Exercise but Not Supplemental Dietary Tryptophan Influences Heart Rate and Respiratory Rate in Sled Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Diets and Study Design
2.3. Exercise Regimen
2.4. Telemetry Jackets
2.5. Statistical Analysis
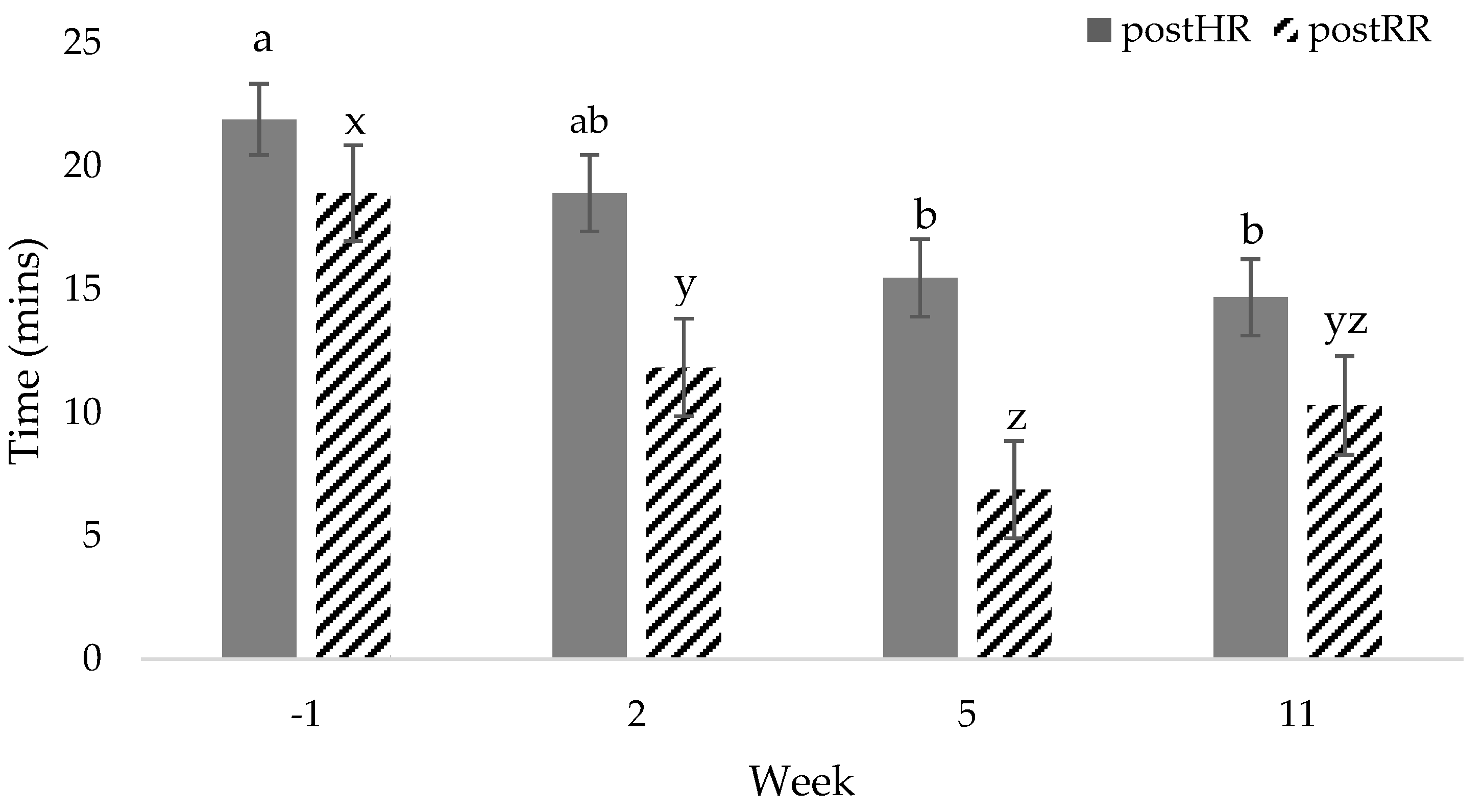
3. Results
3.1. Heart Rate
3.2. Respiratory Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robbins, P.; Ramos, M.; Zanghi, B.; Otto, C.; Robbins, P. Environmental and physiological factors associated with stamina in dogs exercising in high ambient temperatures. Front. Vet. Sci. 2017, 4, 144. [Google Scholar] [CrossRef] [PubMed]
- Orr, N. The feeding of sledge dogs on Antarctic expeditions. Br. J. Nutr. 1966, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. The work output of sledge dogs. J. Physiol. 1957, 137, 210–217. [Google Scholar] [CrossRef]
- Rovira, S.; Munoz, A.; Benito, M. Effect of exercise on physiological, blood and endocrine parameters in search and rescue-trained dogs. Vet. Med. Czech. 2008, 53, 333–346. [Google Scholar] [CrossRef]
- Sonetti, D.; Wetter, T.; Pegelow, D.; Dempsey, J. Effects of respiratory muscle training versus placebo on endurance exercise performance. Respir. Physiol. 2001, 127, 185–199. [Google Scholar] [CrossRef]
- Hall, J.E. Guyton and Hall: Textbook of Medical Physiology, 12th ed.; Saunders Elsevier: Philadelphia, PA, USA, 2011; pp. 1037–1039. [Google Scholar]
- Dunham, C.; Harms, C. Effects of high-intensity interval training on pulmonary function. Eur. J. Appl. Physiol. 2012, 112, 3061–3068. [Google Scholar] [CrossRef]
- Constable, P.; Hinchcliff, K.; Olson, J.; Hamlin, R. Athletic heart syndrome in dogs competing in a long-distance sled race. J. App. Physiol. 1994, 76, 433–438. [Google Scholar] [CrossRef]
- Stepien, R.; Hinchcliff, K.; Constable, P.; Olson, J. Effect of endurance training on cardiac morphology in Alaskan sled dogs. J. Appl. Physiol. 1998, 85, 1368–1375. [Google Scholar] [CrossRef]
- Pluim, B.; Zwinderman, A.; van Der Laarse, A.; van Der Wall, E.; Pluim, B. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation 2000, 101, 336–344. [Google Scholar] [CrossRef]
- Downey, A.; Chenoweth, L.; Townsend, D.; Ranum, J.; Ferguson, C.; Harms, C. Effects of inspiratory muscle training on exercise responses in normoxia and hypoxia. Respir. Physiol. Neurobiol. 2007, 156, 137–146. [Google Scholar] [CrossRef]
- Hodgson, D. Chapter 11-The cardiovascular system: Anatomy, physiology, and adaptations to exercise and training. In The Athletic Horse, 2nd ed.; Hodgson, D., Harrington McKeever, K., McGowan, C., Eds.; Saunders Elsevier: St. Louis, MO, USA, 2014; pp. 162–173. [Google Scholar]
- Sugawara, J.; Murakami, H.; Maeda, S.; Kuno, S.; Matsuda, M. Change in post-exercise vagal reactivation with exercise training and detraining in young men. Eur. J. Appl. Physiol. 2001, 85, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wang, R.; Chen, P.; Huang, S.; Donnelly, J.; Mehlferber, J. Dose–response relationship of cardiorespiratory fitness adaptation to controlled endurance training in sedentary older adults. Eur. J. Prev. Cardiol. 2016, 23, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Davenport, G.; Kelley, E.; Altom, E.; Lepine, A. Effect of diet on hunting performance of English pointers. Vet. Ther. 2001, 2, 10–23. [Google Scholar] [PubMed]
- Kronfeld, D. Diet and the performance of racing sled dogs. J. Am. Vet. Med. Assoc. 1973, 162, 470–473. [Google Scholar] [PubMed]
- Reynolds, A.; Reinhart, D.; Carey, D.; Simmerman, D.; Frank, D.; Kallfelz, F. Effect of protein intake during training on biochemical and performance variables in sled dogs. Am. J. Vet. Res. 1999, 60, 789–795. [Google Scholar] [PubMed]
- Dalton, D. The cardiovascular effects of centrally administered 5-hydroxytryptamine in the conscious normotensive rat. J. Auton. Pharmacol. 1986, 6, 67–75. [Google Scholar] [CrossRef]
- Lalley, P.; Bischoff, A.; Richter, D.; Lalley, P. 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. J. Physiol. 1994, 476, 117–130. [Google Scholar]
- Berger, M.; Gray, J.; Roth, B. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Ruddick, J.; Evans, A.; Nutt, D.; Lightman, S.; Rook, G.; Lowry, C. Tryptophan metabolism in the central nervous system: Medical implications. Expert Rev. Mol. Med. 2006, 8, 1–27. [Google Scholar] [CrossRef]
- O’Mahony, S.; Clarke, G.; Borre, Y.; Dinan, T.; Cryan, J. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Jacobs, L.; Comroe, J.; Jacobs, L. Reflex apnea, bradycardia, and hypotension produced by serotonin and phenyldiguanide acting on the nodose ganglia of the cat. Circ. Res. 1971, 29, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, R.; Shintani, F.; Rowen, H.; Shimomura, K.; Oya, N. Identification of left coronary blood supply of aortic bodies in anesthetized dogs. J. Appl. Physiol. 1971, 30, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, H.; Tomlin, J.; Ward, R. Reflex changes in respiration and heart rate evoked by intravenous and left ventricular injection of 5-HT and capsaicin in anaesthetized rats: A comparison of mechanisms. Lung 1984, 162, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Iovino, L.; Mutolo, D.; Cinelli, E.; Contini, M.; Pantaleo, T.; Bongianni, F. Breathing stimulation mediated by 5-HT1A and 5-HT3 receptors within the preBötzinger complex of the adult rabbit. Brain Res. 2019, 1704, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Templeman, J.R.; Thornton, E.; Cargo-Froom, C.; Squires, E.J.; Swanson, K.S.; Shoveller, A.K. Effects of incremental exercise and dietary tryptophan supplementation on the amino acid metabolism, serotonin status, stool quality, fecal metabolites, and body composition of mid-distance trained sled dogs. J. Anim. Sci. 2020, 98, skaa128. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dogs and Cats, 2nd ed.; National Academic Press: Washington, DC, USA, 2006. [Google Scholar]
- Association of American Feed Control Officials. AAFCO Manual; AAFCO Inc.: West Lafayette, IN, USA, 2016. [Google Scholar]
- Templeman, J.R.; Mansilla, W.D.; Fortener, L.; Shoveller, A.K. Tryptophan requirements in small, medium, and large breed adult dogs using the indicator amino acid oxidation technique. J. Anim. Sci. 2019, 97, 3274–3285. [Google Scholar] [CrossRef]
- Van Citters, R.; Franklin, D. Cardiovascular performance of Alaska sled dogs during exercise. Circ. Res. 1969, 24, 33–42. [Google Scholar] [CrossRef]
- Prior, H.; McMahon, N.; Schofield, J.; Valentin, J. Non-invasive telemetric electrocardiogram assessment in conscious beagle dogs. J. Pharmacol. Tox. Met. 2009, 60, 167–173. [Google Scholar] [CrossRef]
- Zucker, I.; Cornish, K. Reflex cardiovascular and respiratory effects of serotonin in conscious and anesthetized dogs. Circ. Res. 1980, 47, 509–515. [Google Scholar] [CrossRef]
- Chennaoui, M.; Grimaldi, B.; Fillion, M.; Bonnin, A.; Drogou, C.; Fillion, G.; Guezennec, C. Effects of physical training on functional activity of 5-HT1B receptors in rat central nervous system: Role of 5-HT-moduline. N-S Arch. Pharmacol. 2000, 361, 600–604. [Google Scholar] [CrossRef]
- Jakeman, P.; Hawthorn, J.; Maxwell, S.; Kendall, M.; Holder, G. Evidence for downregulation of hypothalamic 5-hydroxytryptamine receptor function in endurance-trained athletes. Exp. Physiol. 1994, 79, 461–464. [Google Scholar] [CrossRef]
- Bender, D. Biochemistry of tryptophan in health and disease. Mol. Aspects Med. 1983, 6, 101–197. [Google Scholar] [CrossRef]
- Tremblay, M.; Copeland, J.; Van Helder, W. Effect of training status and exercise mode on endogenous steroid hormones in men. J. Appl. Physiol. 2004, 96, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Peake, J.; Nosaka, K.; Okutsu, M.; Abbiss, C.; Surriano, R.; Bishop, D.; Quod, M.; Lee, H.; Martin, D.; et al. Changes in markers of muscle damage, inflammation and HSP70 after an Ironman triathlon race. Eur. J. Appl. Physiol. 2006, 98, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Comassi, M.; Vitolo, E.; Pratali, L.; Del Turco, S.; Dellanoce, C.; Rossi, C.; Santini, E.; Solini, A. Acute effects of different degrees of ultra-endurance exercise on systemic inflammatory responses. Intern. Med. J. 2015, 45, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Ordway, G.; Charles, J.; Randall, D.; Billman, G.; Wekstein, D. Heart rate adaptation to exercise training in cardiac-denervated dogs. J. Appl. Physiol. 1982, 52, 1586–1590. [Google Scholar] [CrossRef]
- Billman, G.; Cagnoli, K.; Csepe, T.; Li, N.; Wright, P.; Mohler, P.; Fedorov, V. Exercise training-induced bradycardia: Evidence for enhanced parasympathetic regulation without changes in intrinsic sinoatrial node function. J. Appl. Physiol. 2015, 118, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, H.; Mitchell, J.; Wyatt, H. Influences of physical training on the heart of dogs. Circ. Res. 1974, 35, 883–889. [Google Scholar] [CrossRef]
- Tipton, C.; Carey, R.; Eastin, W.; Erickson, H. A submaximal test for dogs: Evaluation of effects of training, detraining, and cage confinement. J. Appl. Physiol. 1974, 37, 271–275. [Google Scholar] [CrossRef]
- Billman, G.; Kukielka, M. Effect of endurance exercise training on heart rate onset and heart rate recovery responses to submaximal exercise in animals susceptible to ventricular fibrillation. J. Appl. Physiol. 2007, 102, 231–240. [Google Scholar] [CrossRef]
- Boussana, A.; Hue, O.; Matecki, S.; Galy, O.; Ramonatxo, M.; Varray, A.; Le Gallais, D. The effect of cycling followed by running on respiratory muscle performance in elite and competition triathletes. Eur. J. Appl. Physiol. 2002, 87, 441–447. [Google Scholar] [PubMed]
- Di Paco, A.; Dubé, B.; Laveneziana, P. Changes in ventilatory response to exercise in trained athletes: Respiratory physiological benefits beyond cardiovascular performance. Arch. Bronconeumol. 2017, 53, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, C.; Saltin, B. Cardiovascular adaptations to physical training. Annu. Rev. Physiol. 1983, 45, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Amory, H.; Art, T.; Lekeux, P.; Equine Research Funds Asbl. Effects de l’entraînement sur la fonction cardio-respiratoire et sur la température rectale chez le poney; Effects of training and detraining on heart rate, ventilation and thermoregulation during a standardized treadmill exercise in ponies: A preliminary study. J. Anns. Méd. Vét. 1988, 132, 201–216. [Google Scholar]
- Leiner, L.; Fendt, M. Behavioural fear and heart rate responses of horses after exposure to novel objects: Effects of habituation. Appl. Anim. Behav. Sci. 2011, 131, 3–4. [Google Scholar] [CrossRef]
- Saursaunet, V.; Norges Teknisk-Naturvitenskapelige Universitet, F. Effect of Ambient Temperature on Endurance Performance in Cross Country Skiers. Master’s Thesis, Norges teknisk-naturvitenskapelige universitet, Fakultet for samfunnsvitenskap og teknologiledelse; Institutt for bevegelsesvitenskap, Trondheim, Norway, 2010. [Google Scholar]
- Kruk, B.; Kaciuba-Uściłko, H.; Nazar, K.; Greenleaf, J.; Kozłowski, S. Hypothalamic, rectal, and muscle temperatures in exercising dogs: Effect of cooling. J. Appl. Physiol. 1985, 58, 1444–1448. [Google Scholar] [CrossRef]
- Lewis, S.; Foster, R. Effect of Heat on Canines and Felines. Iowa State Univ. Vet. 1976, 38, 117–121. [Google Scholar]
| Activity Parameter 1 | Exercise Challenge | SEM 2 | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| Week −1 | Week 2 | Week 5 | Week 11 | Week | Diet | Week x Diet | ||
| totalHR, bpm 3 | 194 a | 182 b | 179 b,c | 168 c | 3 | <0.01 | 0.10 | 0.57 |
| rHR, bpm | 91 a | 79 b | 71 b | 68 b | 4 | <0.01 | 0.38 | 0.57 |
| wHR, bpm | 270 a | 249 a,b | 261 a,b | 234 b | 9 | <0.01 | 0.10 | 0.17 |
| postHR, bpm | 110 a | 101 a,b | 95 b | 92 b | 4 | <0.01 | 0.55 | 0.85 |
| Activity Parameter 1 | Exercise Challenge | SEM 2 | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| Week −1 | Week 2 | Week 5 | Week 11 | Week | Diet | Week x Diet | ||
| totalHR, % of total | 194 | −10 * | −12 * | −17 * | 2 | <0.01 | 0.10 | 0.57 |
| rHR, % | 91 | −8 | −20 * | −21 * | 4 | <0.01 | 0.30 | 0.81 |
| wHR, % | 270 | −15 * | −5 | −17 * | 3 | <0.01 | 0.18 | 0.06 |
| postHR, % | 110 | −11 | −16 | −19 * | 4 | <0.01 | 0.87 | 0.88 |
| Diet Group 1 | Exercise Challenge | SEM 2 | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| Week −1 | Week 2 | Week 5 | Week 11 | Week | Diet | Week x Diet | ||
| Trt, mins | 19 a | 16 a | 6 b | 12 a,b | 2 | <0.01 | 0.37 | 0.06 |
| Ctl, mins | 18 a | 7 b | 7 b | 8 b | 2 | <0.01 | ||
| Activity Parameter 1 | Exercise Challenge | SEM 2 | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| Week −1 | Week 2 | Week 5 | Week 11 | Week | Diet | Week x Diet | ||
| totalRR, bf 3 | 75 a | 73 a,b | 69 b | 68 b | 1 | <0.01 | 0.36 | 0.81 |
| rRR, bf | 23 a | 19 a,b | 15 c | 16 b,c | 1 | <0.01 | 0.66 | 0.12 |
| wRR, bf | 176 a | 172 a | 174 a | 169 a | 4 | 0.50 | 0.86 | 0.74 |
| postRR, bf | 27 a | 26 a | 18 b | 20 b | 1 | <0.01 | 0.17 | 0.55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thornton, E.; Templeman, J.R.; Bower, M.; Cant, J.P.; Holloway, G.P.; Shoveller, A.K. Exercise but Not Supplemental Dietary Tryptophan Influences Heart Rate and Respiratory Rate in Sled Dogs. Vet. Sci. 2020, 7, 97. https://doi.org/10.3390/vetsci7030097
Thornton E, Templeman JR, Bower M, Cant JP, Holloway GP, Shoveller AK. Exercise but Not Supplemental Dietary Tryptophan Influences Heart Rate and Respiratory Rate in Sled Dogs. Veterinary Sciences. 2020; 7(3):97. https://doi.org/10.3390/vetsci7030097
Chicago/Turabian StyleThornton, Emma, James R. Templeman, Michael Bower, John P. Cant, Graham P. Holloway, and Anna K. Shoveller. 2020. "Exercise but Not Supplemental Dietary Tryptophan Influences Heart Rate and Respiratory Rate in Sled Dogs" Veterinary Sciences 7, no. 3: 97. https://doi.org/10.3390/vetsci7030097
APA StyleThornton, E., Templeman, J. R., Bower, M., Cant, J. P., Holloway, G. P., & Shoveller, A. K. (2020). Exercise but Not Supplemental Dietary Tryptophan Influences Heart Rate and Respiratory Rate in Sled Dogs. Veterinary Sciences, 7(3), 97. https://doi.org/10.3390/vetsci7030097





