Abstract
The antimicrobial activity of a phage mixture and a lactic acid bacterium against Staphylococcus aureus isolates from bovine origin was investigated in vitro with regard to possible applications in the therapy of udder inflammation (mastitis) caused by bacterial infections. The S. aureus isolates used for inoculation derived from quarter foremilk samples of mastitis cases. For the examination of the antimicrobial activity, the reduction of the S. aureus germ density was determined [log10 cfu/mL]. The phage mixture consisted of the three obligatory lytic and S. aureus-specific phages STA1.ST29, EB1.ST11 and EB1.ST27 (1:1:1). The selected Lactobacillus plantarum strain with proven antimicrobial properties and the phage mixture were tested against S. aureus in milk, both alone and in combination. The application of the lactic acid bacterium showed only a low reduction ability for a 24 h incubation period. The bacteriophage mixture as well as its combination with the lactic acid bacterium showed high antimicrobial activity against S. aureus for a 24 h incubation period at 37 °C, with only the phage mixture showing significance.
1. Introduction
The therapy of intramammary infections (IMIs) in conventional dairy farming involves the use of antimicrobial agents for treatment during lactation and for selective drying off [1,2]. A targeted use of antibiotics is necessary to ensure the health and well-being of animals and to achieve a high level of food safety. Nevertheless, bacterial resistance against antimicrobial agents in human as well as veterinary medicine and increasing public concern about the usage of antibiotics in food-producing animals make it necessary to further reduce their application [3,4,5].
Staphylococcus (S.) aureus, a Gram-positive, catalase-positive and coagulase-positive bacterium, represents one of the most important mastitis pathogens [6,7]. Although environment-associated pathogens have become increasingly important, there are still significant aspects to consider, such as the remediation of S. aureus at the herd level, which still represents a long-term and labor-intensive process. As a cow-associated major pathogen, S. aureus is spread between animals, especially during the milking process [7,8,9]. Moreover, characteristics such as low cure rates and a long persistence in the mammary gland make it a difficult-to-treat udder pathogen whose sanitation requires additional hygiene and management efforts [4,7,10]. Control programs covering aspects of milking hygiene, separation of infected animals and therapeutic measures are therefore particularly important [11,12]. Dufour et al. used multilevel regression models to investigate the risk factors associated with prevalence, incidence during lactation and elimination of S. aureus-induced bovine IMI [13]. Key factors related to low prevalence in the herd include a low incidence as the most important factor on herd level and a high elimination rate of S. aureus. The incidence of S. aureus IMI was mainly associated with milking procedures (hygiene measures: wearing gloves, disinfection of teats before milking; good condition of teat ends). These factors are simultaneously associated with the reduction of the prevalence and the elimination of S. aureus [13].
The pathogenicity factors enable S. aureus to perform encapsulation, intracellular occurrence, adhesion to epithelial cells and biofilm formation [14]. This often leads to subclinical or chronic inflammation of the bovine mammary gland, impedes the effect of an antibiotic treatment and might be the cause of insufficient cure rates for an antibiotic treatment during lactation [7,15]. Therefore, antimicrobial alternatives are of particular interest to increase the bacteriological cure (BC) rates during lactation while significantly reducing the application of antibiotics [16].
The use of bacteriophages (phages) for the treatment of bacterial infections (bacteriophage therapy) represents such an alternative approach. Several studies in animal models have already investigated bacteriophages, with promising results regarding a therapeutic use [17,18,19,20]. Phages hold various advantages over conventional antibiotic agents, as they are very host specific and thus protect the physiological bacterial flora [21,22]. Furthermore, new phages can be isolated within a comparatively short time. This advantage, the possibility of combining different phages in the form of a bacteriophage mixture, as well as the possibility to combine phages and antibiotics reduce the risk of a resistance development against single phages [23,24,25]. Various studies indicate a prolonged antibiotic therapy of S. aureus-induced intramammary infections (IMIs) to improve bacteriological cure rates, resulting in an increased use of antimicrobial agents [26,27,28]. For this reason, alternative treatment methods such as phage therapy could be particularly useful to reduce the amount of antibiotics administered.
Lactic acid bacteria (LAB) are a heterogeneous group of Gram-positive, catalase-negative and facultative anaerobic microorganisms which are summarized due to their specific kind of metabolism [29,30]. LAB also possess antimicrobial properties resulting from the colonization of epithelia, the competition for nutrients and the production of organic acids such as lactic acid, free fatty acids as well as hydrogen peroxide, diacetyl, bacteriocins or bacteriocin-like substances [31,32,33]. Furthermore, LAB are able to modulate the host’s immune system [34]. The term “probiotic”, which is closely associated with LAB and bifidobacteria, is used in the sense of “health promoting” and “probiotics” are, by definition, living microorganisms with a consumer health benefit when properly administered [29,31,35]. Several in vitro and in vivo studies on the antimicrobial activity of LAB against S. aureus were published [36,37,38]. A number of studies on the use of LAB for the therapy of bovine udder infections have already been carried out, with promising results [39,40,41]. Crispie et al. (2004) even described the use of a lacticin produced by the probiotic Lactococcus lactis DPC 3147 as effective as a conventional antibiotic treatment for dry cow therapy [42].
Lactobacillus plantarum is a facultative heterofermentative LAB, which is mostly isolated from dairy products, sewage and from human or clinical origin [30,43]. Lb. plantarum strains are known for their probiotic properties, including the formation of a specific bacteriocin, called plantaricin [37,44,45,46].
The aim of the present study was the in vitro investigation of the antimicrobial activity for the combination of an obligately lytic bacteriophage mixture (PM) and the Lb. plantarum strain 118/37 against S. aureus isolates from mastitis cases. The Lb. plantarum strain 118/37 has been extensively tested in previous examinations and has proven its antimicrobial activity against S. aureus [47]. The examinations were conducted regarding a combined application in vivo for the therapy of S. aureus mastitis during lactation.
2. Materials and Methods
2.1. S. aureus Strains and Growth Conditions
The S. aureus isolates 7142 and 10614 used for inoculation of ultra-high temperature (UHT) milk were isolated from two different dairy farms in Germany and provided by the Department of Bioprocess Engineering and Microbiology, University of Applied Sciences and Arts Hannover (Germany). They were obtained from quarter foremilk samples of mastitis cases and were tested as coagulase-positive as well as screened nuc gene-positive. To confirm these results for the present study, species affiliation was performed using matrix-assisted laser desorption time-of-flight-mass spectrometry (MALDI-TOF), resulting in S. aureus for both isolates. The isolates were stored at −80 °C with addition of 20 % glycerin after a 24 h culture in brain heart broth and plated on esculin blood agar (Oxoid Deutschland GmbH, Wesel, Germany) (24 h at 37 °C) before use.
The S. aureus strains ST11, ST27 and ST29, isolated and provided by the Phage Technology Center (PTC) GmbH, Bönen (Germany), were used for routine phage propagation (Table 1). The strains ST11 and ST27 originated from mastitis cases. The phage propagation strains were stored at −80 °C with addition of 20 % glycerin and plated on esculin blood agar (Oxoid Deutschland GmbH, Wesel, Germany) before use. Luria–Bertani (LB) broth (trypton/pepton form casein: 1 g/100 mL; yeast extract, micro-granulated: 0.5 g/100 mL; sodium chloride (NaCl): 0.5 g/100 mL; Carl Roth GmbH & Co. KG, Karlsruhe, Germany) was used for bacterial growth in suspension and for phage plaque assay. LB broth was supplemented with agar (Agar Agar Bioscience; Carl Roth GmbH & Co. KG, Karlsruhe, Germany) at concentrations of 1.5 % for bottom and 0.5 % for top agar and sterile dextrose was added at 1.1 % to the top agar before use. Baird–Parker agar (Carl Roth GmbH & Co. KG, Karlsruhe, Germany) supplemented with egg yolk tellurite-emulsion (Carl Roth GmbH & Co. KG, Karlsruhe, Germany) was used for differential counting of S. aureus germ density.

Table 1.
Bacteriophages and Staphylococcus aureus propagation strains.
2.2. Lactic Acid Bacterium and Growth Conditions
The lactic acid bacterium (LAB) 118/37 identified as Lactobacillus (Lb.) plantarum is an isolate from bovine milk and was provided by the Department of Bioprocess Engineering and Microbiology of the University of Applied Sciences and Arts Hannover (Germany). It has been isolated and described in a research project, tested for its antimicrobial properties and patented. Details can be found at Diepers et al. (2017) [47]. The LAB was stored at −80 °C with addition of 20 % glycerin after a 24 h culture in de Man, Rogosa and Sharpe (MRS) broth (Merck, Darmstadt, Germany). It was cultured twice in MRS broth (37 °C, 24 h, anaerobically) and simultaneously plated on MRS-agar (Merck, Darmstadt, Germany) for optical detection of purity before every use. Species identification based on microbiological identification for Gram-positive, catalase-negative, oxidase-negative (Bactident Oxidase; Merck, Darmstadt, Deutschland), fermentative (OF basal medium with addition of D(+)-glucose monohydrate; Merck, Darmstadt, Deutschland), H2S-negative, immotile rods. Further biochemical and genetic species identification was performed by Diepers et al. (2017) and based on analytic profile index (API) assay and the detection of 16SrDNA sequence according to Kwon et al. (2004) [47,48]. To confirm these results for the present study, species affiliation was performed additionally using MALDI-TOF, resulting in Lb. plantarum for the LAB strain 118/37.
LAB 118/37 was chosen for the present examinations according to safety requirements (no resistance coding genes) and properties such as the proven antimicrobial activity against S. aureus ATCC 12600 and the adhesion to teat canal epithelial cells in vitro [47].
2.3. Bacteriophages
Bacteriophages STA1.ST29, EB1.ST11 and EB1.ST27 were isolated and provided by PTC GmbH, Bönen (Germany). These lytic, sequenced phages belong to the order of Caudovirales and the families of Myoviridae (STA1) and Podoviridae (EB1) (Table 1). Phage STA1 is an isolate of a wastewater facility and is closely related to Phage K, which was isolated and characterized by O’Flaherty in 2004 [49]. Phage EB1 is an isolate from pig manure and is closely related to the phage PSa3, which was isolated and characterized by Kraushaar et al. in 2013 [50]. The genetically distinct phages were grown on different host strains (ST29; ST11 or ST27), which might have led to epigenetic modifications [51] that resulted in a different host range in previous examinations [52].
A bacteriophage mixture (PM) was prepared to enlarge the host range for the examination in UHT milk and with regard to an in vivo use and to decrease the risk of a resistance development against single phages [25]. It contained phages STA1.ST29, EB1.ST11 and EB1.ST27 in equal quantities and a final concentration of 7.4 × 109 pfu/mL. The phages were selected according to previously examined criteria including a broad host range against S. aureus isolates from mastitis cases, good propagation properties and adequate storage stabilities [52]. All phages of the mixture showed lytic activity against the S. aureus isolates 7142 and 10614 in the plaque assay and the phage mixture (PM) showed antimicrobial activity against the S. aureus isolates in pasteurized and raw milk in previous examinations [52].
2.4. Bacteriophage Propagation
For phage propagation, LB broth was inoculated with 1 % of an overnight culture of the respective S. aureus strain (ST11, ST27 or ST29). The optical density at 600 nm (OD600) was determined by a photometer (SPEKOL®1500, Analytik Jena AG, Jena, Germany) every 30 min and one percent of a phage stock solution with a concentration ≥ 1.5 × 1010 pfu/mL (EB1.ST11), ≥ 1.3 × 109 pfu/mL (EB1.ST27), ≥ 1.6 × 109 pfu/mL (STA1.ST29) was added when an OD600 of 0.3 was reached. The OD600 was measured at half-hourly intervals until a decrease could be seen. Chloroform was added at 1 ‰ and the solution was centrifuged (10,000× g for 20 min at room temperature) before supernatant was removed and sterile filtered (using a filter with a pore size of 0.45 µm, Minisart®NML Plus/NY Plus, Sartorius, Göttingen, Germany). The plaque forming units per milliliter (pfu/mL) were determined by using a double-layer agar technique modified to the one described by Sambrook and Russel (Sambrook and Russel 2001). Briefly, 100 µL of the phage serial dilution (Ringer’s solution; Merck KGaA, Darmstadt, Germany) was added to 100 µL overnight culture of the respective S. aureus propagation strain and incubated for 5 min at room temperature. After addition of 5 mL top agar, the assays were mixed by a Vortex Mixer (Vortex Genie®2; Scientific Industries, Bohemia, NY, USA) and poured onto a petri dish (Ø94 × 16 mm) prepared with 10 mL LB—bottom agar. The inverted plates were incubated for 18 h at 37 °C until the plaque forming units per milliliter (pfu/mL) were determined. The phage solution was stored in the dark at + 6 °C until further investigation.
2.5. Antimicrobial Activity of a Phage Mixture and a Lactic Acid Bacterium in Milk
With regard to a future use in the therapy of S. aureus mastitis in dairy cows, we investigated the antimicrobial activity of a phage mixture (PM) and a lactic acid bacterium in milk for an approximation to in vivo conditions. The antimicrobial activity was examined for a single in comparison to a combined use of the PM (STA1.ST29, EB1.ST11 and EB1.ST27) and the Lb. plantarum strain 118/37 (LAB) against two S. aureus isolates from mastitis cases, respectively, and was determined by the reduction of the S. aureus germ density [cfu/mL]. Baird–Parker agar plates were used to prevent contamination by the lactic acid bacterium, representing a selective culture medium for the isolation of coagulase-positive Staphylococcus species. The S. aureus isolates 7142 and 10614 were tested for their typical growth (black colonies surrounded by a clear zone) on Baird–Parker agar before trial. Commercial ultra-high temperature (UHT) milk was used to achieve high reproducibility and to prevent contamination by the natural milk microflora. The milk was tested for absence of S. aureus and antibiotic residues by direct plating and a Brilliant Black Reduction Test (Delvotest®BR Brilliant, DSM; MILKU Tierhygiene GmbH, Bovenden, Germany) prior to examination.
The UHT milk was inoculated with S. aureus isolates 7142 or 10614 respectively at an average concentration of 1.3 × 105 cfu/mL. Depending on the approach a) the PM at a concentration of 1.3 × 109 pfu/mL, b) the LAB (Lb. plantarum 118/37) at a concentration of 6.6 × 106 cfu/mL or c) a combination of PM and LAB was added, resulting in a total amount of 7 mL for each approach. Previous examinations [52] have shown the best reduction ability for the concentrations of S. aureus isolates (7142; 10614) and PM, which were also used in the present trials. For the application of S. aureus and LAB, a ratio of 1:10 was used according to the findings of previous studies [53,54].
The pH value was constantly neutralized to prevent germ reduction solely due to acid formation by the LAB and in order to investigate the effect of other products such as antimicrobial peptides produced by the LAB. NaOH (1 mol/L) was used to achieve a constant pH value between 6.0 and 7.5. A fourth approach was solely used to determine the pH values and the amount of NaOH to be added.
Samples were taken after 30 min, after 12 h and after 24 h of incubation at 37 °C under aerobic conditions. Serial dilutions (RINGER, Merck, Darmstadt, Germany) were examined for their S. aureus germ density by using the spatula method with 100 µL sample material on Baird–Parker agar plates with addition of egg yolk tellurite (Baird-Parker-Agar, Basis, ROTH; egg yolk tellurite, ROTH) according to DIN EN ISO 6888-1. The 24 h incubation period was chosen according to common growth characteristics of LAB in milk and to investigate a possible long-term effect of the LAB on S. aureus [55,56]. To obtain statistically relevant data, the trial was carried out in triplicate including controls without phages and LAB (solely S. aureus isolate 7142 or 10,614) and negative controls (UHT milk without additives; solely LAB).
2.6. Statistical Analysis
Three measurements were collected for each data point. A statistical calculation was performed to determine the influence of the PM, the LAB and their combination on the reduction of the S. aureus germ density in UHT milk. The values of the S. aureus germ density (cfu/mL) were log transformed (log10) and one was added to approximate normal distribution. The data were analyzed by ANOVA using SPSS 25.0 (IBM SPSS 25.0.0.0., Armonk, NY, USA). p values < 0.05 were considered significant.
3. Results
Antimicrobial Activity of a Phage Mixture and a Lactic Acid Bacterium in Milk
The combination of a phage mixture and a lactic acid bacterium as well as their solitary use were investigated in UHT milk for their antimicrobial activity against S. aureus isolates 7142 and 10614 (initial concentration of 1.3 × 105 cfu/mL), respectively (Figure 1). Samples were taken after 30 min, 12 h and after 24 h. The germ density was calculated as cfu/mL and is given as a logarithm of the common mean values of the two S. aureus isolates [log10 cfu/mL] used for inoculation in the different approaches, as the statistical calculation showed no significant difference between the two isolates (p > 0.05).
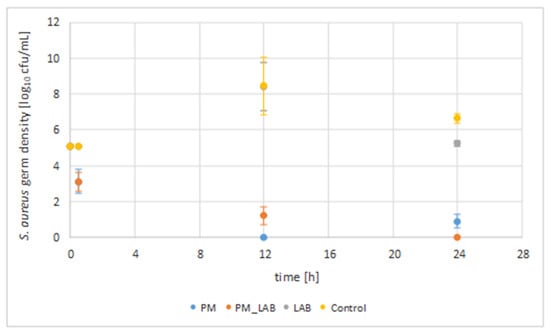
Figure 1.
S. aureus germ density for the application of a phage mixture, a lactic acid bacterium and their combination. The S. aureus germ density [log10 cfu/mL] for 100 µL sample material is given with standard errors. The common mean values of the two S. aureus isolates 7142 and 10614 are given (SEM 5.25 log cfu/mL ± 0.15 SEM) as the statistical calculation showed no significant difference between the two S. aureus isolates (p > 0.05). * Time of incubation at 37 °C in ultra-high temperature (UHT) milk. PM = phage mixture (STA1.ST29; EB1.ST11; EB1.ST27) (p < 0.05); LAB = lactic acid bacterium (118/37, Lb. plantarum) (p > 0.05); PM + LAB = combined application of the phage mixture and the lactic acid bacterium (p > 0.05).
The control without phages and LAB showed an average increase of 3.3 log units in S. aureus germ density for incubation at 37 °C to an average value of 8.5 log cfu/mL (SEM 5.25 log cfu/mL ± 0.15 SEM) after 12 h. After 24 h of incubation, an average germ density of 6.7 log cfu/mL (SEM 5.25 log cfu/mL ± 0.15 SEM) of S. aureus was observed.
The sole addition of PM resulted in a reduction (p < 0.05) compared to the initial germ density (5.1 log cfu/mL) by an average of 1.9 log units reduction to a value of 3.2 log cfu/mL (SEM 5.25 log cfu/mL ± 0.15 SEM) after 30 min, a reduction by 5.1 log units to a value of 0.0 log cfu/mL (SEM 5.25 log cfu/mL ± 0.15 SEM) after 12 h and a reduction by averagely 4.2 log units to a value of 0.9 log cfu/mL (SEM 5.25 log cfu/mL ± 0.15 SEM) after 24 h of incubation at 37 °C (Table 2). Compared to the control without phages and LAB at the same time, the reduction of the S. aureus germ density (p < 0.05) was 1.9 log units (30 min), 8.5 log units (12 h) and 5.8 log units (24 h).

Table 2.
Reduction of S. aureus germ density in log10 units.
The addition of the LAB alone did not lead to a reduction of the microbial density compared to the initial value for a 24 h incubation period, but to an increase by 3.3 log units (12 h) to a value of 8.4 log cfu/mL (SEM 5.25 log cfu/mL ± 0.15 SEM) and an increase by 0.1 log units (24 h) to a value of 5.2 log cfu/mL (SEM 5.25 log cfu/mL ± 0.15 SEM). Nevertheless, a reduction by <1 log unit after 12 h and by averagely 1.5 log units was shown compared to the control without phages and LAB after 24 h (p > 0.05).
The combined application of PM and LAB resulted in a reduction of the initial germ density (p > 0.05) by an average of 2.0 log units (30 min) to a value of 3.1 log cfu/mL (SEM 5.25 log cfu/mL ± 0.15 SEM), by 3.9 log units (12 h) to a value of 1.2 log cfu/mL (SEM 5.25 log cfu/mL ± 0.15 SEM) and by 5.1 log units (24 h) to a value of 0.0 log cfu/mL (SEM 5.25 log cfu/mL ± 0.15 SEM). Compared to the control without phages and LAB at the same time, the combined application led to a reduction (p > 0.05) by 2.0 log units (30 min), 7.3 log units (12 h) and 6.7 log units (24 h).
In conclusion, it can be said that the application of PM led to a considerable reduction of the bacterial density over a period of 24 h in contrast to the sole application of LAB. The statistical calculation showed significance for the use of PM (p < 0.05), but no significance for the application of the LAB or the combination of both (p > 0.05). Thus, it can be concluded that the addition of LAB had no additional effect on the reduction of the S. aureus germ density for an incubation period of 24 h.
4. Discussion
S. aureus strains possess certain pathogenicity factors that hamper antibiotic treatment and lead to insufficient bacteriological cure rates during lactation [7,15]. Research into alternatives for the treatment of S. aureus mastitis is therefore an important component in improving cure rates, reducing antibiotic consumption and thus reducing the risk of developing bacterial resistance. Lytic phages and probiotic lactic acid bacteria are regarded as alternative approaches in the therapy of bacterial infections.
For this reason, we investigated the combination of a three-component lytic bacteriophage mixture of sequenced Myo- and Podoviridae and a wild-type Lb. plantarum strain against S. aureus isolates from mastitis cases. Since obligatory lytic phages usually lead to a rapid reduction of pathogens, while lactic acid bacteria proliferate more slowly, but can establish themselves in the target tissue for longer, this combination was assumed to have an advantage for the treatment of S. aureus mastitis compared to use alone. Thus, an incubation period of 24 h was chosen considering the slower growth and the delayed production of antimicrobial peptides by the LAB. The present in vitro examinations were conducted with regard to a combined use in mastitis therapy. The S. aureus isolates used for inoculation were already investigated in a previous study and the PM also used in the present study was able to significantly reduce the S. aureus isolates in pasteurized and raw milk [52].
Bacteriophages are viruses that exclusively target prokaryotic cells. Obligately lytic phages in particular are able to proliferate inside and lyse the host cell within a short time. Phages commonly show very host-specific targeting, which leads to protection of the apathogenic flora in the target tissue when administered in vivo [21]. Several examinations in animal models have already demonstrated that the use of bacteriophages in vivo leads to a significant pathogen reduction without adverse effects on the treated animals [17,18,20].
Similar to any antibacterial agent, bacterial resistance can develop against phages. However, the possibility for a comparatively rapid isolation of new phages, the use of obligately lytic and sequenced phages with a lack of lysogenic potential, the usage of several phages in a mixture and finally the opportunity for a combined use of phages and antimicrobial agents reduce the risk for resistance development and represent advantages over common antibiotics [23,25,57,58]. In the present study, we used a mixture of obligately lytic, sequenced phages to fulfil functional and safety requirements. The phages of the mixture were already investigated in a previous study, resulting in a broad host spectrum against S. aureus isolates from mastitis cases [52].
Lactic acid bacteria also possess the ability to inhibit the growth of pathogens by the colonization of epithelia, the competition for nutrients and the production of antimicrobial active substances [30,31,32]. Lb. plantarum strains are known for their probiotic properties partly due to their production of a bacteriocin called plantaricin [44,45,46]. The literature describes general properties of lactic acid bacteria intended to be used as probiotics. These include a proven antagonistic activity against pathogens, the adhesion to and the persistence on epithelia, as well as the survival and a maintained functionality in the target tissue [34]. In addition, the modulation of the host’s immune response represents an important probiotic property [34,59,60]. Safety aspects include the absence of resistance-coding genes and a general susceptibility to antibiotics in case of treating immunosuppressed patients [34]. The LAB 118/37 used in the present study was isolated from milk and was already examined by Diepers et al. (2017) [47]. This Lb. plantarum strain was selected for the present examinations based on its properties, such as a proven antimicrobial activity against S. aureus, the possibility of using milk as a nutritional substrate and the ability to adhere to mammary gland canal epithelial cells in vitro [47]. In addition, its lack of resistance-coding genes fulfills an important safety requirement [34,47].
In the present study, we investigated the antimicrobial activity of a single and a combined use of the PM and the LAB against S. aureus isolates in UHT milk. The S. aureus isolates originated from quarter foremilk samples of mastitis cases and thus present typical mastitis causing pathogens. The antimicrobial activity was evaluated for the reduction of the S. aureus germ density in relation to the initial germ density (t0h), and in relation to the values of the control culture without phages after the same incubation period. The statistical calculation showed significance only for the use of PM. The evaluation of the results has already shown an effect on the germ density at the beginning of the experiment (t30min). Both the PM (p < 0.05) and the combination of PM and LAB (p > 0.05) led to a reduction of the S. aureus germ density in UHT milk after 30 min compared to the initial value, which increased to an average reduction by 5.1 log units (PM) (p < 0.05) and 3.9 log units (PM + LAB) (p > 0.05) after 12 h. Compared to the initial values, the reduction ability decreased slightly after 24 h when using PM alone (reduction by 4.2 log units; p < 0.05), but increased further when combining PM and LAB after 24 h incubation (reduction by 5.1 log units; p > 0.05). The highest reduction compared to the initial values was thus shown after 12 h for the use of PM (p < 0.05) and after 24 h for the combination of PM and LAB (p > 0.05).
A constant reduction was also shown in comparison to the controls without phages, with the highest reduction by 8.5 log units for PM (p < 0.05) and by 7.3 log units for the combination (p > 0.05) after 12 h. The use of the LAB alone led to no reduction when compared to the initial values for an incubation period of 24 h, but to a slight increase in germ density. Nevertheless, compared to the control culture without phages, the LAB led to a reduction of the S. aureus germ density by 1.5 log units after 24 h (p > 0.05).
It can therefore be said that for an incubation period of 24 h, only the use of PM led to a significant reduction in germ density (p < 0.05). The combination of PM and LAB was more successful than the use of LAB alone in terms of antimicrobial activity against S. aureus in UHT milk, but both did not show significance (p > 0.05).
The results of the present investigations are partly comparable to the results of Woo and Ahn (2014) for their examination of a combined use of phage SA11 and a Lactobacillus (Lb.) rhamnosus strain against Staphylococcus aureus for modeled intestinal conditions in vitro at different pH values [61]. Phage SA11 inhibited the growth of antibiotic-sensitive and -resistant S. aureus strains if compared to the control cultures after 10 h, whereas the single use of Lb. rhamnosus led to an inhibition of the germ density first after 20 h in comparison to the corresponding values of the control culture without phages. Thus, the authors suggested that phage lytic activity was predominant after 10 h of incubation and the antimicrobial activity of LAB after 20 h. The authors described the highest reduction ability for a combined application of phage and LAB after 10 h and 20 h [61].
In our study, the use of the PM resulted in a reduction ability already after 30 min (p < 0.05). It has to be pointed out that the reduction was shown at each sampling time compared to the initial values as well as to the control culture. The combination of PM and LAB also led to a reduction after 30 min, after 12 h and after 24 h, but without significance (p > 0.05). From this, it can be concluded that the addition of LAB did not show an increase or synergism for an incubation period of 24 h. The sole use of the LAB in the present study led to a reduction only compared to the values of the control culture without phages first after 24 h of incubation (p > 0.05), which is comparable to the results of Woo and Ahn (2014) and will be related to the fact that the proliferation, the synthesis of antimicrobial peptides and consequently the antimicrobial activity of LAB requires more time than that of obligately lytic phages. This could also be a possible reason explaining why the combination with LAB and the use of LAB alone did not lead to a significant result (p > 0.05). As with all microorganisms, there is a lag phase, where bacteria adapt to growth conditions, and so the effect of the LAB probably started when the present measurements were finished after 24 h. Therefore, an extension of the observation time would be necessary. A further in vivo trial could be used to investigate whether the LAB, by being established in the udder, could be more effective and whether a prolonged incubation period in vivo may potentially lead to a significant effect of the LAB against S. aureus. In any case, our results show that there are no negative interferences between the PM and the LAB which would have led to a lower reduction in the combined application, underlining the possibility of a combined use in further trials. In this combination, lytic phages could thus achieve a short-term effect, whereas the LAB could offer the advantage of a long-term effect by establishing itself in the target tissue. At this point, further examinations are necessary to investigate this context more detailed in vivo.
Further, it is possible that incubation under anaerobic conditions would have had an influence on the antimicrobial activity of the Lb. plantarum strain. However, the present in vitro investigations were conducted against the background of in vivo use in the treatment of mastitis cases and as LAB represent anaerobic but aerotolerant microorganisms, the approaches were incubated under aerobic conditions.
Although the resistance situation of antibiotics for mastitis therapy in Germany can still be described as moderate, the isolation of resistant S. aureus strains from mastitis cases and tank milk has been published [9,62,63]. Since the development of antibiotic resistance follows a natural process and any application and in particular the improper administration of antibacterial agents can lead to the development of resistance in bacteria, alternative solutions for the treatment of bacterial infections are needed [3,5]. The use of probiotic lactic acid bacteria as well as phages in therapy represents such alternative approaches and offers several advantages. These include, among others, the protection of the apathogenic flora when using phages [21], the modulation of the immune system by LAB as a probiotic property [34] as well as a self-limitation of phages at low S. aureus concentrations [17] and, in contrast, a possible long-term effect of LAB by establishing themselves in the udder. There are already investigations on a combined use of phages and probiotics against different pathogens [61,64,65]. Dini et al. (2016) demonstrated pathogen reduction in the treatment of in vitro EHEC infection using the phage CA933P and a probiotic mixture, with a diminishing cytotoxic effect. The authors additionally demonstrated freeze drying as a method of storage with no significant reduction in concentration of the two components [66]. We determined a storability of the phages used in the present study for a period of six month at +6 °C without a substantial loss of efficacy (< 1 log unit), determined for pfu/mL in periodically performed plaque assays of the phage solution after propagation and sterile filtration. This storage stability represents an important factor with regard to its therapeutic use [67]. Another study investigated the use of phage P22 and a mixture of four bacteriocin-producing Lactobacilli for the treatment of Salmonella infection in chicken, resulting in the total elimination of intestinal Salmonella after 48 h [64]. Accordingly, the investigation of phages and LAB against pathogens is not new but, to our knowledge, the present study is the first examination of a combined use with regard to bovine mastitis therapy.
Further studies are needed to investigate the efficacy of a combined application of phages and LAB for the treatment of S. aureus mastitis more detailed in vivo. For this case, the combination of the phage mixture and the LAB 118/37 could be applied into the teat canal of healthy udders for a tolerability assessment, followed by the investigation of the BC rates of an intramammary treatment in comparison to treatment with common antibiotic agents. Further examinations could also deal with a prophylactic use in the cleaning of milking equipment. D’Accolti et al. (2018) already demonstrated that a combination of phages (Staphylococcal phage and Pyophage) and the probiotic PCHS detergent including spores of three Bacillus species led to decontamination of hard surfaces in the hospital environment (hospital-acquired pathogens, e.g., antibiotic-sensitive and -resistant S. aureus) [65]. The authors also noted that the probiotic detergent not only had a positive effect on the maintenance of phage efficacy, but also led to an increased activity compared to the suspension in PBS. Thus, they suggested the phage–probiotic combination for use as a routine disinfectant [65]. In the present study, the LAB had no adverse effect on the activity of the phages when used in combination and an extended incubation period in further investigations could possibly lead to a significant influence on the S. aureus germ density.
It is a fact that any use of antibiotics promotes the development of bacterial resistance and some bacterial infections, such as S. aureus infections, whether in animals or humans, may not respond adequately to antibiotics [5]. These problems make it necessary to research alternatives for the therapy of bacterial infections. Against the background of a limited number of studies on a combination of phages and LAB and no prior investigation into mastitis therapy, the present study provides a basis for future investigations. Nevertheless, further in vivo studies are necessary to find adequate application doses and intervals as in vitro investigations are not fully able to reproduce in vivo conditions due to the complex anatomical and physiological conditions of the bovine udder.
5. Conclusions
The antimicrobial activity of a single application as well as the combination of a Lb. plantarum strain with proven antimicrobial properties and a lytic three-component phage mixture was investigated in milk against S. aureus isolates from bovine mastitis. The single application of the phage mixture showed the most efficient reduction of S. aureus germ density after 12 h (p < 0.05). The combination of the phage mixture and the lactic acid bacterium showed the highest antimicrobial activity after 24 h (p > 0.05). Statistical calculations showed that only the phage mixture had a significant effect on the S. aureus germ density for an incubation period of 24 h.
Author Contributions
Conceptualization, I.T. and V.K.; methodology, I.T. and V.K.; investigation, I.T.; writing—original draft preparation, I.T.; writing—review and editing, I.T. and V.K.; visualization, I.T.; supervision, V.K.; project administration, V.K.; funding acquisition, V.K. All authors have read and agreed to the published version of the manuscript.
Funding
The Steinbeis Research Center Milk Science, Kirchlengern, Germany, financially supported this study.
Acknowledgments
The PTC (Phage Technology Center) GmbH, Bönen, Germany, provided the bacteriophages used in this study. The authors would like to thank the laboratory personnel of the Microbiology Work Group of the University of Applied Sciences and Arts Hannover. Isabel Titze is the recipient of a Hanns Seidel Foundation Fellowship for talented doctoral students, founded by the German Federal Ministry of Education and Research (BMBF).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Merle, R.; Mollenhauer, Y.; Hajek, P.; Robanus, M.; Hegger-Gravenhorst, C.; Honscha, W.; Käsbohrer, A.; Kreienbrock, L. Monitoring of antibiotic consumption in cattle on agricultural farms. Berl. Munch. Tierarztl. Wochenschr. 2013, 126, 318–325. [Google Scholar] [PubMed]
- Wise, R.; Hart, T.; Cars, O.; Streulens, M.; Helmuth, R.; Huovinen, P.; Sprenger, M. Antimicrobial resistance. BMJ 1998, 317, 609–610. [Google Scholar] [CrossRef] [PubMed]
- GVA. Guidelines for Combating Bovine Mastitis as a Stock Problem, 5th ed.; German Veterinary Association: Gießen, Germany, 2012. [Google Scholar]
- WHO. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Paris, France, 2014. [Google Scholar]
- Que, Y.-A.; Moreillon, P. Staphylococcus aureus (including staphylococcal toxic shock). In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2015; pp. 2237–2271. [Google Scholar]
- Barkema, H.; Schukken, Y.; Zadoks, R. Invited review: The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J. Dairy Sci. 2006, 89, 1877–1895. [Google Scholar] [CrossRef]
- Sol, H.W.; Barkema, Y.H. Schukken. Factors Associated with Cure after Therapy of Clinical Mastitis Caused by Staphylococcus aureus. J. Dairy Sci. 2000, 83, 278–284. [Google Scholar] [CrossRef]
- GERMAP. GERMAP 2015–Bericht über den Antibiotikaverbrauch und die Verbreitung von Antibiotikaresistenzen in der Human-und Veterinärmedizin in Deutschland; Bundesamt für Verbraucherschutz: Berlin, Germany, 2016; ISBN 978-3-9818383-0-5. [Google Scholar]
- IDF. Economic consequences of mastitis. In Bulletin No 394; International Dairy Federation: Brussels, Belgium, 2005. [Google Scholar]
- Zadoks, R.N.; van Leeuwen, W.B.; Kreft, D.; Fox, L.K.; Barkema, H.W.; Schukken, Y.H.; van Belkum, A. Comparison of Staphylococcus aureus Isolates from Bovine and Human Skin, Milking Equipment, and Bovine Milk by Phage Typing, Pulsed-Field Gel Electrophoresis, and Binary Typing. J. Clin. Microbiol. 2002, 40, 3894–3902. [Google Scholar] [CrossRef]
- Dodd, F.; Neave, F.J. Mastitis Control; National Institute Research Dairy: London, UK, 1970; pp. 21–60. [Google Scholar]
- Dufour, S.; Dohoo, I.; Barkema, H.; DesCôteaux, L.; DeVries, T.; Reyher, K.; Roy, J.-P.; Scholl, D. Manageable risk factors associated with the lactational incidence, elimination, and prevalence of Staphylococcus aureus intramammary infections in dairy cows. J. Dairy Sci. 2012, 95, 1283–1300. [Google Scholar] [CrossRef]
- Schönborn, S.; Krömker, V. Detection of the biofilm component polysaccharide intercellular adhesin in Staphylococcus aureus infected cow udders. Vet. Microbiol. 2016, 196, 126–128. [Google Scholar] [CrossRef]
- Linder, M.P.; Paduch, J.H.; Grieger, A.S.; Mansion-de-Vries, E.; Nicole, K.; Zinke, C.; Teich, K.; Krömker, V. Heilungsraten chronischer subklinischer Staphylococcus aureus-Mastitiden nach antibiotischer Therapie bei laktierenden Milchkühen. Berl. Münch. Tierärztl. Wschr. 2013. [Google Scholar] [CrossRef]
- Francoz, D.; Wellemans, V.; Dupré, J.; Roy, J.; Labelle, F.; Lacasse, P.; Dufour, S. Invited review: A systematic review and qualitative analysis of treatments other than conventional antimicrobials for clinical mastitis in dairy cows. J. Dairy Sci. 2017, 100, 7751–7770. [Google Scholar] [CrossRef]
- Capparelli, R.; Parlato, M.; Borriello, G.; Salvatore, P.; Iannelli, D. Experimental phage therapy against Staphylococcus aureus in mice. Antimicrob. Agents Chemother. 2007, 51, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Wills, Q.F.; Kerrigan, C.; Soothill, J.S. Experimental bacteriophage protection against Staphylococcus aureus abscesses in a rabbit model. Antimicrob. Agents Chemother. 2005, 49, 1220–1221. [Google Scholar] [CrossRef] [PubMed]
- Takemura-Uchiyama, I.; Uchiyama, J.; Osanai, M.; Morimoto, N.; Asagiri, T.; Ujihara, T.; Daibata, M.; Sugiura, T.; Matsuzaki, S. Experimental phage therapy against lethal lung-derived septicemia caused by Staphylococcus aureus in mice. Microbes. Infect. 2014, 16, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Yasuda, M.; Nishikawa, H.; Kuroda, M.; Ujihara, T.; Shuin, T.; Shen, Y.; Jin, Z.; Fujimoto, S.; Nasimuzzaman, M.D.; et al. Experimental Protection of Mice against Lethal Staphylococcus aureus Infection by Novel Bacteriophage ϕMR11. J. Infect. Dis. 2003, 187, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Carlton, R.M. Phage therapy: Past history and future prospects. Arch. Immunol. Et. Exp. Engl. Ed. 1999, 47, 267–274. [Google Scholar] [CrossRef]
- Hamza, A.; Perveen, S.; Abbas, Z.; Ur Rehman, S. The Lytic SA Phage Demonstrate Bactericidal Activity against Mastitis Causing Staphylococcus aureus. Open Life Sci. 2016, 11. [Google Scholar] [CrossRef]
- Santos, S.B.; Carvalho, C.M.; Sillankorva, S.; Nicolau, A.; Ferreira, E.C.; Azeredo, J. The use of antibiotics to improve phage detection and enumeration by the double-layer agar technique. BMC Microbiol. 2009, 9, 148. [Google Scholar] [CrossRef]
- Kirby, A.E. Synergistic action of gentamicin and bacteriophage in a continuous culture population of Staphylococcus aureus. PLoS ONE 2012, 7, e51017. [Google Scholar] [CrossRef]
- Garcia, P.; Madera, C.; Martinez, B.; Rodriguez, A.; Evaristo Suarez, J. Prevalence of bacteriophages infecting Staphylococcus aureus in dairy samples and their potential as biocontrol agents. J. Dairy Sci. 2009, 92, 3019–3026. [Google Scholar] [CrossRef]
- Krömker, V.; Paduch, J.-H.; Klocke, D.; Friedrich, J.; Zinke, C. Efficacy of extended intramammary therapy to treat moderate and severe clinical mastitis in lactating dairy cows. Berl. Und. Munch. Tierarztl. Wochenschr. 2010, 123, 147–152. [Google Scholar]
- Swinkels, J.M.; Kromker, V.; Lam, T.J. Efficacy of standard vs. extended intramammary cefquinome treatment of clinical mastitis in cows with persistent high somatic cell counts. J. Dairy Res. 2014, 81, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Truchetti, G.; Bouchard, É.; DesCôteaux, L.; Scholl, D.; Roy, J.-P. Efficacy of extended intramammary ceftiofur therapy against mild to moderate clinical mastitis in Holstein dairy cows: A randomized clinical trial. Can. J. Vet. Res. 2014, 78, 31–37. [Google Scholar] [PubMed]
- Pfeiler, E.A.; Klaenhammer, T.R. The genomics of lactic acid bacteria. Trends Microbiol. 2007, 15, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, W.H.; Wood, B.J. Lactic Acid Bacteria: Biodiversity and Taxonomy; John Wiley & Sons: Oxford, UK, 2014; ISBN 978-1-4443-3383-1. [Google Scholar]
- Vandenbergh, P.A. Lactic acid bacteria, their metabolic products and interference with microbial growth. FEMS Microbiol. Rev. 1993, 12, 221–237. [Google Scholar] [CrossRef]
- De Vuyst, L.; Vandamme, E.J. Bacteriocins of Lactic Acid Bacteria: Microbiology, Genetics and Applications; Springer: New York, NY, USA, 2012; ISBN 978-1-4613-6146-6. [Google Scholar] [CrossRef]
- Höltzel, A.; Gänzle, M.G.; Nicholson, G.J.; Hammes, W.P.; Jung, G. The first low molecular weight antibiotic from lactic acid bacteria: Reutericyclin, a new tetramic acid. Angew. Chem. Int. Ed. 2000, 39, 2766–2768. [Google Scholar] [CrossRef]
- Saarela, M.; Mogensen, G.; Fonden, R.; Mättö, J.; Mattila-Sandholm, T. Probiotic bacteria: Safety, functional and technological properties. J. Biotechnol. 2000, 84, 197–215. [Google Scholar] [CrossRef]
- FAO, W. Probiotics in food: Health and nutritional properties and guidelines for evaluation. FAO Food Nutr. Pap. 2006, 85, 2. [Google Scholar]
- Kao, C.T.; Frazier, W. Effect of lactic acid bacteria on growth of Staphylococcus aureus. Appl. Environ. Microbiol. 1966, 14, 251–255. [Google Scholar] [CrossRef]
- Andersson, R. Inhibition of Staphylococcus aureus and spheroplasts of Gram-negative bacteria by an antagonistic compound produced by a strain of Lactobacillus plantarum. Int. J. Food Microbiol. 1986, 3, 149–160. [Google Scholar] [CrossRef]
- Espeche, M.C.; Pellegrino, M.; Frola, I.; Larriestra, A.; Bogni, C.; Nader-Macías, M.F. Lactic acid bacteria from raw milk as potentially beneficial strains to prevent bovine mastitis. Anaerobe 2012, 18, 103–109. [Google Scholar] [CrossRef]
- Crispie, F.; Alonso-Gómez, M.; O’Loughlin, C.; Klostermann, K.; Flynn, J.; Arkins, S.; Meaney, W.; Ross, R.P.; Hill, C. Intramammary infusion of a live culture for treatment of bovine mastitis: Effect of live lactococci on the mammary immune response. J. Dairy Res. 2008, 75, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Frola, I.D.; Pellegrino, M.S.; Espeche, M.C.; Giraudo, J.A.; Nader-Macias, M.E.; Bogni, C.I. Effects of intramammary inoculation of Lactobacillus perolens CRL1724 in lactating cows’ udders. J. Dairy Res. 2012, 79, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Klostermann, K.; Crispie, F.; Flynn, J.; Ross, R.P.; Hill, C.; Meaney, W. Intramammary infusion of a live culture of Lactococcus lactis for treatment of bovine mastitis: Comparison with antibiotic treatment in field trials. J. Dairy Res. 2008, 75, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Crispie, F.; Flynn, J.; Ross, R.P.; Hill, C.; Meaney, W.J. Dry cow therapy with a non-antibiotic intramammary teat seal-a review. Ir. Vet. J. 2004, 57, 412. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, M.; Moraes, P.; Perin, L.; Viçosa, G.; Carvalho, K.; Júnior, A.S.; Nero, L. Molecular identification of naturally occurring bacteriocinogenic and bacteriocinogenic-like lactic acid bacteria in raw milk and soft cheese. J. Dairy Sci. 2010, 93, 2880–2886. [Google Scholar] [CrossRef]
- Anderssen, E.L.; Diep, D.B.; Nes, I.F.; Eijsink, V.G.; Nissen-Meyer, J. Antagonistic activity of Lactobacillus plantarum C11: Two new two-peptide bacteriocins, plantaricins EF and JK, and the induction factor plantaricin A. Appl. Environ. Microbiol. 1998, 64, 2269–2272. [Google Scholar] [CrossRef]
- Gonzalez, B.; Arca, P.; Mayo, B.; Suárez, J.E. Detection, purification, and partial characterization of plantaricin C, a bacteriocin produced by a Lactobacillus plantarum strain of dairy origin. Appl. Environ. Microbiol. 1994, 60, 2158–2163. [Google Scholar] [CrossRef]
- Chen And, H.; Hoover, D. Bacteriocins and their food applications. Compr. Rev. Food Sci. Food Saf. 2003, 2, 82–100. [Google Scholar] [CrossRef]
- Diepers, A.-C.; Krömker, V.; Zinke, C.; Wente, N.; Pan, L.; Paulsen, K.; Paduch, J.-H. In vitro ability of lactic acid bacteria to inhibit mastitis-causing pathogens. Sustain. Chem. Pharm. 2017, 5, 84–92. [Google Scholar] [CrossRef]
- Kwon, H.-S.; Yang, E.-H.; Yeon, S.-W.; Kang, B.-H.; Kim, T.-Y. Rapid identification of probiotic Lactobacillus species by multiplex PCR using species-specific primers based on the region extending from 16S rRNA through 23S rRNA. FEMS Microbiol. Lett. 2004, 239, 267–275. [Google Scholar] [CrossRef]
- O’Flaherty, S.; Coffey, A.; Edwards, R.; Meaney, W.; Fitzgerald, G.F.; Ross, R.P. Genome of Staphylococcal Phage K: A New Lineage of Myoviridae Infecting Gram-Positive Bacteria with a Low G+C Content. J. Bacteriol. 2004, 186, 2862–2871. [Google Scholar] [CrossRef]
- Kraushaar, B.; Thanh, M.D.; Hammerl, J.A.; Reetz, J.; Fetsch, A.; Hertwig, S. Isolation and characterization of phages with lytic activity against methicillin-resistant Staphylococcus aureus strains belonging to clonal complex 398. Arch. Virol. 2013, 158, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- Arber, W. Host-controlled modification of bacteriophage. Ann. Rev. Microbiol. 1965, 19, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Titze, I.; Lehnherr, T.; Lehnherr, H.; Krömker, V. Efficacy of bacteriophages against Staphylococcus aureus isolates from bovine mastitis. Pharmaceuticals 2020, 35. [Google Scholar] [CrossRef] [PubMed]
- Haines, W.C.; Harmon, L. Effect of variations in conditions of incubation upon inhibition of Staphylococcus aureus by Pediococcus cerevisiae and Streptococcus lactis. Appl. Environ. Microbiol. 1973, 25, 169–172. [Google Scholar] [CrossRef]
- Charlier, C.; Cretenet, M.; Even, S.; Le Loir, Y. Interactions between Staphylococcus aureus and lactic acid bacteria: An old story with new perspectives. Int. J. Food Microbiol. 2009, 131, 30–39. [Google Scholar] [CrossRef]
- Anas, M.; Eddine, H.J.; Mebrouk, K. Antimicrobial activity of Lactobacillus species isolated from Algerian raw goat’s milk against Staphylococcus aureus. World J. Dairy Food Sci. 2008, 3, 39–49. [Google Scholar]
- Guessas, B.; Hadadji, M.; Saidi, N.; Kihal, M. Inhibition of Staphylococcus aureus growth by lactic acid bacteria in milk. In African Crop Science Conference Proceedings; African Crop Science Society: El-Minia, Egypt, 2007; Volume 8, pp. 1159–1163. [Google Scholar]
- Hyman, P. Phages for Phage Therapy: Isolation, Characterization, and Host Range Breadth. Pharmaceuticals 2019, 12. [Google Scholar] [CrossRef]
- Comeau, A.M.; Tetart, F.; Trojet, S.N.; Prere, M.F.; Krisch, H.M. Phage-Antibiotic Synergy (PAS): Beta-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS ONE 2007, 2, e799. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Salminen, S.J. The health effects of cultured milk products with viable and non-viable bacteria. Int. Dairy J. 1998, 8, 749–758. [Google Scholar] [CrossRef]
- Schiffrin, E.J.; Brassart, D.; Servin, A.L.; Rochat, F.; Donnet-Hughes, A. Immune modulation of blood leukocytes in humans by lactic acid bacteria: criteria for strain selection. Am. J. Clin. Nutr. 1997, 66, 515S–520S. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Ahn, J. Assessment of synergistic combination potential of probiotic and bacteriophage against antibiotic-resistant Staphylococcus aureus exposed to simulated intestinal conditions. Arch. Microbiol. 2014, 196, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Spohr, M.; Rau, J.; Friedrich, A.; Klittich, G.; Fetsch, A.; Guerra, B.; Hammerl, J.A.; Tenhagen, B.-A. Methicillin-Resistant Staphylococcus aureus (MRSA) in Three Dairy Herds in Southwest Germany. Zoonoses Public Health 2011, 58, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Kreausukon, K.; Fetsch, A.; Kraushaar, B.; Alt, K.; Muller, K.; Kromker, V.; Zessin, K.H.; Kasbohrer, A.; Tenhagen, B.A. Prevalence, antimicrobial resistance, and molecular characterization of methicillin-resistant Staphylococcus aureus from bulk tank milk of dairy herds. J. Dairy Sci. 2012, 95, 4382–4388. [Google Scholar] [CrossRef]
- Gonçalves, G.A.M.; Lima, E.T.L.; Donato, T.C.; Rocha, T.S.; Álvarez, L.E.C.; Sequeira, J.L.; Andreatti Filho, R.L. Eradication of Salmonella Typhimurium in broiler chicks by combined use of P22 bacteriophage and probiotic. Microbiol. Res. 2011, 2, e2. [Google Scholar] [CrossRef]
- D’Accolti, M.; Soffritti, I.; Piffanelli, M.; Bisi, M.; Mazzacane, S.; Caselli, E. Efficient removal of hospital pathogens from hard surfaces by a combined use of bacteriophages and probiotics: Potential as sanitizing agents. Infect. Drug Resist. 2018, 11, 1015. [Google Scholar] [CrossRef]
- Dini, C.; Bolla, P.A.; de Urraza, P.J. Treatment of in vitro enterohemorrhagic Escherichia coli infection using phage and probiotics. J. Appl. Microbiol. 2016, 121, 78–88. [Google Scholar] [CrossRef]
- Łobocka, M.; Hejnowicz, M.S.; Gagała, U.; Weber-Dabrowska, B.; Wegrzyn, G.; Dadlez, M. The first step to bacteriophage therapy—How to choose the correct phage. In Phage Therapy: Current Research and Applications; Borysowski, J., Miedzybrodzki, R., Górski, A., Eds.; Caister Academic Press: Norfolk, UK, 2014; pp. 23–69. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).