Effect of Sulfur and Urea Fortification of Fresh Cassava Root in Fermented Total Mixed Ration on the Improvement Milk Quality of Tropical Lactating Cows
Abstract
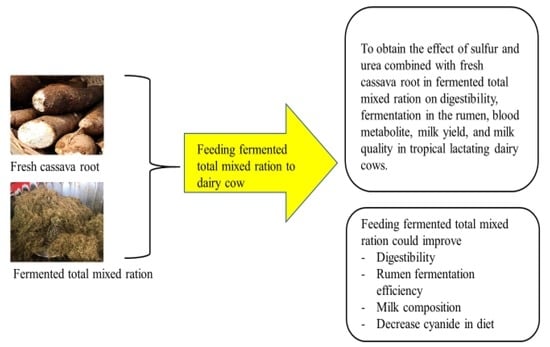
1. Introduction
2. Materials and Methods
2.1. Animal and Facility Details
2.2. Cows, Feed and Treatments
2.3. Sample Collection and Chemical Analysis
2.4. Calculations and Statistical Analysis
3. Results and Discussion
3.1. Chemical Contents in the Diets
3.2. Nutrient Intake and Digestibility
3.3. Rumen Characteristics and Blood Profiles
3.4. Nitrogen Balance and Purine Derivatives
3.5. Volatile Fatty Acid (VFA) Concentration in the Rumen
3.6. Milk Production, Composition, Somatic Cells and Thiocyanate Concentration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bach, A.; Cabrera, V. Robotic milking: Feeding strategies and economic returns. J. Dairy Sci. 2017, 100, 7720–7728. [Google Scholar] [CrossRef] [PubMed]
- Cherdthong, A.; Khonkhaeng, B.; Seankamsorn, A.; Supapong, C.; Wanapat, M.; Gunun, N.; Gunun, P.; Chanjula, P.; Polyorach, S. Effects of feeding fresh cassava root with high-sulfur feed block on feed utilization, rumen fermentation and blood metabolites in Thai native cattle. Trop. Anim. Health Prod. 2018, 50, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Cipollone, R.; Ascenzi, P.; Frangipani, E.; Visca, P. Cyanide detoxification by recombinant bacterial rhodanese. Chemosphere 2006, 63, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Larson, B. Precaution When Utilizing Sorghum/Sudan Crops as Cattle Feed; College of Agriculture Food and Natural Resources, University of Missouri: Columbia, MO, USA, 2006. [Google Scholar]
- Silva, C.J.D.; Leonel, F.D.P.; Pereira, J.C.M.; Costa, G.; Moreira, L.M.; Oliveira, T.S.D.; Abreu, C.L.D. Sulfur sources in protein supplements for ruminants. Rev. Bras. Zootec. 2014, 43, 537–543. [Google Scholar] [CrossRef]
- Van Soest, P. Minerals. In Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994; pp. 122–139. [Google Scholar]
- Cherdthong, A.; Wanapat, M.; Wachirapakorn, C. Influence of urea calcium mixture supplementation on ruminal fermentation characteristics of beef cattle fed on concentrates containing high levels of cassava chips and rice straw. Anim. Feed Sci. Technol. 2011, 163, 43–51. [Google Scholar] [CrossRef]
- Tahboub, Y.R.; Galijasevic, S.; Diamond, M.P.; Abu-Soud, H.M. Thiocyanate modulates the catalytic activity of mammalian peroxidases. J. Biol. Chem. 2005, 28, 26129–26136. [Google Scholar] [CrossRef]
- Minervini, F.; Algaron, F.; Rizzello, C.G.; Fox, P.F.; Monnet, V.; Gobbetti, M. Angiotensin I-converting-enzyme-inhibitory and antibacterial peptides from Lactobacillus helveticus PR4 proteinase-hydrolyzed caseins of milk from six species. Appl. Environ. Microbiol. 2003, 69, 5297–5305. [Google Scholar] [CrossRef]
- Bafort, F.; Parisi, O.; Perraudin, J.P.; Jijakli, M.H. Mode of action of lactoperoxidase as related to its antimicrobial activity: A review. Enzym. Res. 2014, 2014, 517164. [Google Scholar] [CrossRef]
- Soto-Blanco, B.; Górniak, S.L. Milk transfer of cyanide and thiocyanate: Cyanide exposure by lactation in goats. Vet. Res. 2003, 34, 213–220. [Google Scholar] [CrossRef]
- Supapong, C.; Cherdthong, A.; Wanapat, M.; Chanjula, P.; Uriyapongson, S. Effects of sulfur levels in fermented total mixed ration containing fresh cassava root on feed utilization, rumen characteristics, microbial protein synthesis, and blood metabolites in Thai native beef cattle. Animals 2019, 9, 261. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Van Keulen, J.; Young, B.A. Evaluation of acid insoluble ash as a neutral marker in ruminant digestibility studies. J. Anim. Sci. 1977, 44, 282–287. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Bradbury, J.H.; Egan, S.M.; Lynch, M.J. Analysis of cyanide in cassava using acid hydrolysis of cyanogenic glucosides. J. Sci. Food Agric. 1991, 55, 277–290. [Google Scholar] [CrossRef]
- Chen, X.B.; Gomes, M.J. Estimation of Microbial Protein Supply to Sheep and Cattle Based on Urinary Excretion of Purine Derivatives—An Overview of the Technical Details; Occasional Publication 1992; International Feed Resources Unit, Rowel Research Institute: Aberdeen, UK, 1995. [Google Scholar]
- Agricultural Research Council (ARC). The Nutrient Requirements of Ruminant Livestock; Commonwealth Agricultural Bureaux: Farnham Royal, UK, 1991. [Google Scholar]
- Robinson, P.H.; Givens, I.D.; Getachew, G. Evaluation of NRC, UC Davis and ADAS approaches to estimate the metabolizable energy values of feeds atmaintenance energy intake from equations utilizing chemical assays and in vitro determinations. Anim. Feed Sci. Technol. 2004, 114, 75–90. [Google Scholar] [CrossRef]
- Jacob, B.M.; Antony, K.E.; Sreekumar, B.; Haridas, M. Thiocyanate mediated antifungal and antibacterial property of goat milk lactoperoxidase. Life Sci. 2000, 66, 2433–2439. [Google Scholar] [CrossRef]
- Crocker, C.L. Rapid determination of urea nitrogen in serum or plasma without deproteinization. Am. J. Med. Technol. 1967, 33, 361–365. [Google Scholar] [PubMed]
- Lambert, J.L.; Ramasamy, J.; Paukstelis, J.F. Stable reagents for the colorimetric determination of cyanide by modified Konig reactions. Anal. Chem. 1975, 47, 916–918. [Google Scholar] [CrossRef]
- Galyean, M. Laboratory Procedure in Animal Nutrition Research; Department of Animal and Life Science, New Mexico State University: Las Cruces, NM, USA, 1989; pp. 107–122. [Google Scholar]
- Samuel, M.; Sagathewan, S.; Thomus, J.; Mathen, G. An HPLC method for estimation of volatile fatty acids of rumen fluid. Indian J. Anim. Sci. 1997, 67, 805–807. [Google Scholar]
- Majak, W.; Cheng, K.J. Cyanogenesis in bovine rumen fluid and pure cultures of rumen bacteria. J. Anim. Sci. 1984, 59, 784–790. [Google Scholar] [CrossRef]
- Kimaryo, V.M.; Massawe, G.A. The use of a starter culture in the fermentation of cassava for the production of “Kivunde”, a traditional Tanzanian food product. Int. J. Food Microbiol. 2000, 56, 179–190. [Google Scholar] [CrossRef]
- Boonnop, K.; Wanapat, M.; Nontaso, N.; Wanapat, S. Enriching nutritive value of cassava root by yeast fermentation. Sci. Agric. 2009, 66, 616–620. [Google Scholar] [CrossRef]
- Gunawan, S.; Widjaja, T.; Zullaikah, S.; Ernawati, L.; Istianah, N.; Aparamarta, H.W.; Prasetyoko, D. Effect of fermenting cassava with Lactobacillus plantarum, Saccharomyces cereviseae, and Rhizopus oryzae on the chemical composition of their flour. Int. Food Res. J. 2015, 22, 1280–1287. [Google Scholar]
- McSweeney, C.S.; Denman, S.E. Effect of sulfur supplements on cellulolytic rumen micro-organisms and microbial protein synthesis in cattle fed a high fibre diet. J. Appl. Microbiol. 2007, 103, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Van Amburgh, M.E.; Tedeschi, L.O. Rumen balance and rates of fiber digestion. In Proceedings of the Cornell Nutrition Conference for Feed Manufacturers, Rochester, NY, USA, 19–21 October 2010; New York State College of Agriculture & Life Sciences, Cornell University: Rochester, NY, USA, 2000; pp. 150–166. [Google Scholar]
- Promkot, C.; Wanapat, M.; Wachirapakorn, C.; Navanukraw, C. Influence of sulfur on fresh cassava foliage and cassava hay incubated in rumen fluid of beef cattle. Asian-Australas. J. Anim. Sci. 2007, 20, 1424–1432. [Google Scholar] [CrossRef]
- Cherdthong, A.; Wanapat, M.; Saenkamsorn, A.; Supapong, C.; Anantasook, N.; Gunun, P. Improving rumen ecology and microbial population by dried rumen digesta in beef cattle. Trop. Anim. Health Prod. 2015, 47, 921–926. [Google Scholar] [CrossRef]
- Nocek, J.E.; Russell, J.B. Protein and energy as on integrated system. Relationship of ruminal protein and carbohydrate availability to microbial synthesis and milk production. J. Dairy Sci. 1988, 71, 2070–2083. [Google Scholar] [CrossRef]
- Khampa, S.; Wanapat, M.; Wachirapakorn, C.; Nontaso, N.; Wattiaux, M. Effects of urea level and sodium DL-malate in concentrate containing high cassava chip on ruminal fermentation efficiency, microbial protein synthesis in lactating dairy cows raised under tropical condition. Asian-Australas. J. Anim. Sci. 2006, 19, 837–844. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Dairy Cattle, 7th ed.; National Academic Press: Washington, DC, USA, 2001. [Google Scholar]
- Uwituze, S.; Parsons, G.L.; Schneider, C.J.; Karges, K.K.; Gibson, M.L.; Hollis, L.C.; Higgins, J.J.; Drouillard, J.S. Evaluation of sulfur content of dried distillers grains with solubles in finishing diets based on steam-flaked corn or dry–rolled corn. J. Anim. Sci. 2011, 89, 2582–2591. [Google Scholar] [CrossRef]
- Supapong, C.; Cherdthong, A. Effect of sulfur concentrations in fermented total mixed rations containing fresh cassava root on rumen fermentation. Anim. Prod. Sci. 2020, 16, 1429–1434. [Google Scholar] [CrossRef]
- Khosravi, M.; Rouzbehan, Y.; Rezaei, M.; Rezaei, J. Total replacement of corn silage with sorghum silage improves milk fatty acid profile and antioxidant capacity of Holstein dairy cows. J. Dairy Sci. 2018, 12, 10953–10961. [Google Scholar] [CrossRef]
- Chanjula, P.; Wanapat, M.; Wachirapakorn, C.; Rowlinson, P. Effect of synchronizing starch sources and protein (NPN) in the rumen on feed intake, rumen microbial fermentation, nutrient utilization and performance of lactating dairy cows. Asian-Australas. J. Anim. Sci. 2004, 17, 1400–1410. [Google Scholar] [CrossRef]
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Gould, D.H. Update on sulfur-related polioencephalomalacia. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 481–496. [Google Scholar] [CrossRef]
- Bal, M.A.; Ozturk, D. Effects of sulfur containing supplements on ruminal fermentation and microbial protein synthesis. J. Anim. Vet. Sci. 2006, 1, 33–36. [Google Scholar]
- Weiss, W.P. A 100-year review: From ascorbic acid to zinc-Mineral and vitamin nutrition of dairy cows. J. Dairy Sci. 2017, 100, 10045–10060. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Iglesias, C.; Calsamiglia, S.; Devant, M. Effect of amount of concentrate offered in automatic milking systems on milking frequency, feeding behavior, and milk production of dairy cattle consuming high amounts of corn silage. J. Dairy Sci. 2007, 90, 5049–5055. [Google Scholar] [CrossRef]
- Knika, M.I.; Zmiev, V.V. Effect of feed sulfur on the composition and technological properties of milk. Dokl. Vses. Akad. Sel’skokhoz. Nauk. 1973, 8, 26–28. [Google Scholar]
- Dageaw, G.; Cherdthong, A.; Wanapat, M.; Seankamsorn, A.; Khonkhaeng, B.; Supapong, C.; Sumadong, P.; Prachumchai, R. A new approach for utilization of fresh cassava root in dairy cow supplemented with feed block containing high sulfur. J. Agric. Res. Ext. 2018, 35, 117–125. [Google Scholar]
- Dageaw, G.; Cherdthong, A.; Wanapat, M.; Chanjula, P. Effect of fresh cassava root proportion and feed block containing high sulfur on in vitro gas production kinetics, hydrocyanic acid concentration and fermentation characteristics. Anim. Prod. Sci. 2020, 60, 659–664. [Google Scholar] [CrossRef]
- Srisaikham, S.; Suksombat, W.; Lounglawan, P. Fresh cassava peel in dairy cattle diet: Effects on milk production, hygienic quality of raw milk and somatic cell counts. J. Sci. Technol. 2018, 40, 977–984. [Google Scholar]
- Isobe, N.; Kubota, H.; Yamasaki, A.; Yoshimura, Y. Lactoperoxidase activity in milk is correlated with somatic cell count in dairy cows. J. Dairy Sci. 2011, 94, 3868–3874. [Google Scholar] [CrossRef]
- Hung, L.V.; Wanapat, M.; Cherdthong, A. Effects of Leucaena leaf pellet on bacterial diversity and microbial protein synthesis in swamp buffalo fed on rice straw. Livest. Sci. 2013, 151, 188–197. [Google Scholar] [CrossRef]
- Wanapat, M.; Boonnop, K.; Promkot, C.; Cherdthong, A. Effects of alternative protein sources on rumen microbes and productivity of dairy cows. Maejo Int. J. Sci. Technol. 2011, 5, 13–23. [Google Scholar]
- Cherdthong, A.; Wanapat, M.; Kongmun, P.; Pilajun, R.; Khejornsart, P. Rumen fermentation, microbial protein synthesis and cellulolytic bacterial population of swamp buffaloes as affected by roughage to concentrate ratio. J. Anim. Vet. Adv. 2010, 9, 1667–1675. [Google Scholar] [CrossRef]
| Item | 1% Sulfur | 2% Sulfur | Fresh Cassava Root | ||
|---|---|---|---|---|---|
| 1.25% Urea | 2.5% Urea | 1.25% Urea | 2.5% Urea | ||
| Ingredients, %DM | |||||
| Rice straw | 40.0 | 40.0 | 40.0 | 40.0 | |
| Fresh cassava root | 40.0 | 40.0 | 40.0 | 40.0 | |
| Soybean meal | 5.0 | 5.0 | 5.0 | 5.0 | |
| Palm kernel meal | 4.7 | 3.5 | 3.7 | 2.5 | |
| Rice bran | 3.0 | 3.0 | 3.0 | 3.0 | |
| Urea | 1.3 | 2.5 | 1.3 | 2.5 | |
| Pure sulfur | 1.0 | 1.0 | 2.0 | 2.0 | |
| Mineral premix | 1.0 | 1.0 | 1.0 | 1.0 | |
| Molasses, liquid | 3.0 | 3.0 | 3.0 | 3.0 | |
| Salt | 1.0 | 1.0 | 1.0 | 1.0 | |
| Chemical composition | |||||
| Dry matter, % | 55.2 | 55.6 | 55.4 | 55.3 | 32.0 |
| Organic matter, %DM | 96.3 | 96.6 | 95.6 | 95.8 | 96.3 |
| Ash, %DM | 3.7 | 3.4 | 4.4 | 4.2 | 3.7 |
| Crude protein, %DM | 9.6 | 13.0 | 9.8 | 13.1 | 2.8 |
| Neutral detergent fiber, %DM | 38.1 | 38.1 | 38.0 | 38.1 | 7.8 |
| Acid detergent fiber, %DM | 24.2 | 24.2 | 24.1 | 24.2 | 5.7 |
| pH | 5.09 | 5.38 | 4.91 | 5.15 | |
| Hydrocyanic acid, ppm | 0.76 | 0.70 | 0.76 | 0.74 | 110.00 |
| Item | 1.0% Sulfur | 2.0% Sulfur | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 1.25% Urea | 2.5% Urea | 1.25% Urea | 2.5% Urea | S | U | S*U | ||
| Dry matter intake | ||||||||
| % BW | 2.5 | 2.8 | 2.8 | 3.0 | 0.51 | 0.45 | 0.35 | 0.85 |
| g/kg BW0.75 | 118.9 a | 132.0 b | 129.3 b | 139.1 b | 3.37 | 0.05 | 0.05 | 0.89 |
| Nutrient intake, kg/day | ||||||||
| Organic matter | 12.0 | 13.4 | 12.9 | 13.9 | 1.05 | 0.54 | 0.29 | 0.88 |
| Crude protein | 1.2 a | 1.8 b | 1.3 a | 1.9 b | 0.34 | 0.41 | 0.01 | 1.00 |
| Neutral detergent fiber | 4.8 | 5.3 | 5.1 | 5.6 | 0.66 | 0.50 | 0.30 | 0.96 |
| Acid detergent fiber | 3.0 | 3.4 | 3.3 | 3.5 | 0.53 | 0.47 | 0.29 | 0.90 |
| Estimated energy intake | ||||||||
| DOMI d, kg/day | 8.4 | 9.6 | 9.2 | 10.0 | 0.88 | 0.48 | 0.24 | 0.83 |
| DOMR e, kg/day | 5.5 | 6.2 | 6.0 | 6.5 | 0.71 | 0.45 | 0.23 | 0.81 |
| ME, MJ/day | 32.0 | 36.4 | 34.8 | 38.0 | 2.72 | 0.47 | 0.23 | 0.85 |
| Nutrient digestibility, % | ||||||||
| Dry matter | 61.0 a | 66.3 b | 68.5 b | 69.0 b | 1.07 | 0.01 | 0.03 | 0.06 |
| Organic matter | 70.0 a | 71.5 b | 71.0 b | 71.8 b | 0.33 | 0.15 | 0.02 | 0.37 |
| Crude protein | 61.0 a | 68.3 b | 63.5 a | 69.0 b | 1.40 | 0.42 | 0.01 | 0.66 |
| Neutral detergent fiber | 59.5 | 59.0 | 59.8 | 60.3 | 1.44 | 0.73 | 1.00 | 0.81 |
| Acid detergent fiber | 38.0 | 37.3 | 38.5 | 40.3 | 1.25 | 0.29 | 0.76 | 0.44 |
| Item | 1% Sulfur | 2% Sulfur | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 1.25% Urea | 2.5% Urea | 1.25% Urea | 2.5% Urea | S | U | S*U | ||
| Rumen ecology | ||||||||
| Ruminal pH | ||||||||
| 0 h post feeding | 6.6 | 6.6 | 6.8 | 6.7 | 0.33 | 0.24 | 0.24 | 0.58 |
| 4 h post feeding | 6.4 | 6.4 | 6.4 | 6.2 | 0.30 | 0.44 | 0.44 | 0.30 |
| Ruminal temperature, °C | ||||||||
| 0 h post feeding | 38.9 | 38.9 | 39.2 | 39.4 | 0.56 | 0.21 | 0.72 | 0.60 |
| 4 h post feeding | 39.2 | 39.3 | 39.6 | 39.4 | 0.35 | 0.09 | 0.84 | 0.32 |
| NH3-N concentration, mg/dL | ||||||||
| 0 h post feeding | 8.3 a | 10.0 b | 9.3 a | 11.0 b | 0.54 | 0.11 | 0.01 | 0.93 |
| 4 h post feeding | 16.0 a | 23.0 b | 16.2 a | 22.9 b | 0.50 | 0.81 | 0.01 | 0.53 |
| Ruminal microbes, cell/mL | ||||||||
| Protozoa, ×106 | ||||||||
| 0 h post feeding | 9.5 | 9.5 | 10.0 | 9.0 | 0.45 | 1.00 | 0.43 | 0.33 |
| 4 h post feeding | 12.5 | 12.8 | 13.3 | 12.5 | 0.67 | 0.58 | 0.58 | 0.28 |
| Bacteria, ×109 | ||||||||
| 0 h post feeding | 30.8 | 31.8 | 31.0 | 32.0 | 0.70 | 0.62 | 0.06 | 1.00 |
| 4 h post feeding | 41.3 a | 42.3 a | 42.0 a | 43.8 b | 0.48 | 0.03 | 0.01 | 0.43 |
| Blood metabolites | ||||||||
| Blood urea-N concentration, mg/dL | ||||||||
| 0 h post feeding | 10.3 | 12.0 | 10.8 | 13.0 | 1.38 | 0.70 | 0.32 | 0.90 |
| 4 h post feeding | 11.0 a | 14.0 bc | 12.3 ab | 15.0 c | 1.03 | 0.31 | 0.02 | 0.91 |
| Blood thiocyanate concentration, mg/dL | ||||||||
| 0 h post feeding | 12.6 | 12.7 | 15.0 | 14.6 | 1.21 | 0.17 | 0.91 | 0.87 |
| 4 h post feeding | 12.5 a | 14.0 ab | 15.8 bc | 17.6 c | 0.97 | 0.01 | 0.12 | 0.85 |
| Item | 1.0% Sulfur | 2.0% Sulfur | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 1.25% Urea | 2.5% Urea | 1.25% Urea | 2.5% Urea | S | U | S*U | ||
| N balance, g/day | ||||||||
| N intake | 209.1 a | 230.6 b | 225.3 b | 243.7 b | 4.35 | 0.45 | 0.05 | 0.94 |
| N excretion | ||||||||
| Feces | 82.5 | 77.1 | 78.5 | 80.5 | 3.07 | 0.98 | 0.86 | 0.70 |
| N balance | ||||||||
| Absorption | 126.6 a | 153.4 b | 146.9 b | 163.1 c | 3.46 | 0.24 | 0.05 | 0.67 |
| Purine derivative, mmol/day | ||||||||
| Allantoin, mmol/day | ||||||||
| Excretion | 194.6 a | 218.8 c | 203.8 b | 243.6 c | 1.66 | 0.01 | 0.01 | 0.02 |
| Absorption | 195.8 a | 224.2 c | 206.7 b | 253.4 c | 1.80 | 0.01 | 0.01 | 0.02 |
| Creatinine, mg/dL | 26.9 a | 26.1 ab | 26.1 ab | 25.4 b | 0.48 | 0.01 | 0.01 | 0.83 |
| MCP, g/day | 664.5 a | 747.2 c | 696.2 b | 831.8 c | 3.07 | 0.01 | 0.01 | 0.02 |
| MCP (g/digest OM kg) | 74.1 | 78.7 | 76.8 | 86.1 | 4.86 | 0.20 | 0.13 | 0.34 |
| EMNS (gN/kg OMDR) | 11.1 a | 11.1 a | 13.1 a | 14.9 b | 1.03 | 0.02 | 0.46 | 0.42 |
| Item | 1.0% Sulfur | 2.0% Sulfur | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 1.25% Urea | 2.5% Urea | 1.25% Urea | 2.5% Urea | S | U | S*U | ||
| Total VFA, mM | ||||||||
| 0 h post feeding | 106.0 | 107.5 | 107.1 | 106.9 | 0.65 | 0.58 | 0.17 | 0.06 |
| 4 h post feeding | 108.1 a | 129.4 bc | 126.6 b | 133.3 c | 1.21 | 0.01 | 0.01 | 0.01 |
| VFA profiles, mol/100 mol | ||||||||
| Acetic acid | ||||||||
| 0 h post feeding | 64.8 | 64.0 | 64.8 | 64.2 | 0.69 | 0.78 | 0.16 | 0.86 |
| 4 h post feeding | 65.0 a | 58.9 bc | 60.8 b | 56.2 c | 0.83 | 0.01 | 0.01 | 0.01 |
| Propionic acid | ||||||||
| 0 h post feeding | 24.0 a | 26.1 b | 24.1 a | 26.4 b | 0.65 | 0.71 | 0.05 | 0.84 |
| 4 h post feeding | 25.0 a | 29.6 b | 29.3 b | 29.9 b | 0.58 | 0.01 | 0.01 | 0.01 |
| Butyric acid | ||||||||
| 0 h post feeding | 11.2 a | 9.0 ab | 10.1 ab | 8.5 b | 0.78 | 0.63 | 0.03 | 0.78 |
| 4 h post feeding | 10.0 a | 12.5 bc | 10.9 ab | 14.9 c | 0.85 | 0.04 | 0.01 | 0.32 |
| Item | 1% Sulfur | 2% Sulfur | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 1.25% Urea | 2.5% Urea | 1.25% Urea | 2.5% Urea | S | U | S*U | ||
| Milk production | ||||||||
| Milk yield, kg/day | 12.4 | 12.5 | 12.8 | 13.0 | 0.83 | 0.55 | 0.81 | 0.92 |
| 3.5% FCM, kg/day | 13.0 | 13.4 | 13.6 | 13.9 | 1.01 | 0.61 | 0.75 | 0.96 |
| Milk composition, % | ||||||||
| Fat | 3.55 a | 3.63 ab | 3.66 b | 3.68 b | 0.03 | 0.01 | 0.01 | 0.22 |
| Protein | 3.22 | 3.44 | 3.33 | 3.50 | 0.13 | 0.15 | 0.07 | 0.25 |
| Lactose | 4.29 | 4.34 | 4.36 | 4.41 | 0.11 | 0.55 | 0.66 | 1.00 |
| Solids-not-fat | 8.21 | 8.48 | 8.39 | 8.61 | 0.15 | 0.11 | 0.07 | 0.30 |
| Total solids | 11.76 a | 12.11 a | 12.05 a | 12.29 b | 0.16 | 0.06 | 0.04 | 0.25 |
| Milk thiocyanate, ppm | 5.02 a | 6.02 a | 11.87 b | 10.48 b | 1.42 | 0.02 | 0.92 | 0.56 |
| Somatic cell count, cell/mL | 262,833 a | 287,833 a | 226,500 b | 223,541 b | 146.95 | 0.04 | 0.62 | 0.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supapong, C.; Cherdthong, A. Effect of Sulfur and Urea Fortification of Fresh Cassava Root in Fermented Total Mixed Ration on the Improvement Milk Quality of Tropical Lactating Cows. Vet. Sci. 2020, 7, 98. https://doi.org/10.3390/vetsci7030098
Supapong C, Cherdthong A. Effect of Sulfur and Urea Fortification of Fresh Cassava Root in Fermented Total Mixed Ration on the Improvement Milk Quality of Tropical Lactating Cows. Veterinary Sciences. 2020; 7(3):98. https://doi.org/10.3390/vetsci7030098
Chicago/Turabian StyleSupapong, Chanadol, and Anusorn Cherdthong. 2020. "Effect of Sulfur and Urea Fortification of Fresh Cassava Root in Fermented Total Mixed Ration on the Improvement Milk Quality of Tropical Lactating Cows" Veterinary Sciences 7, no. 3: 98. https://doi.org/10.3390/vetsci7030098
APA StyleSupapong, C., & Cherdthong, A. (2020). Effect of Sulfur and Urea Fortification of Fresh Cassava Root in Fermented Total Mixed Ration on the Improvement Milk Quality of Tropical Lactating Cows. Veterinary Sciences, 7(3), 98. https://doi.org/10.3390/vetsci7030098