Dystocia in a Captive Reared Agouti (Dasyprocta leporina) in Trinidad and Tobago, West Indies
Abstract
1. Introduction
2. Material and Method
2.1. Housing of the Agouti (Dasyprocta leporina)
2.2. Clinical Signs
3. Results
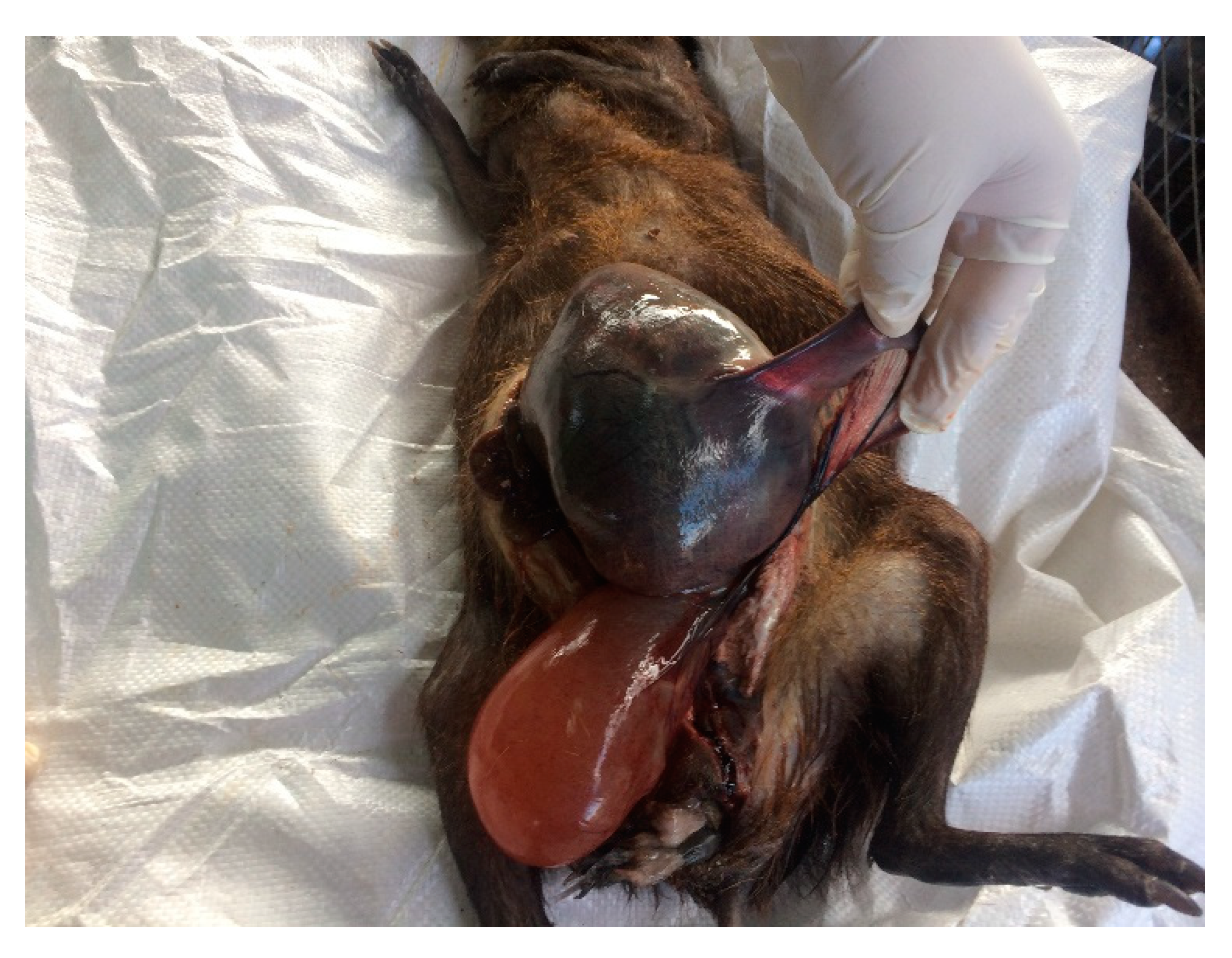
3.1. Post Mortem Examination
3.2. Treatment
3.3. Necropsy Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brown-Uddenberg, R.C.; Garcia, G.W.; Baptiste, Q.S.; Counand, T.; Adogwa, A.O.; Sampson, T. The Agouti (Dasyprocta leporina, D. aguti) Booklet and Producers’ Manual; GWG Publications: Champs Fleurs, Trinidad and Tobago, 2014; Available online: http://ostasp.brinkster.net/ (accessed on 17 September 2017).
- Hardouin, J.; Thys, E.; Joiris, V.; Fielding, D. Mini-livestock breeding with indigenous species in the tropics. Livest. Res. Rural Dev. 2003, 15, 4. Available online: http://www.lrrd.org/lrrd15/4/hard154.htm (accessed on 11 October 2019).
- Mollineau, W.M.; Adogwa, A.O.; Young, K.; Jasper, N.; Garcia, G.W. The Gross anatomy of the male reproductive system of a neo-tropical rodent: The Agouti (Dasyprocta leporina). Anat. Histol. Embryol. 2006, 35, 47–52. [Google Scholar] [CrossRef]
- Mollineau, W.M.; Adogwa, A.O.; Garcia, G.W. The Gross and Micro Anatomy of the Accessory Sex Glands of the Male Agouti (Dasyprocta leporina). Anat. Histol. Y Embryol. 2009, 38, 204–207. [Google Scholar] [CrossRef]
- Mollineau, W.M.; Adogwa, A.O.; Garcia, G.W. A Preliminary technique for electro-ejaculation of agouti (Dasyprocta leporina). Anim. Reprod. Sci. 2008, 108, 92–97. [Google Scholar] [CrossRef]
- Mollineau, W.M.; Adogwa, A.O.; Garcia, G.W. Spermatozoa morphologies and fructose and citric acid concentrations in agouti (Dasyprocta leporina) semen. Anim. Reprod. Sci. 2008, 105, 378–383. [Google Scholar] [CrossRef]
- Mollineau, W.M.; Adogwa, A.O.; Garcia, G.W. Liquid and Frozen storage of Agouti (Dasyprocta leporina) Semen extended with UHT Milk, Unpasteurized Coconut Water and Pasteurized Coconut Water. Vet. Med. Int. 2011, 5, 702635. [Google Scholar] [CrossRef] [PubMed]
- Mollineau, W.M.; Adogwa, A.O.; Garcia, G.W. Improving the efficiency of the preliminary electro-ejaculation technique developed for semen collection from the Agouti (Dasyprocta leporina). J. Zoo Wildl. Med. 2010, 41, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Mollineau, W.M.; Adogwa, A.O.; Garcia, G.W. Anatomical stages of penile erection in the agouti (Dasyprocta leporina) induced by electro-ejaculation. Anat. Histol. Embryol. 2012, 41, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.; Legall, G.; Garcia, G.W. Towards the determination of a “Weaning Age” for the intensive production of the Agouti (Dasyprocta leporina). Livest. Res. Rural Dev. 2018, 30. Available online: http://www.lrrd.org/lrrd30/10/riyad30173.html (accessed on 11 October 2019).
- Garcia, G.W.; Baptiste, Q.S.; Kakuni, M.; Arishima, K.; Makita, T. The Digestive System of the Agouti (Dasyprocta leporina)—Gross Anatomy and Histology. Jpn. J. Zoo Wildl. Med. 2000, 5, 55–66. [Google Scholar] [CrossRef]
- Henry, O. Frugivory and the Importance of Seeds in the Diet of the Orange-Rumped Agouti (Dasyprocta leporina) in French Guiana. J. Trop. Ecol. 1999, 15, 291–300. [Google Scholar] [CrossRef]
- Silvius, K.M.; Fragoso, J.M.V. Red-Rumped Agouti (Dasyprocta leporina) Home Range Use in an Amazonian Forest: Implications for the Aggregated Distribution of Forest Trees. Biotropica 2003, 35, 74–83. [Google Scholar] [CrossRef]
- Silvius, K.M. Spatio-Temporal Patterns of Palm Endocarp Use by Three Amazonian Forest Mammals: Granivory or ‘Grubivory’? J. Trop. Ecol. 2002, 18, 707–723. [Google Scholar] [CrossRef]
- Dookie, B.; Jones, K.R.; Mohammed, R.; Garcia, G.W. Feed particle size preference and feed wastage in Agouti (Dasyprocta leporina) reared intensively in the Republic of Trinidad and Tobago. Livest. Res. Rural Dev. 2018, 30, 1–6. [Google Scholar]
- Lall, K.R.; Jones, K.R.; Garcia, G.W. Nutrition of Six Selected Neo-Tropical Mammals in Trinidad and Tobago with the Potential for Domestication. Vet. Sci. 2018, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Figueira, L.; Zucaratto, R.; Pires, A.S.; Cid, B.; Fernandez, F.A.S. Carrion Consumption by Dasyprocta leporina (Rodentia: Dasyproctidae) and a Review of Meat Use by Agoutis. Braz. J. Biol. 2014, 74, 585–587. [Google Scholar] [CrossRef]
- Jones, K.R.; Lall, K.R.; Garcia, G.W. Omnivorous Behaviour of the Agouti (Dasyprocta leporina): A Neotropical Rodent with the Potential for Domestication. Scientifica 2019, 5, 3759783. [Google Scholar] [CrossRef]
- Lall, K.R.; Jones, K.R.; Garcia, G.W. Infectious Diseases of Six Non-Domesticated Neo-Tropical Animals in Trinidad and Tobago. Int. J. Trop. Vet. Biomed. Res. 2018, 3, 1–31. [Google Scholar] [CrossRef]
- Jones, K.R.; Garcia, G.W. Gastrointestinal parasites of domesticated animals introduced into the Neo-tropics (New World Tropics). Concepts Dairy Vet. Sci. 2018, 1, 56–78. [Google Scholar]
- Jones, K.R.; Garcia, G.W. Endoparasites of domesticated animals that originated in the neo-tropics (new world tropics). Vet. Sci. 2019, 6, 24. [Google Scholar] [CrossRef]
- Jones, K.R.; Garcia, G.W. Endoparasites of selective native non-domesticated mammals in the neo-tropics (new world tropics). Vet. Sci. 2019, 6, 87. [Google Scholar]
- Suepaul, R.; Charles, R.; Dziva, F. Aerobic microflora and endoparasites of freshly shot wild Agouti (Dasyprocta leporina) in Trindad, West Indies. J. Zoo Wildl. Med. 2016, 47, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.R.; Garcia, G.W. A survey of the gastrointestinal parasites present in the Agouti (Dasyprocta leporina) reared intensively in Trinidad. Livest. Res. Rural Dev. 2017, 29, 1–7. [Google Scholar]
- Jones, K.R.; Garcia, G.W. Observations on endoparasitic load in captive reared agoutis (Dasyprocta leporina) without anthelmintic exposure in Trinidad, Republic of Trinidad and Tobago. Livest. Res. Rural Dev. 2018, 30, 1–7. [Google Scholar]
- Griffiths, H.J. Studies on Strongyloides agoutii from the agouti (Dasyptocta agouti). Can. J. Res. D 1940, 18, 173–190. [Google Scholar] [CrossRef]
- Cameron, T.W.M.; Reesal, M.R. Studies on the endoparasitic fauna of Trinidad mammals. Can. J. Zool. 1951, 29, 276–289. [Google Scholar] [CrossRef]
- Lainson, R.; Carneiro, L.; Silveira, F.T. Observations on the Eineria species of the Dasyprocta leporina (Linnaeus, 1758) (Rodentia: Dasyproctidae) for the state of Para, North Brazil. Mem. Inst. De Oswaldo Cruz 2007, 102, 183–189. [Google Scholar] [CrossRef]
- de Thoisy, B.; Michel, J.-C.; Vogel, I.; Vie, J.-C. A survey of hemoparasite infection in free ranging mammals and reptiles in French Guina. J. Parasitol. 2000, 86, 1035–1040. [Google Scholar] [CrossRef]
- Ayala, S.C.; D’ Alessandro, A.; Mackenzie, R.; Angel, D. Hemoparasites in 830 wild animals from Eastern Llanos of Colombia. J. Parasitol. 1973, 59, 52–59. [Google Scholar] [CrossRef]
- Jones, K.R.; Lall, K.R.; Garcia, G.W. Haematological and Serum biochemical values of the agouti (Dasyprocta leporina) reared intensively in Trinidad, Republic of Trinidad and Tobago. Livest. Res. Rural Dev. 2019, 31, 1–8. [Google Scholar]
- Baas, E.J.; Potkay, S.; Bacher, J. The agouti (Dasyprocta sp.) in biomedical research and captivity. Lab. Anim. Sci. 1976, 26, 788–796. [Google Scholar] [PubMed]
- Jones, K.R.; Garcia, G.W. Haematology and Serum Biochemistry in the Agouti (Dasyprocta spp.): A Neo-Tropical Rodent with the Potential for Domestication. Concepts Dairy Vet. Sci. 2019, 3, 48–51. [Google Scholar]
- Jones, K.R.; Garcia, G.W. Understanding of the Blood and Serum values of the Agouti (Dasyprocta spp.): A Rodent of the Neo-Tropics with the potential to be domesticated. Trop. Agric. 2019, in press. [Google Scholar]
- Kenny, D.; Cambre, R.C.; Lewandowski, A.; Pelto, J.A.; Iribeck, N.A.; Wilson, H.; Mierau, G.W.; Sill, M.G.; Garcia, A.P. Suspected vitamin D3 toxicity in pacas (Cuniculus paca) and agoutis (Dasyprocta aguti). J. Zoo Wildl. Med. 1993, 24, 129–139. [Google Scholar]
- Anderson, K.M.; Lewandowski, A.; Dennis, P.M. Suspected hypervitaminosis in red-rumped agouti (Dasyprocta leporina) receiving a commercial rodent diet. J. Zoo Wildl. Med. 2018, 49, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.D.; Garcia, G.W. Perimortality in a Captive Reared Agouti (Dasyprocta leporina). Wildl. Biol. Pract. 2015, 11, 70–74. [Google Scholar]
- Batista, J.S.; Freitas, C.J.A.; Brilhante, F.S.; Viana, G.A.; Olinda, R.G.; Cavalcante, T.V.; de Paiva, K.A.R.; de Oliveira, M.F. Pathological changes of the genital system of agoutis (Dasyprocta aguti Linnaeus, 1758) females bred in captivity. Braz. Vet. J. 2016, 36, 634–641. [Google Scholar]
- Batista, J.S.; Olinda, R.G.; Silva, T.M.F.; Rodriguez, C.M.F.; Oliveira, A.F.; Queiroz, S.A.C.; Morais, S.R.L.; Oliveira, M.F. Diseases of agouti (Dasyprocta aguti) raised in captivity diagnosed by pathological examination. Braz. Vet. J. 2010, 30, 497–502. [Google Scholar]
- Sankar, P.; Mandal, D.; Kumar, V.; Mondal, M. Dystocia in rabbits and its surgical management. Explor. Anim. Med Res. 2017, 7, 216–217. [Google Scholar]
- Dickie, E. Dystocia in a rabbit (Oryctolagus cuniculus). Can. Vet. J. 2011, 52, 80–83. [Google Scholar]
- Narver, H.L. Oxytocin in the treatment if dystocia in mice. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 10–17. [Google Scholar]
- Mayor, P.; Bodmer, R.E.; Lopez-Bejar, M. Functional anatomy of the female genital organs of the wild black agouti (Dasyprocta fuliginosa) female in the Peruvian Amazon. Anim. Reprod. Sci. 2011, 123, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.D.; Adogwa, A.O.; Mollineau, W.M.; Garcia, G.W. Gross and microscopic anatomy of the reproductive tract of the female agouti (Dasyprocta leporina): A neotropical rodent with the potential for domestication. Trop. Agric. (Trinidad) 2014, 91, 38–46. [Google Scholar]
- Matamoros, Y. Anatomia e histologia del Sistema reproductor del tepezcuinte (Cuniculus paca). Rev. De Biol. Trop. 1981, 29, 155–164. [Google Scholar]
- Miglino, M.A.; dos Santos, T.C.; Kanashiro, C.; dos Santos Ferraz, R.H. Morphology and Reproductive Physiology of Female Capybaras. In Capybara: Biology, Use and Conservation of an Exceptional Neotropical Species; Moreira, J.B., Ferraz, K.M.P.M.B., Herrera, E.A., Macdonald, D.W., Eds.; Springer Science + Media Business: New York, NY, USA, 2013. [Google Scholar]
- Enders, R.K. Parturition in the agouti with notes on several pregnant uteri. J. Mammal. 1931, 12, 390–396. [Google Scholar] [CrossRef]
- Weir, B.J. Some observations on reproduction in the female agouti, Dasyprocta aguti. J. Reprod. Fertil. 1971, 24, 203–211. [Google Scholar] [CrossRef][Green Version]
- Souza, F.C.A.; Alves, F.R.; Fortes, E.A.M.; Ferraz, M.S.; Machado Junior, A.A.N.; Menezes, D.J.A.; de Carvalho, M.A.M. Pregnancy in Hystricomorpha: Gestational age and embryonic-fetal development of agouti (Dasyprocta prymnolopha, Wagler 1831) estimated by ultrasonography. Theriogenology 2012, 78, 1278–1285. [Google Scholar] [CrossRef]
- Brown, C.E. Rearing Wild Animals in Captivity, Gestational Periods. J. Mammal. 1936, 17, 10–13. [Google Scholar] [CrossRef]
- Pachaly, J.R.; Acco, A.; Lange, R.R.; Noguiera, T.M.R.; Noguiera, M.F.; Ciffoni, E.M.G. Order Rodentia (Rodents), Biology, Medicine and Surgery of South American Mammals, 2nd ed.; Fowler, M.E., Cubas, Z.S., Eds.; Iowa State University Press: Ames, IA, USA, 2001; pp. 225–237. [Google Scholar]
- Clark, J.D.; Olfert, E.D. Rodents (Rodentia). In Zoo and Wildlife Medicine, 2nd ed.; Fowler, M.E., Philadelphia, W.B., Eds.; Saunders: Philadelphia, PA, USA, 1986; pp. 728–737. [Google Scholar]
- Fortes, E.A.M.; Ferraz, M.S.; Bezerra, D.O.; Conde Junior, A.M.; Cabral, R.M.; Sousa, F.C.A.; Almeida, H.M.; Pessoa, G.T.; Menezes, D.J.A.; Guerra, S.P.L.; et al. Prenatal development of the agouti (Dasyprocta prymnolopha, Wagler, 1831): External features and growth curves. Anim. Reprod. Sci. 2012, 140, 195–205. [Google Scholar] [CrossRef]
- Brown-Uddenberg, R.C. Conceptualisation of an Intensive Production Model for the Agouti (Dasyprocta leporina) a Neotropical Rodent in Trinidad. Ph.D. Thesis, University of the West Indies, West Indies, Kingston, Jamaica, 2001. [Google Scholar]
- Jones, K.R.; Garcia, G.W. Anthelmintic usage on the reproductive parameter in captive reared agoutis (Dasyprocta leporina) in Trindad and Tobago, West Indies. Trop. Agric. 2020, 97. in press. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, K.R.; Lall, K.R.; Garcia, G.W. Dystocia in a Captive Reared Agouti (Dasyprocta leporina) in Trinidad and Tobago, West Indies. Vet. Sci. 2020, 7, 30. https://doi.org/10.3390/vetsci7010030
Jones KR, Lall KR, Garcia GW. Dystocia in a Captive Reared Agouti (Dasyprocta leporina) in Trinidad and Tobago, West Indies. Veterinary Sciences. 2020; 7(1):30. https://doi.org/10.3390/vetsci7010030
Chicago/Turabian StyleJones, Kegan Romelle, Kavita Ranjeeta Lall, and Gary Wayne Garcia. 2020. "Dystocia in a Captive Reared Agouti (Dasyprocta leporina) in Trinidad and Tobago, West Indies" Veterinary Sciences 7, no. 1: 30. https://doi.org/10.3390/vetsci7010030
APA StyleJones, K. R., Lall, K. R., & Garcia, G. W. (2020). Dystocia in a Captive Reared Agouti (Dasyprocta leporina) in Trinidad and Tobago, West Indies. Veterinary Sciences, 7(1), 30. https://doi.org/10.3390/vetsci7010030





