1. Introduction
Nosema ceranae and
N. apis are obligate microsporidian parasites of the honey bee ventriculus, causing chronic infections [
1]. The life cycle of
N. ceranae and
N. apis are similar with spores being ingested by young bees and then germinating in the ventriculus, penetrating epithelial cells, reproducing and rupturing host cells, and reentering the gut in as little as six days [
2].
N. ceranae has replaced
N. apis as the most prevalent microsporidian in honey bee colonies in Asia, Europe, and the Americas [
3,
4,
5,
6] and infection levels by
N. ceranae can be significantly higher than those of
N. apis [
7]. The impact of
N. ceranae on colonies can be severe due to bee depopulation, reduced brood rearing, slow spring development, reduced honey stores, and higher winter mortality [
8,
9,
10,
11,
12].
For over 60 years, fumagillin has been the only registered antibiotic for
Nosema spp. infections of honey bees in North America [
13]. While effective, fumagillin poses concerns for food safety due to the contamination of hive products, such as honey and wax [
14,
15]. Additionally, the commercial formulation of fumagillin sold in North America was recently discontinued. One alternative to control
Nosema spp. infections of honey bees could be feeding bees prebiotics and probiotics.
Prebiotics are non-digestible food ingredients that benefit animals by selectively stimulating the growth and activity of certain microbes in the digestive tract while sometimes inhibiting others [
16]. For example, chitosan, a deacetylated form of chitin, is a prebiotic that can be directly antimicrobial, such as inhibition of the growth of
Serratia marcescens, a bacterial pathogen of
Bombyx mori, both in vitro and in vivo [
17], and can also stimulate the honey bee immune system [
18]. Administration of chitosan to honey bees resulted in a significant decrease in the number of
N. ceranae spores and an increase in survivorship of the infected bees [
19]. Many components of essential oils are prebiotics, such as eugenol from clove oil, that thickened the inner mucus layer of mice, increasing resistance to the enteric pathogen
Citrobacter rodentium [
20]. Flavones can also be prebiotics, such as naringenin from citrus fruits, that has anti-inflammatory properties increasing the abundance and activity of antioxidants, scavenging ROS and other free radicals, and decreasing the levels and activity of pro-inflammatory cytokines in mice [
21]. Feeding it to caged honey bees in the laboratory resulted in a 64% reduction in
N. ceranae spores with bees fed naringenin living as long as non-infected control bees and significantly longer than infected bees [
22]. Other prebiotics have also shown promise for the control of
N. ceranae infections in honey bees in laboratory studies. For example, Nanetti et al. [
23] found that administration of defatted seed meals from
Brassica nigra in the diet of honey bees inhibits
N. ceranae replication and increases the lifespan of the insects. Part of the antimicrobial activity of these seed meals seems to be associated to the production of isothiocyanates in the gut of the bees. However, the previously mentioned prebiotics have not been tested in colonies in field studies.
Probiotics are living microbial food supplements that benefit animals by altering the balance of intestinal microorganisms [
24]. One example is
Enterococcus faecium, which is found in the intestines of a range of animals, including honey bees, although it is not one of their core gut bacteria [
25]. Feeding
E. faecium, for example, affected the intestinal microbial flora and the immune response of mice [
26].
E. faecium also produces lactic acid in the midgut of the honey bee [
25], which causes thickening of the peritrophic membrane in the ventriculus epithelium [
27]. As
Nosema must go through this membrane to infect epithelial cells, this may be a mode of defense against
Nosema infection [
28]. Moreover, addition of
E. faecium to pollen substitutes increased digestibility by honey bees and reduced their mortality [
29]. There are many commercially formulated products containing
E. faecium, such as Protexin
®, that contains a single strain of
E. faecium marketed for improving the microbiota and gut health of farm animals. However, it also appears to function in honey bees as Borges et al. [
30] found that treating honey bees with Protexin
® decreased
Nosema spore numbers by >58% and significantly increased survival relative to both
N. ceranae-inoculated and non-inoculated bees. However, this probiotic has not been tested in hives in field studies.
The objective of this study is to determine the effect of the prebiotics, eugenol, chitosan and naringenin, and the commercial probiotic Protexin® (E. faecium) on N. ceranae infections, bee populations, honey production, and overwintering survival of honey bee colonies. The hypothesis is that feeding pre/probiotics to honey bees infected with N. ceranae would result in a significant reduction in N. ceranae infection and increased colony populations, honey yields, and over-wintering survival.
4. Discussion
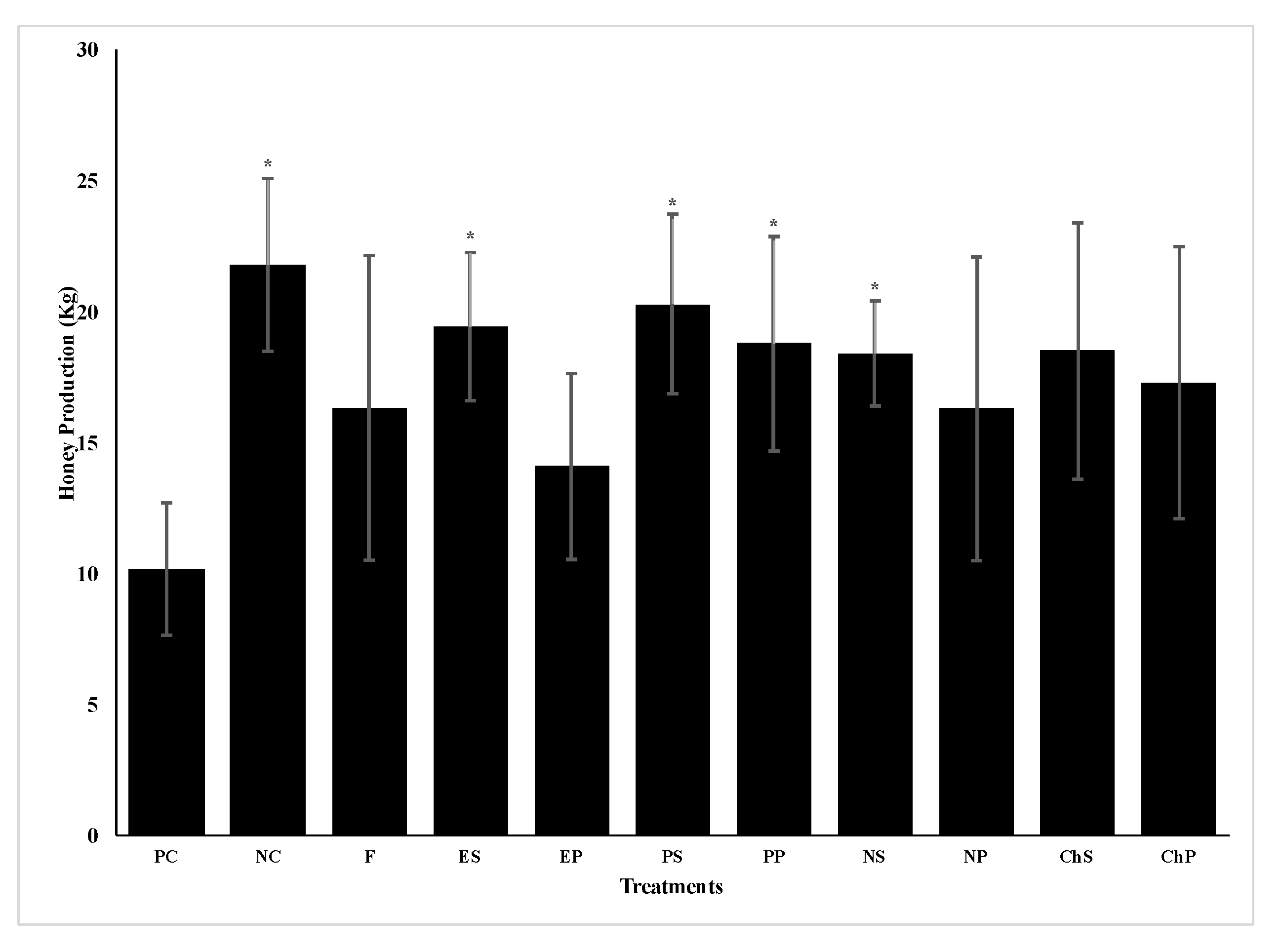
Analyses of the positive and negative control colonies in spring 2017 showed that while the negative control colonies started the experiment without infection by
N. ceranae, they were rapidly infected when placed in an apiary next to infected colonies. This was expected as Higes et al. [
34] also found that
N. ceranae negative colonies became rapidly infected after being placed adjacent to infected hives due to bee drift or spore movement by the wind [
38]. Thus, negative control colonies contained spores at the time of the fall 2017 treatments as well as in the following spring. However, despite becoming infected, colonies of the negative control produced twice as much honey as colonies of the positive control that had high
N. ceranae infection levels at the beginning of the study. Other studies of field colonies infected with different levels of
N. ceranae infection also found significant reductions in honey yield in highly infected colonies [
8,
12].
Nosema ceranae infection can reduce honey yields because infected bees have impaired nutrient absorption and the pathogen uses large amounts of energy from their hosts, resulting in the bees having significantly less energy for activities such as foraging behavior [
39]. Infection may also result in smaller colonies [
12,
19,
39]. While some studies have shown that
N. ceranae infections reduce the lifespan of bees [
12,
19], other studies have reported no significant reduction in lifespan due to infection [
40,
41]. This study also indicates no reduction in lifespan as colony populations were comparable based on a lack of significant differences in the number of frames with brood or adult bees between negative and positive control colonies in late spring 2017. As honey production is a highly variable trait (CV >65%) [
42] affected by many environmental factors, an experiment with a much larger sample size is necessary to confirm the effect of
N. ceranae infections on colony honey yields. However, due to the limited number of colonies with the greater replicates in 2018, no negative control colonies were included in the second year of this study.
Fumagillin was included in this study as a standard control treatment for the 2017 experiment. However, this treatment was not associated with a significant decrease in
N. ceranae spores after the spring 2017 treatment. This suggests that fumagillin was ineffective in bees with an established infection, which shows that the antibiotic on which North American beekeepers once relied can be ineffective when applied as per the label for spring treatment. A study by Huang et al. [
13] showed that
N. ceranae infections were not affected by treating honey bees with fumagillin, which agrees with our results. Spring fumagillin treatment also did not increase the number of frames with brood or adult bees, nor honey yield compared to the positive control. In contrast, fall fumagillin treatment significantly decreased the number of
N. ceranae spores per bee three weeks later, and a large but non-significant decrease the following spring, suggesting there may be a factor of seasonality in the efficacy of this antibiotic. Considering its variable effectiveness in this study as well as the health risks of fumagillin, such as antibiotic resistance and contamination of honey and wax [
15], it is clear that alternatives to this antibiotic are needed than can match or exceed its effectiveness.
Compared to the pre-treatment levels, spring 2017 eugenol P treatment was associated with a reduction of spores per bee by >89% and fall 2017 eugenol S treatment reduced spores per bee by >45% three weeks later (although the spore numbers were slightly higher by the following spring), thus demonstrating its pathogen control effectiveness in this study. Eugenol in sugar syrup also decreased
N. ceranae spore numbers in caged bees [
30]. Eugenol may have reduced
N. ceranae spore numbers in bees by interfering with enzymes involved in fungal spore germination, such as it does against
Aspergillus species [
43], or it may have reduced the chance of
N. ceranae infection by thickening the inner layer of the intestinal tract, such as it does in mice increasing resistance to the enteric pathogen
Citrobacter rodentium [
20]. As
N. ceranae spores must penetrate this layer membrane to infect epithelial cells, thickening it might explain part of the results seen in this study. Further investigation could reveal if eugenol has similar effects on
N. ceranae spore germination or the thickness of the midgut lining of the honey bee. Despite reduced spore numbers per bee, none of the spring or fall eugenol treatments significantly affected brood or adult bee populations. However, spring eugenol S treatment significantly increased honey production compared to the positive control. Similar to the difference between the positive and negative control colonies, honey production may have increased as reduced infection in eugenol treated bees may have allowed for greater foraging.
Among the treatments in this study, Protexin
® P was most effective in the spring 2017 treatment in reducing
N. ceranae spore numbers, whereas Protexin
® S was the most effective for fall 2017 treatment three weeks later, as well as resulting in one of the lowest spore numbers by the following spring among the treatments. Thus, Protexin
® was also tested for a second year. Protexin
® P spring treatment was also effective against
N. ceranae in 2018, although the Protexin
® S fall treatment was only effective in reducing spore numbers the following spring and not the fall. Thus, Protexin
® was more consistent in its ability to control
N. ceranae infection in the field than fumagillin, the only registered product for control. Similarly, including it in the sugar syrup of caged honey bees reduced
N. ceranae infection [
30,
44]. This could be related to the ability of
E. faecium to produce antimicrobial bacteriocin-like compounds [
25] that might aid in the prevention of
N. ceranae infection in bees.
E. faecium also produces lactic acid in the gut of honey bees [
25], which has been shown to reduce
N. ceranae infection when produced by other microorganisms in the digestive tube of honey bees [
45,
46]. Additionally, lactic acid can cause thickening of the peritrophic membrane in the gut epithelium of honey bees [
47]. A thicker membrane could reduce the chances of
N. ceranae spores infecting epithelial cells of honey bees. However, it may have other benefits to the bee. Feeding caged honey bees
E. faecium resulted in increased life span of bees [
30]. This may help explain why, in both years of this study, Protexin
® P spring treatment increased the number of adult bees in colonies, and Protexin
® S fall 2018 treatment increased adult bee populations that survived over winter compared to the positive control. However, Protexin
® P did not increase the number of brood frames, although Protexin
® S fall treatment did for overwintering colonies in both years of the study, but only significantly in the second year. This implies that Protexin
® is likely extending the life span of the bees, rather than allowing the colony to rear more bees, except in the overwintered bees. This result is consistent with the increased life span of bees treated with Protexin
® reported by Borges et al. [
30]. Regardless of the impact of
N. ceranae infection on bee life span, Protexin
® not only reduced
N. ceranae proliferation, but increased bee survivorship compared to infected and non-infected bees [
30], which appeared to occur in this study. Additionally, colonies treated with Protexin
® S and Protexin
® P produced significantly more honey than positive control colonies in 2017, with production similar to that of a healthy colony. While this could be due to the increased adult bee population, Protexin
® treatment did not increase honey production in 2018, even though it also increased adult bee populations. Honey yields can be quite variable making it difficult to find treatment effects. For example, Fanciotti et al. [
48] found that colonies of honey bees fed
Lactobacillus salivarius yielded significantly more honey than the control in one year, but not in a subsequent year. Larger numbers of colonies over many years of study will be required to confirm the effect of probiotics on honey yields. Overall, Protexin
® treatment of honey bee colonies in this study was consistently associated with the most positive effects on bees, making it the most successful compound at minimizing the damaging effects of
N. ceranae.Naringenin S spring 2017 treatment significantly reduced
N. ceranae infection, whereas naringenin S fall 2017 treatment had a nearly significant reduction in infection in the following spring. Thus, it was tested again in the subsequent year using more colonies, showing a significant effect with both naringenin P spring 2018 treatment at three weeks later and naringenin S fall 2018 treatment in the following spring. Naringenin treatment in sugar syrup similarly reduced
N. ceranae spore numbers in caged honey bees [
22]. Reduced multiplication of
N. ceranae with naringenin could be due to its anti-inflammatory activity that increases the abundance of antioxidants and decreases the levels of pro-inflammatory cytokines, thus increasing host resistance [
21]. Although spring treatment with naringenin was not associated with larger adult or brood bee populations in 2017, it was significantly related with more adult bees in the larger-scale experiment in 2018. Honey production changes with naringenin treatment group were significantly higher in 2017 but not 2018, which could reflect the high variability in this parameter as mentioned previously with Protexin
®.
Chitosan was never associated with a significant reduction in spore numbers in this study, although chitosan P fall 2017 treatment resulted in a large reduction in spore numbers the following spring. Chitosan can act as a pathogen-associated molecular pattern, binding to pattern recognition receptors to trigger an immune response against pathogens [
49]. The ineffectiveness of chitosan in this study was surprising as studies had found that feeding chitosan in sugar syrup reduced
N. ceranae infections in caged honey bees [
19,
22] and also decreased infections of
N. apis in a laboratory study [
18]. Chitosan S spring 2017 treatment did not increase the amount of adult bees and actually reduced the amount of brood, while chitosan fall 2017 treatment gave highly variable results. Borges et al. [
22] did not find higher adult bee survival with chitosan treatment, whereas both Saltykova et al. [
18] and Valizadeh et al. [
19] reported higher survival with chitosan. Perhaps the positive effects observed in studies in the laboratory are not reflective of what would occur in the field. A possible explanation for the negative effect of chitosan on brood number is that it may have initiated an immune response causing the bees to use excess energy and thus decrease temporarily the energy for rearing brood.
In this study, all the pre/probiotics were administered to honey bee colonies by either incorporating them into sucrose syrup or a protein patty in 2017. This was done to determine if that could impact their effectiveness. The pattern seen across most treatments was that compounds administered in the protein patty worked better in the spring, whereas the sugar syrup worked better in the fall. Thus, only selected compounds in protein patty in the spring and sugar syrup in the fall were tested in 2018. The benefit of patties in spring could be related to the rapid consumption of the treated syrup compared to the patty, thus limiting the period of treatment. Corby-Harris et al. [
50] found that microbes reproduced well and were efficiently distributed within a hive when administered in pollen patties during spring. The benefit of syrup in fall could be related to the slow patty consumption by bees in the fall, with some colonies never finishing their patties prior to overwintering, whereas the syrup was consistently completely consumed prior to overwintering providing full treatment. Therefore, the most effective administration method may be due to feeding compounds in a form that the colony will completely but not too rapidly consume based on the time of year (patties in the spring and syrup in the fall).