4% |
| Cryptosporidium spp. | 4.17% (SD ± 2.08%) | 2.8–11.1% |
| Buxtonella spp. | 4.17% (SD ± 2.50%) | 2.8–11.1% |
| Taenia spp. | 4.17% (SD ± 5.42%) | 2.8–11.1% |
| Parascaris equorum | 2.08% (SD ± 2.92%) | 2.9–7.0% |
| Microsporidium spp. | 2.08% (SD ± 2.92%) | 2.9–7.0% |
| Strongyloides spp. | 2.08% (SD ± 3.96%) | 2.9–7.0% |
| Trichonema spp. | 2.08% (SD ± 3.96%) | 2.9–7.0% |
| Mesocestoides spp. | 2.08% (SD ± 1.46%) | 2.9–7.0% |
| Dicroelium spp. | 2.08% (SD ± 1.46%) | 2.9–7.0% |
| Tremarctos ornatus | ||
| Eimeria spp. | 30.0% (SD ± 7.07%) | 18.2–41.8% |
| Ascaris spp. | 21.7% (SD ± 5.11%) | 11.1–32.3% |
| Ancylostoma spp. | 15.0% (SD ± 3.54%) | 5.8–24.2% |
| Baylisascaris spp. | 13.3% (SD ± 3.14%) | 4.6–22.1% |
| Cryptosporidium spp. | 10.0% (SD ± 2.36%) | 2.3–17.7% |
| Balantidium coli | 5.0% (SD ± 1.18%) | 0.6–10.6% |
| Anaplocephalidae spp. | 3.3% (SD ± 0.79%) | 1.3–8.0% |
| Acanthamoeba spp. | 1.7% (SD ± 0.39%) | 1.6–5.0% |
| Dientamoeba spp. | 1.7% (SD ± 0.39%) | 1.6–5.0% |
| Diphyllobotrium spp. | 1.7% (SD ± 0.39%) | 1.6–5.0% |
| Fluke | 1.7% (SD ± 0.39%) | 1.6–5.0% |
| Giardia spp. | 1.7% (SD ± 0.39%) | 1.6–5.0% |
| Paramphistomum spp. | 1.7% (SD ± 0.39%) | 1.6–5.0% |
| Parascaris spp. | 1.7% (SD ± 0.39%) | 1.6–5.0% |
| Stephanurus spp. | 1.7% (SD ± 0.39%) | 1.6–5.0% |
| Strongylus spp. | 1.7% (SD ± 0.39%) | 1.6–5.0% |
| Buxtonella spp. | 1.7% (SD ± 0.39%) | 1.6–5.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zárate Rodriguez, P.T.; Collazos-Escobar, L.F.; Benavides-Montaño, J.A. Endoparasites Infecting Domestic Animals and Spectacled Bears (Tremarctos ornatus) in the Rural High Mountains of Colombia. Vet. Sci. 2022, 9, 537. https://doi.org/10.3390/vetsci9100537
Zárate Rodriguez PT, Collazos-Escobar LF, Benavides-Montaño JA. Endoparasites Infecting Domestic Animals and Spectacled Bears (Tremarctos ornatus) in the Rural High Mountains of Colombia. Veterinary Sciences. 2022; 9(10):537. https://doi.org/10.3390/vetsci9100537
Chicago/Turabian StyleZárate Rodriguez, Paula Tatiana, Luisa Fernanda Collazos-Escobar, and Javier Antonio Benavides-Montaño. 2022. "Endoparasites Infecting Domestic Animals and Spectacled Bears (Tremarctos ornatus) in the Rural High Mountains of Colombia" Veterinary Sciences 9, no. 10: 537. https://doi.org/10.3390/vetsci9100537
APA StyleZárate Rodriguez, P. T., Collazos-Escobar, L. F., & Benavides-Montaño, J. A. (2022). Endoparasites Infecting Domestic Animals and Spectacled Bears (Tremarctos ornatus) in the Rural High Mountains of Colombia. Veterinary Sciences, 9(10), 537. https://doi.org/10.3390/vetsci9100537






