Conjunctival Inverted Papilloma Progressing to Carcinoma. First Report in Horse
Abstract
1. Introduction
2. Materials and Methods
3. Results
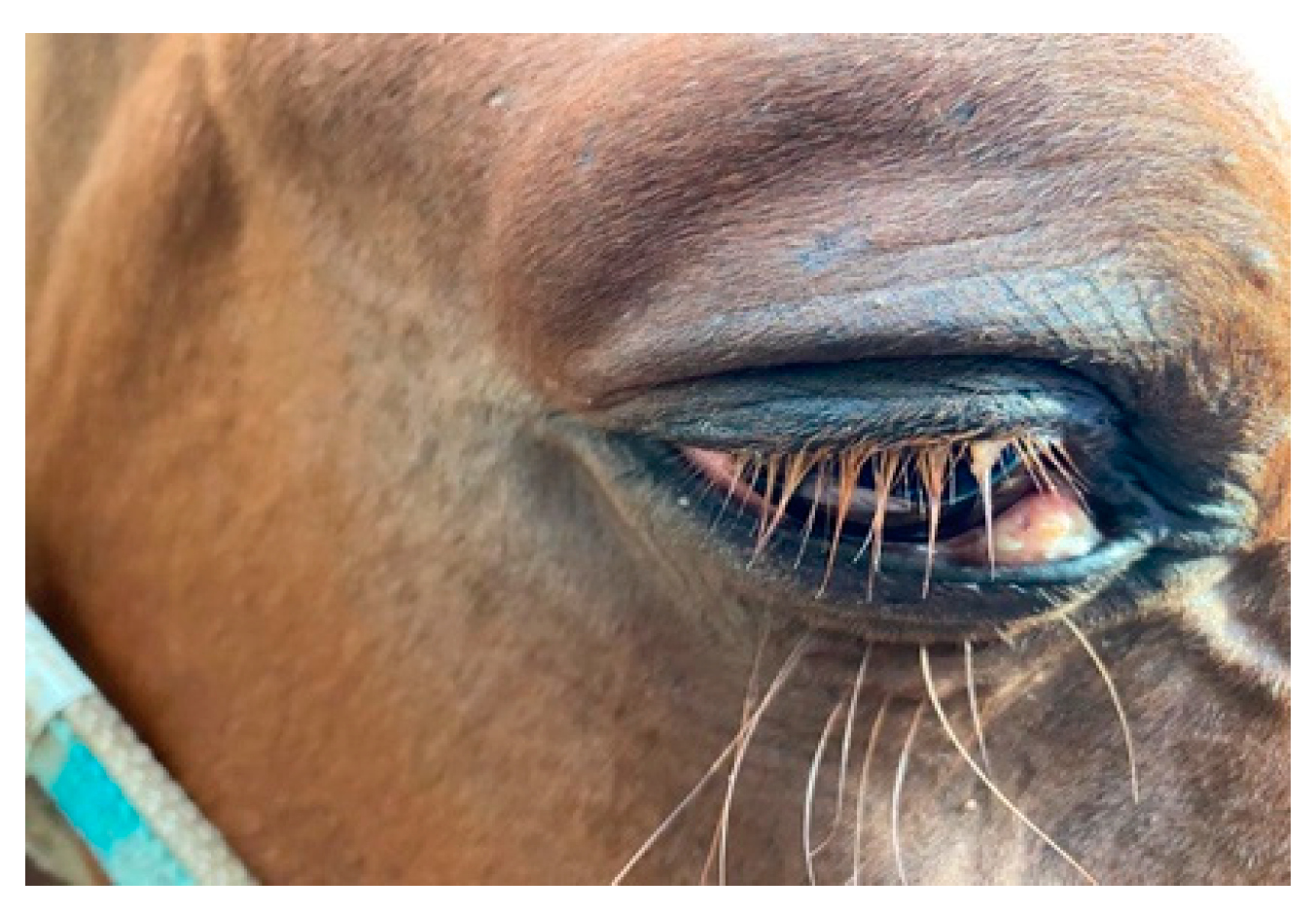
3.1. Ophthalmological Finding
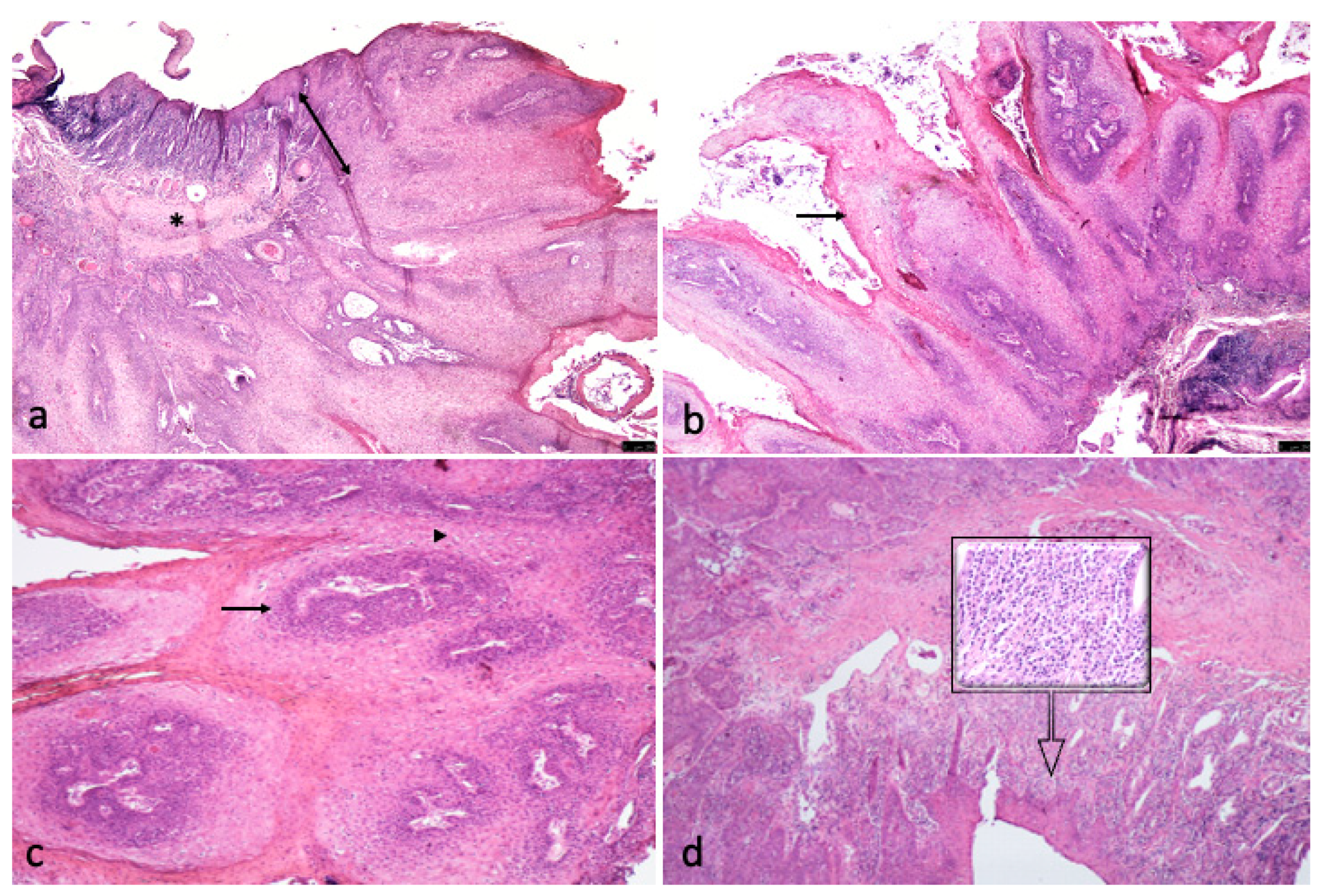
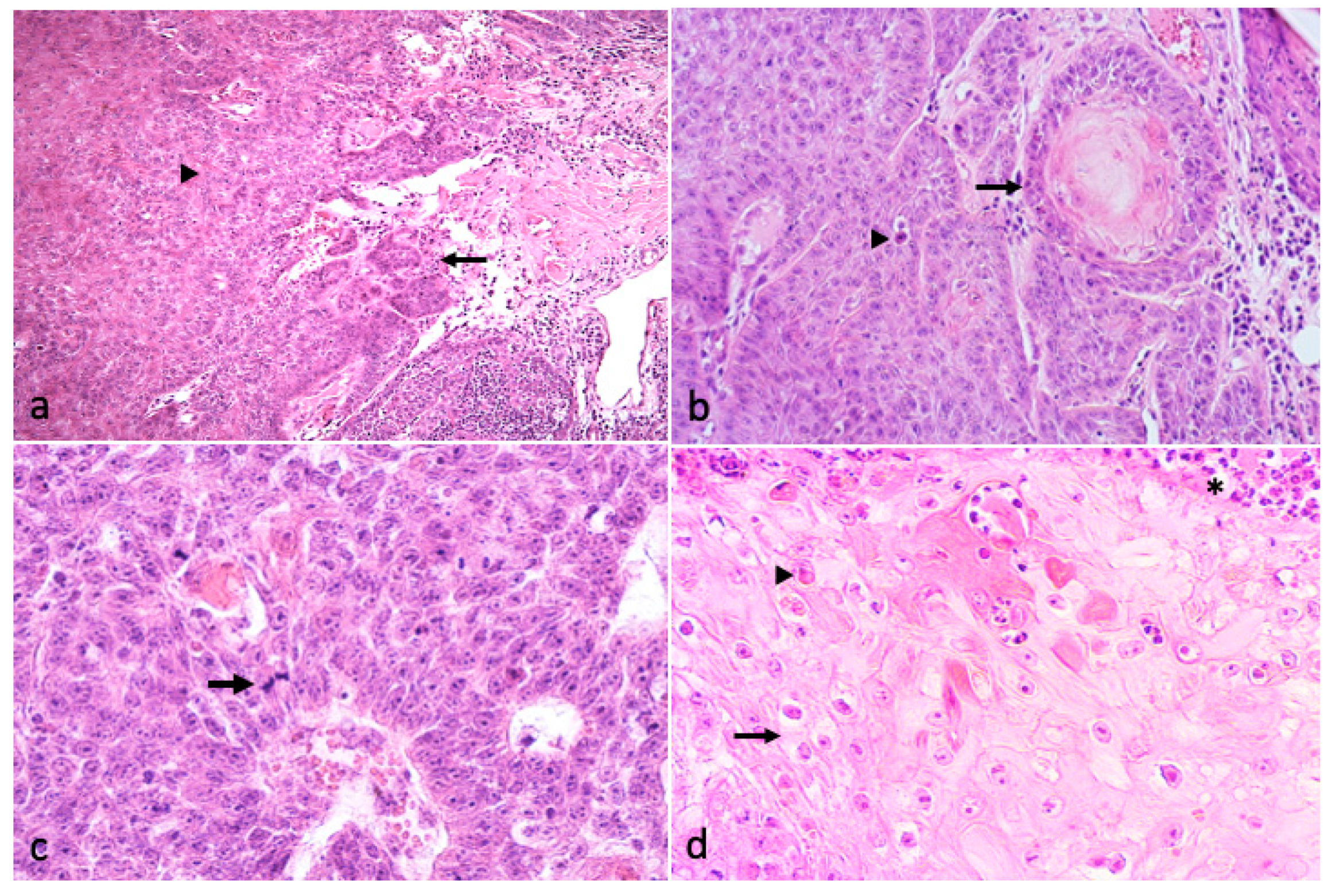
3.2. Histological Finding
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cook, R.H.; Olson, C. Experimental transmission of cutaneous papilloma of the horse. Am. J. Pathol. 1951, 27, 1087–1097. [Google Scholar] [PubMed]
- Theilen, G.H.; Madewell, B.R. (Eds.) Papillomatosis and fibromatosis. In Veterinary Cancer Medicine; Lea &Febinger: Philadelphia, PA, USA, 1987. [Google Scholar]
- Dugan, S.J. Ocular neoplasia. Vet. Clin. N. Am. Equine Pract. 1992, 8, 609–626. [Google Scholar] [CrossRef]
- Rebhun, W.C. Diseases of the Ocular System, 4th ed.; American Veterinary Publications: Goleta, CA, USA, 1991; p. 1083. [Google Scholar]
- Goldschmidt, M.H.; Munday, J.S.; Scruggs, J.L.; Klopfleisch, R.; Kiupel, M. Volume 1: Epithelial Tumors of the Skin. In Surgical Pathology of Tumors of Domestic Animals; Matti Kiupel, Ed.; C.L. Davis-Thompson DVM Foundation: Gurnee, IL, USA, 2019. [Google Scholar]
- Campbell, K.L.; Sundberg, J.P.; Goldschmidt, M.H.; Knupp, C.; Reichmann, M.E. Cutaneous inverted papillomas in dogs. Vet. Pathol. 1988, 25, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lu, H.; Zhang, H.; Sun, X.; Hu, L.; Wang, D. Squamous cell carcinoma associated with inverted papilloma: Recurrence and prognostic factors. Oncol. Lett. 2020, 19, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Buergelt, C.D.; Del Piero, F. Color Atlas of Equine Pathology; John Wiley & Sons: Ames, IA, USA, 2013; p. 538. [Google Scholar]
- Meuten, D.J. Tumors in Domestic Animals; John Wiley & Sons: Ames, IA, USA, 2017. [Google Scholar]
- Krouse, J.H. Endoscopic treatment of inverted papilloma: Safety and efficacy. Am. J. Otolaryngol. 2001, 22, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Melroy, C.T.; Senior, B.A. Benign sinonasal neoplasms: A focus on inverting papilloma. Otolaryngol. Clin. N. Am. 2006, 39, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Lawson, W.; Le Benger, J.; Som, P.; Bernard, P.J.; Biller, H.F. Inverted papilloma: An analysis of 87 cases. Laryngoscope 1989, 99, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Lawson, W.; Ho, B.T.; Shaari, C.M.; Biller, H.F. Inverted papilloma: A report of 112 cases. Laryngoscope 1995, 105, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.E.; Tobler, K.; Brandes, K.; Breithardt, K.; Ordeix, L.; Von Bomhard, W.; Favrot, C. Canine inverted papillomas associated with DNA of four different papillomaviruses. Vet. Dermatol. 2010, 21, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Shinya, K.; Awakura, T.; Narama, I.; Maeda, H.; Umemura, T. Cutaneous papillomatosis associated with papillomavirus infection in a dog. J. Comp. Pathol. 1993, 108, 103–107. [Google Scholar] [CrossRef]
- Thaiwong, T.; Sledge, D.G.; Wise, A.G.; Olstad, K.; Maes, R.K.; Kiupel, M. Malignant transformation of canine oral papillomavirus (CPV1)-associated papillomas in dogs: An emerging concern? Papillomavirus Res. 2018, 6, 83–89. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biondi, V.; Passantino, A.; Pugliese, M.; Monti, S.; Sfacteria, A.; Di Pietro, S. Conjunctival Inverted Papilloma Progressing to Carcinoma. First Report in Horse. Vet. Sci. 2021, 8, 108. https://doi.org/10.3390/vetsci8060108
Biondi V, Passantino A, Pugliese M, Monti S, Sfacteria A, Di Pietro S. Conjunctival Inverted Papilloma Progressing to Carcinoma. First Report in Horse. Veterinary Sciences. 2021; 8(6):108. https://doi.org/10.3390/vetsci8060108
Chicago/Turabian StyleBiondi, Vito, Annamaria Passantino, Michela Pugliese, Salvatore Monti, Alessandra Sfacteria, and Simona Di Pietro. 2021. "Conjunctival Inverted Papilloma Progressing to Carcinoma. First Report in Horse" Veterinary Sciences 8, no. 6: 108. https://doi.org/10.3390/vetsci8060108
APA StyleBiondi, V., Passantino, A., Pugliese, M., Monti, S., Sfacteria, A., & Di Pietro, S. (2021). Conjunctival Inverted Papilloma Progressing to Carcinoma. First Report in Horse. Veterinary Sciences, 8(6), 108. https://doi.org/10.3390/vetsci8060108








