The Role of Phosphatidylinositol Mannosides in the Serological Diagnosis of Mycobacterial Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. Statement of Ethical Approval
2.3. Mycobacterial Antigen Preparations
2.4. Mycobacterial Cultures used for Antigen Preparations
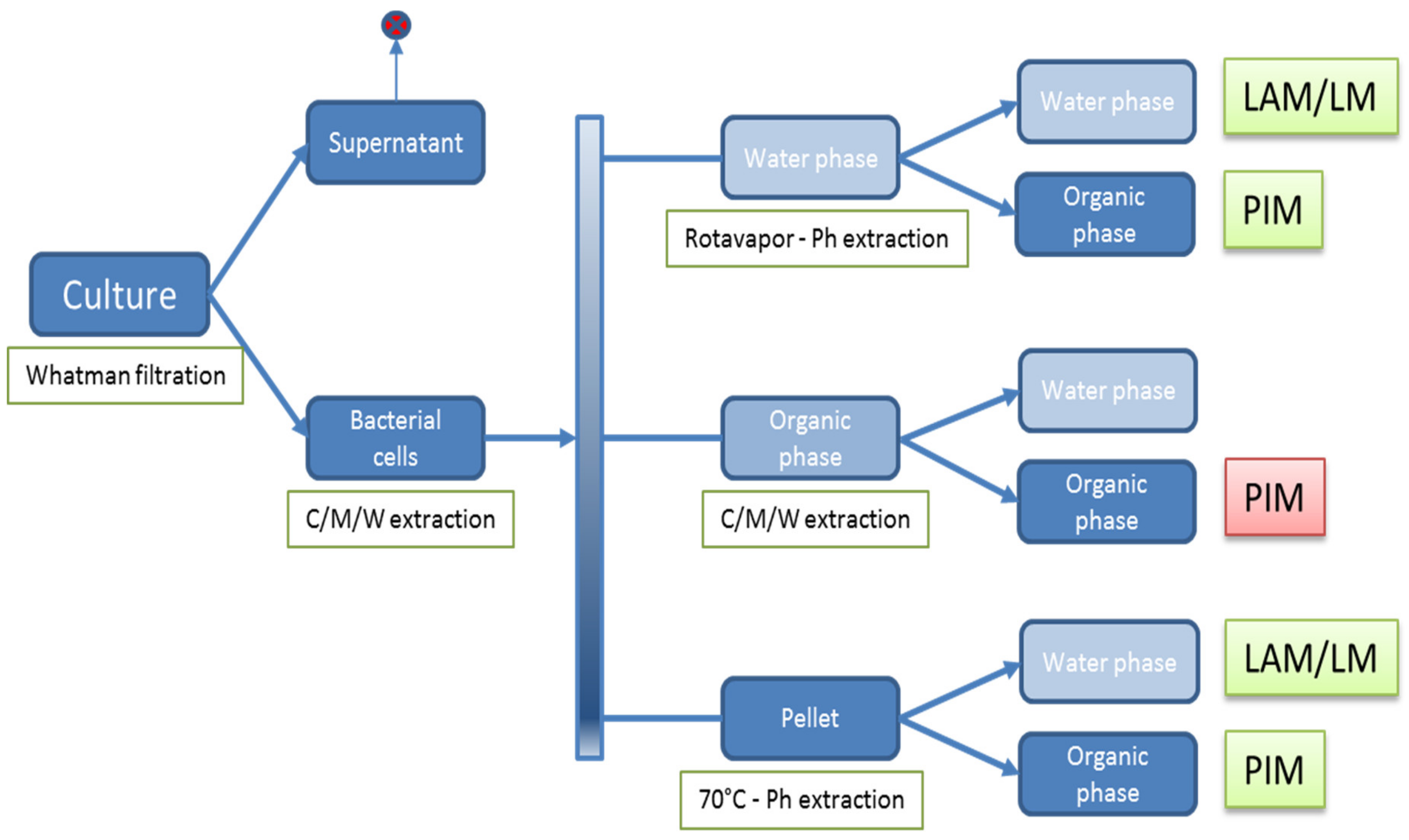
2.5. Isolation of Polar (glyco)lipids
2.6. Thin-Layer Chromatography Analysis of Lipids
2.7. Immune TLC
2.8. Reference Reagents
2.9. Recombinant Protein and PPD ELISA
2.10. PIM ELISA
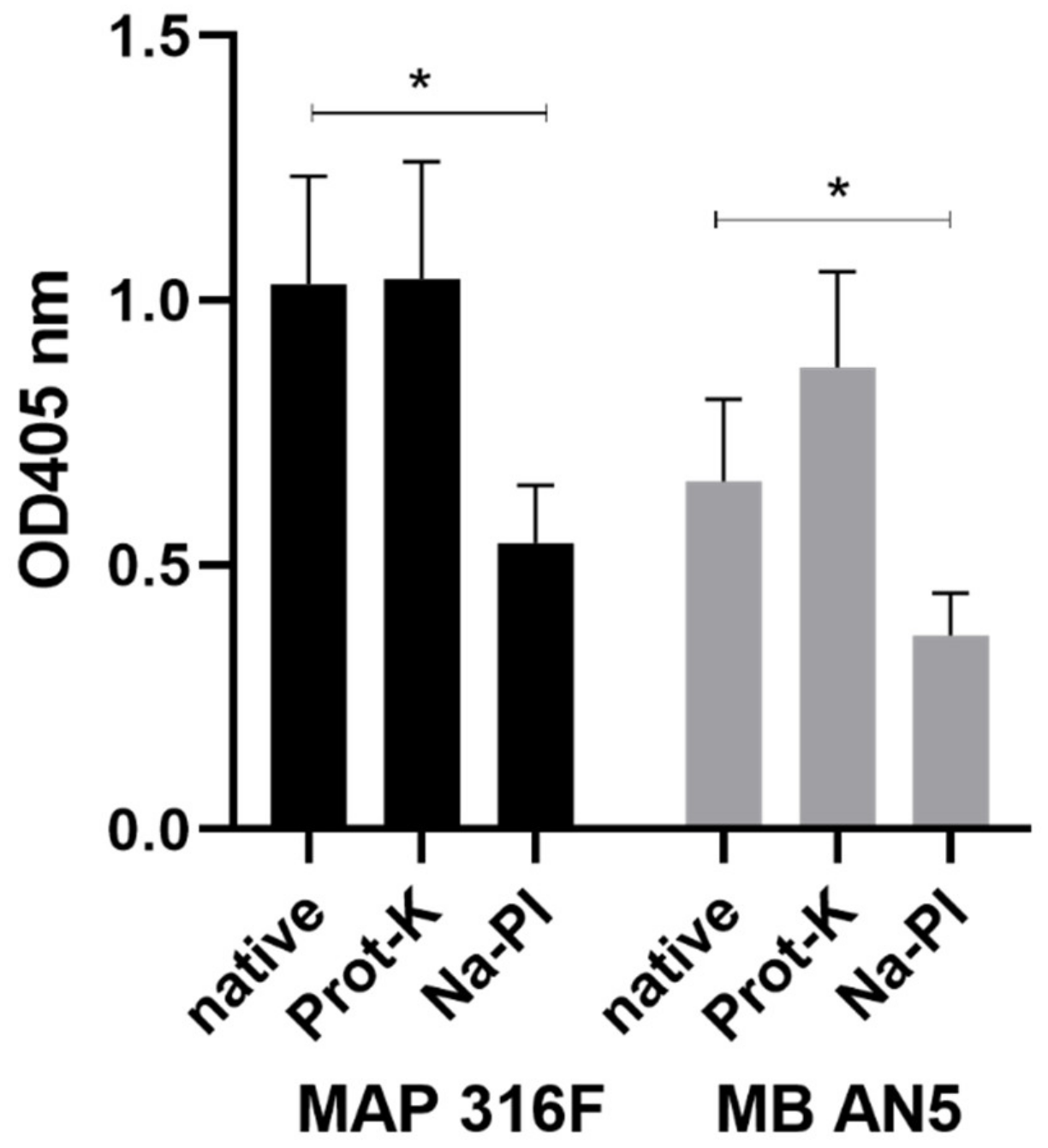
2.11. Antigenic Structures in PIM
2.12. Statistics
3. Results
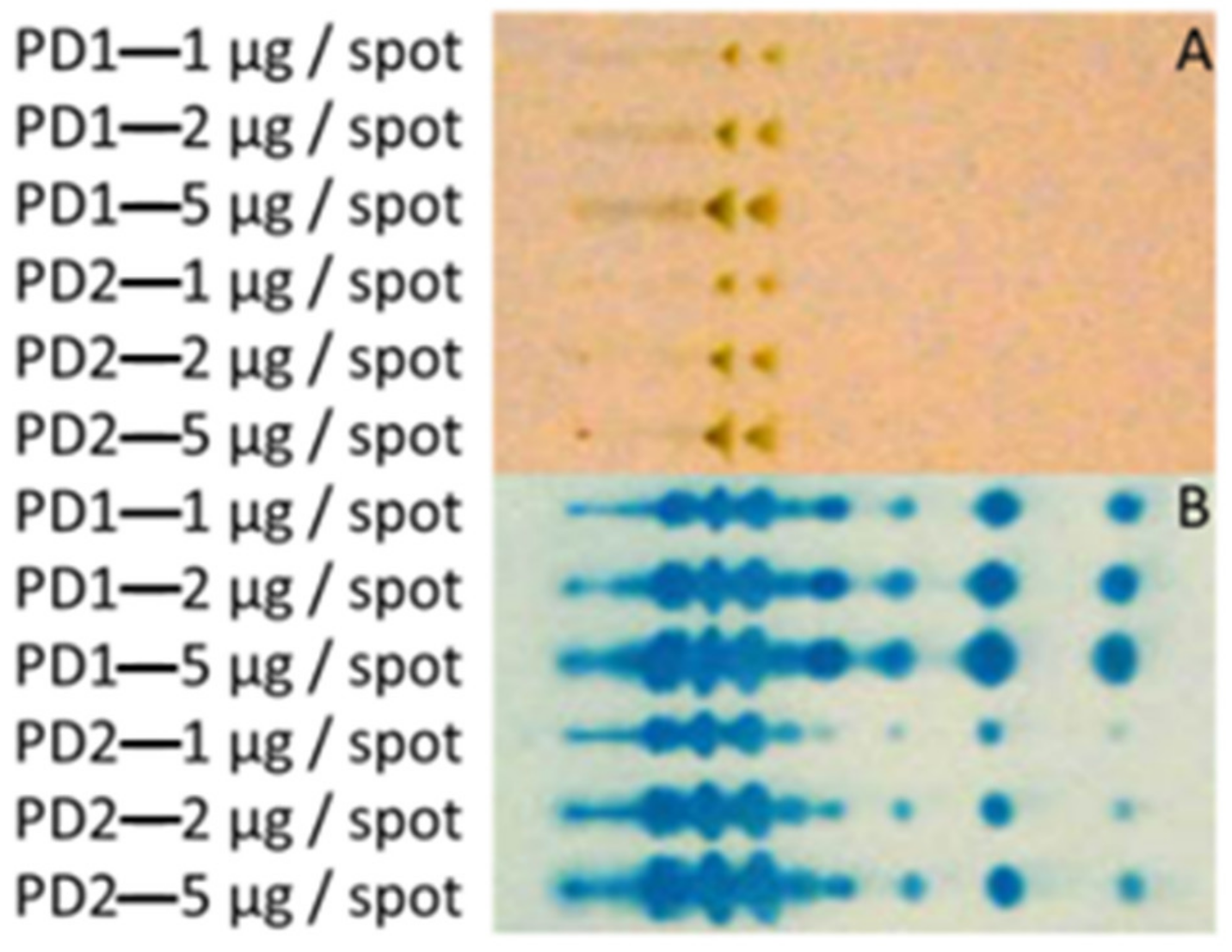
3.1. Extraction of Polar GLs and PIM Detection with an Improved Immune TLC
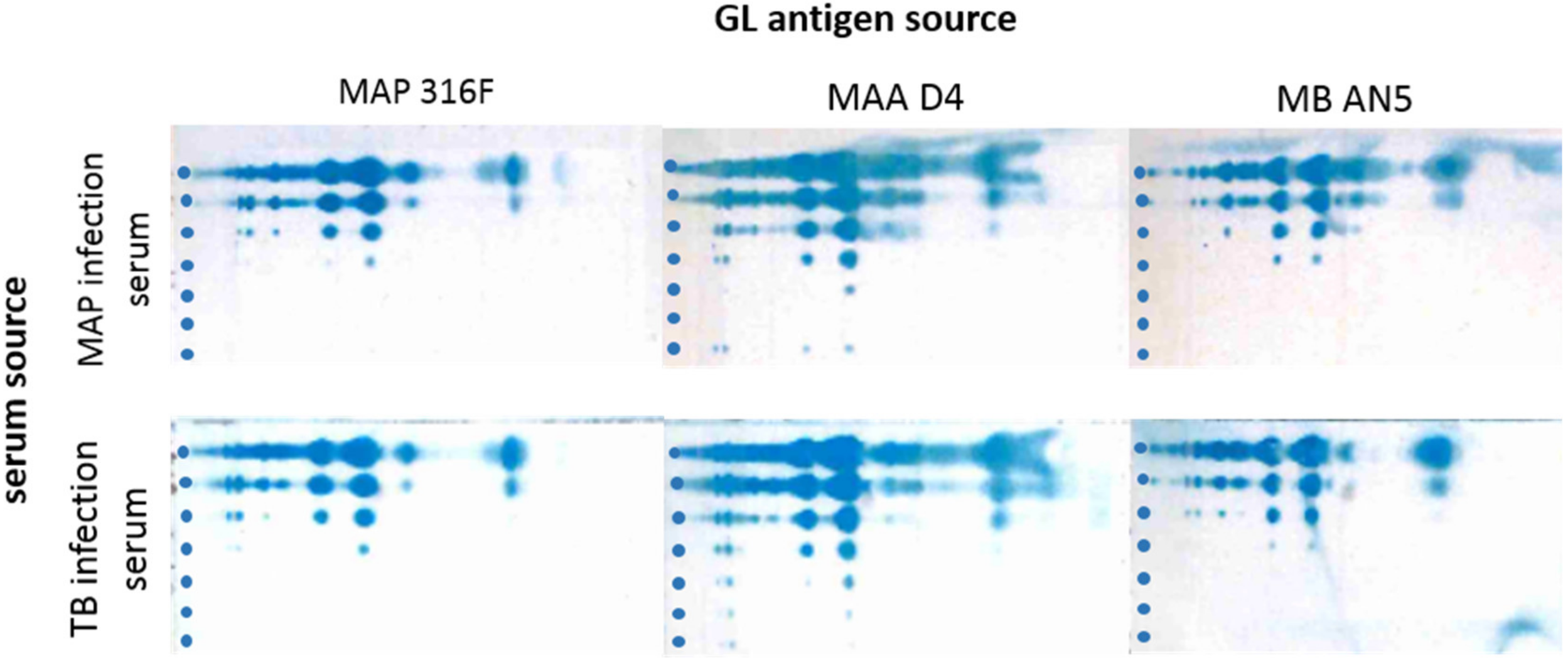
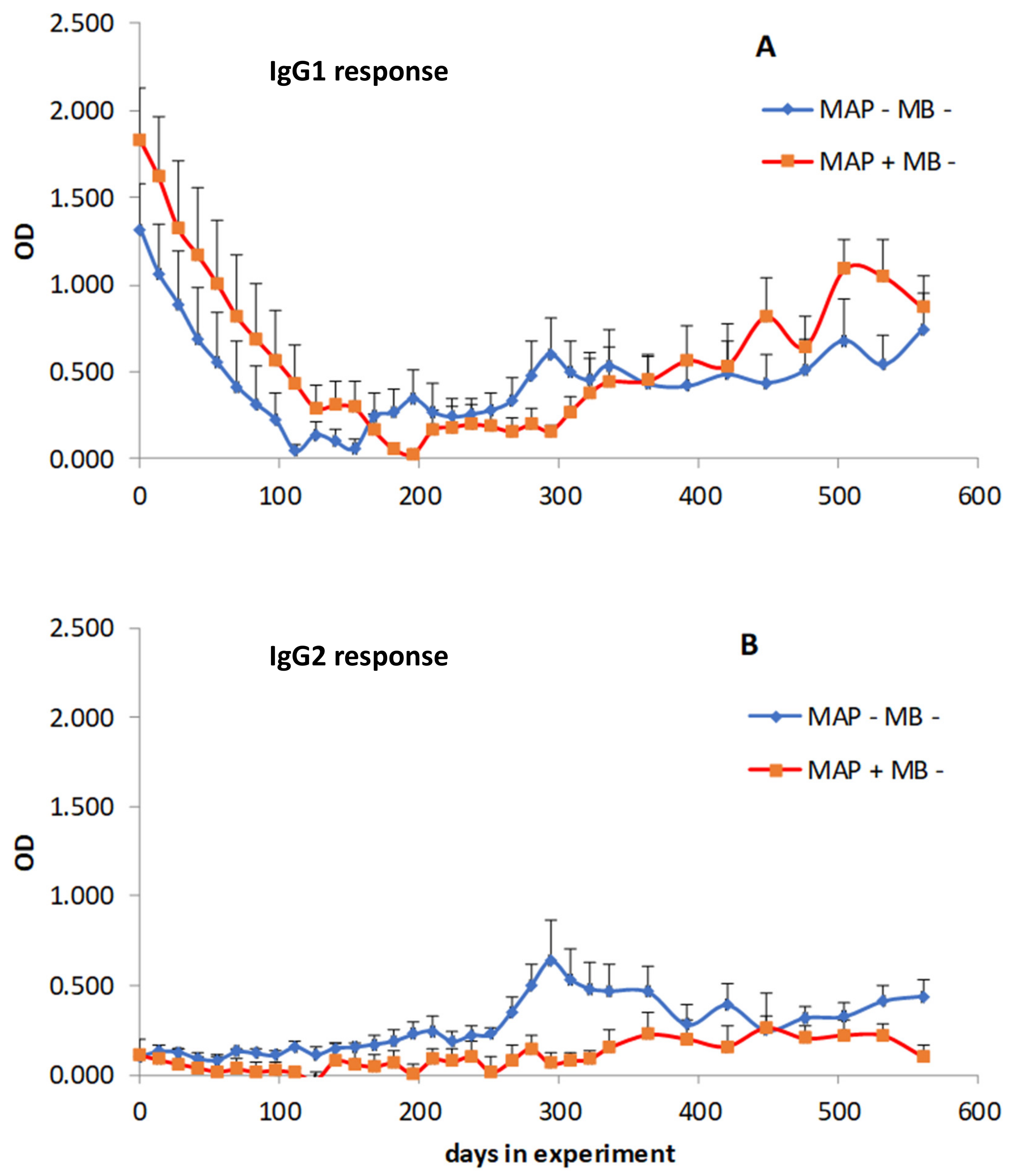
3.2. Cross-reactions of Immune Sera from MAP and MB Infected Cows
3.3. Development and Characterization of the PIM ELISA
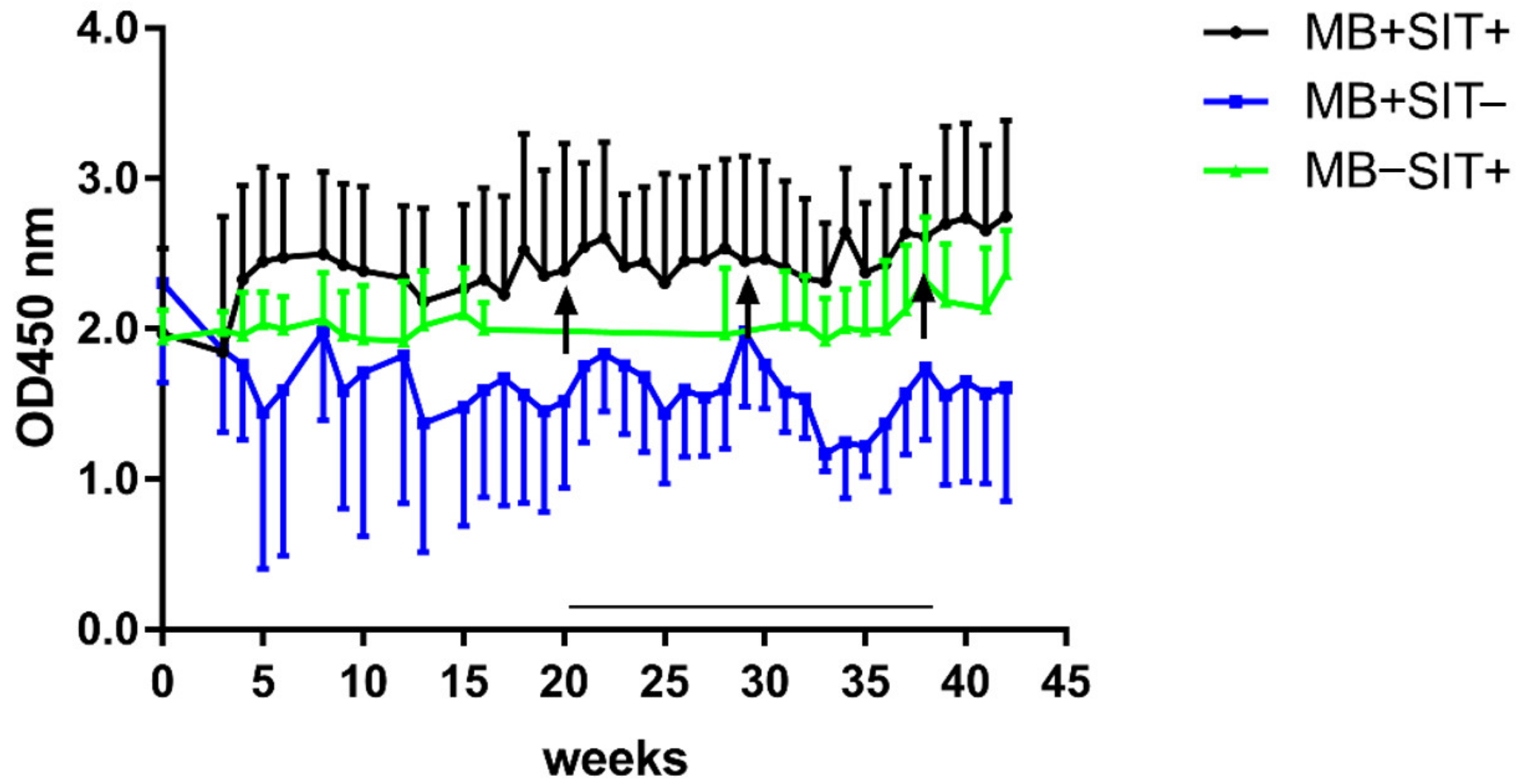
3.4. Effects of Skin Test and Mycobacterial Exposure on PIM Specific Antibody Responses in Cattle
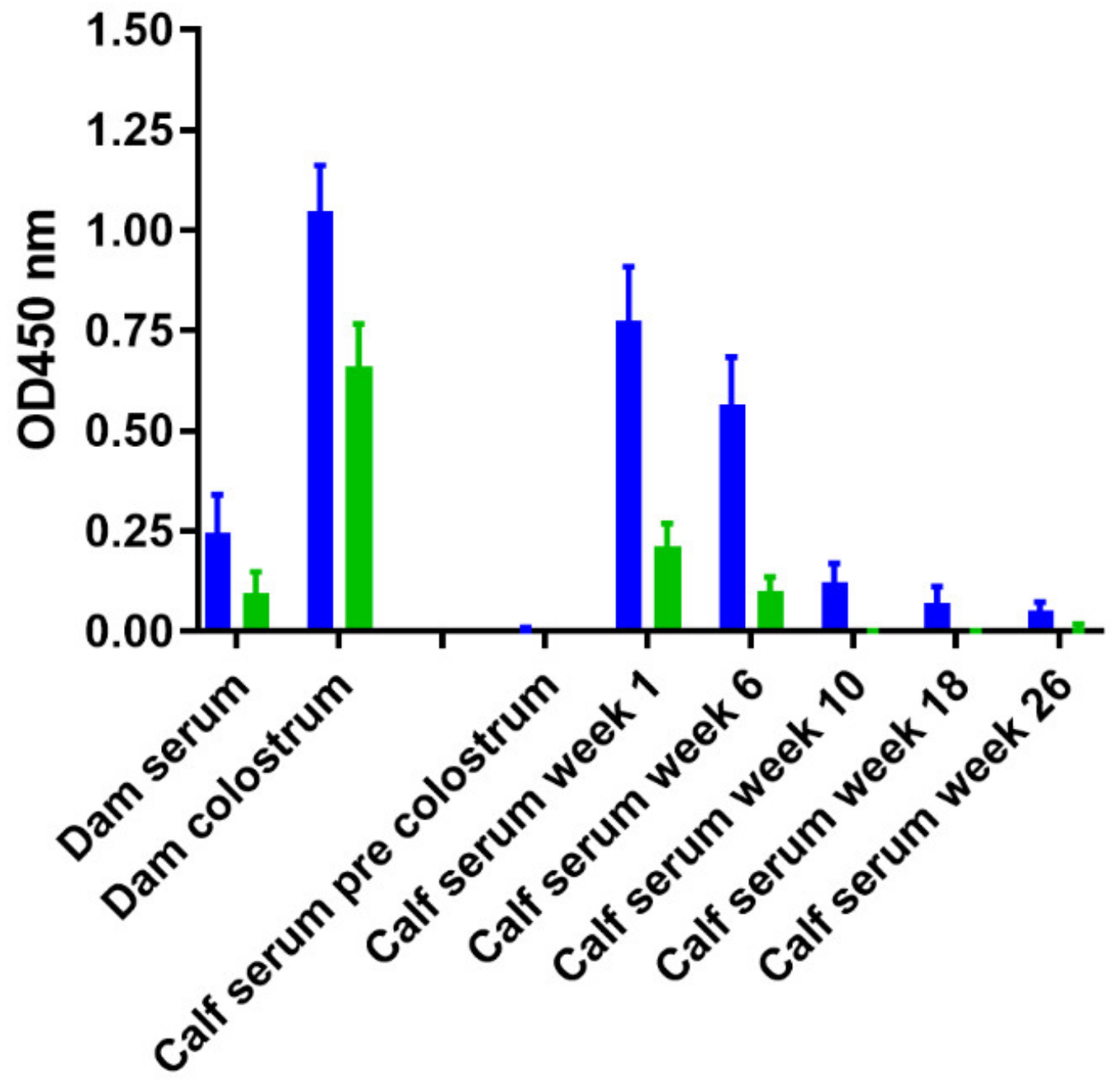
3.5. Effect of Age and M. Avium Subsp. Paratuberculosis Infection Status on PIM Reactivity
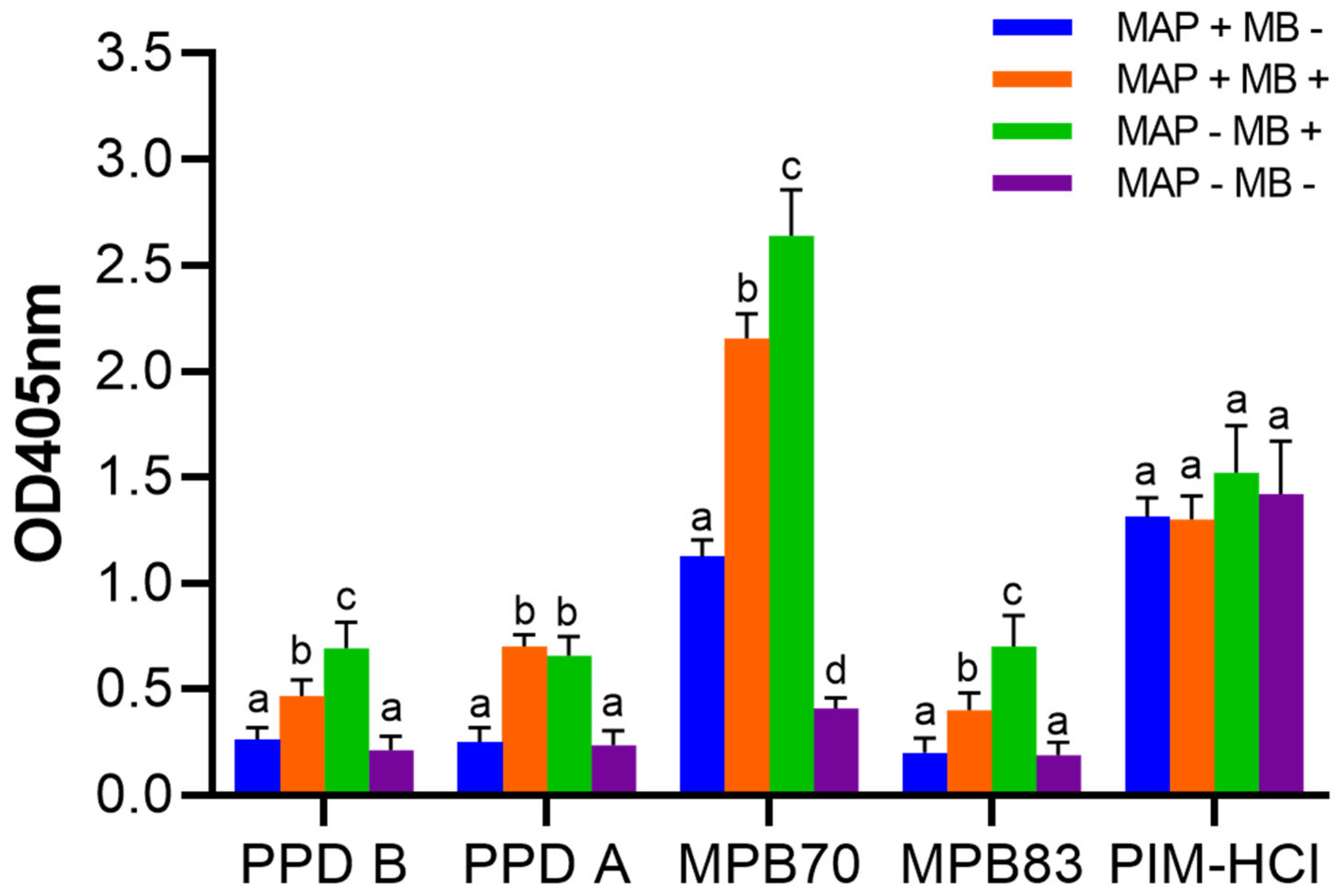
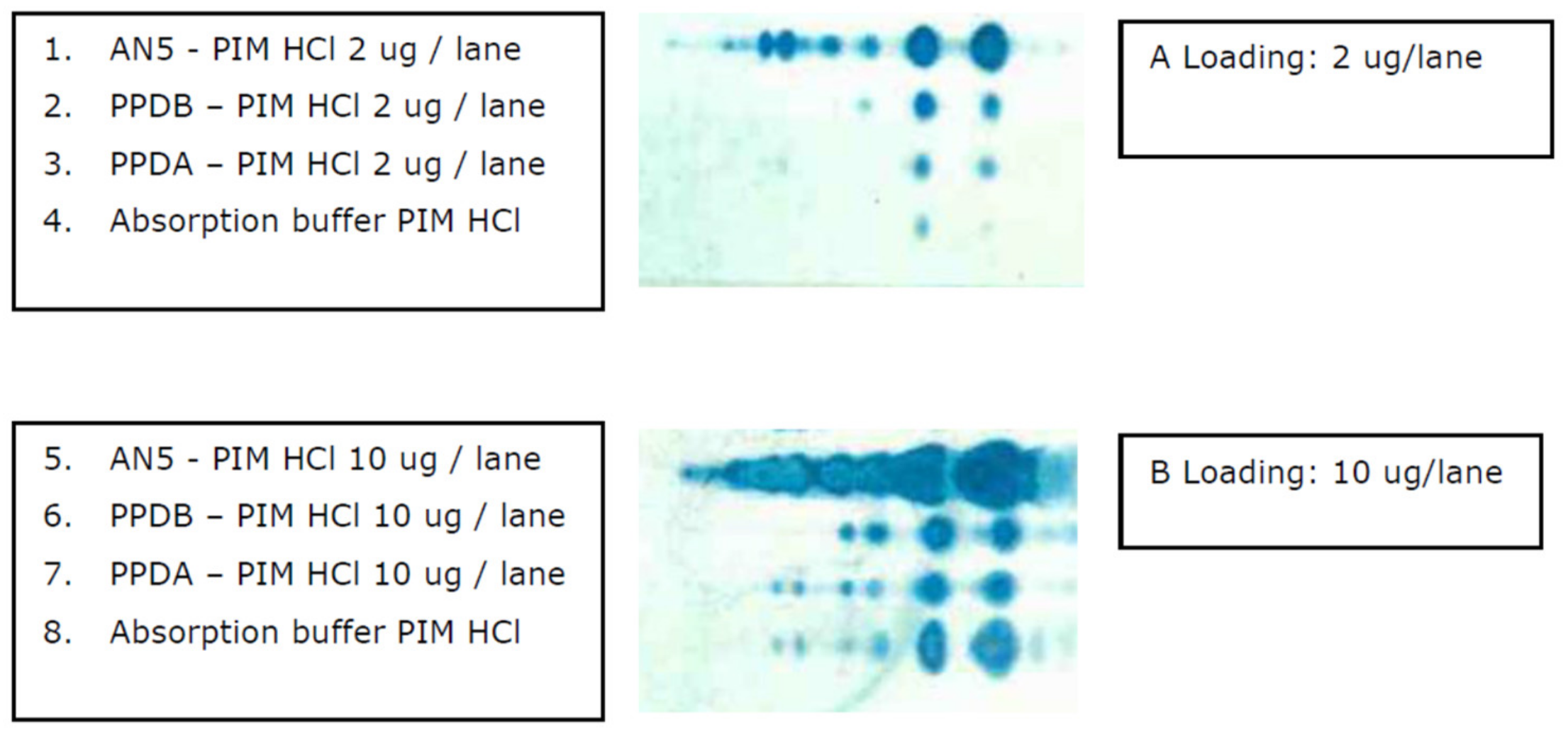
3.6. The Presence of PIM in Commonly used Mycobacterial Antigen Preparations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- More, S.; Botner, A.; Butterworth, A.; Calistri, P.; Depner, K.; Edwards, S.; Garin-Bastuji, B.; Good, M.; Schmidt, C.G.; Michel, V.; et al. Assessment of listing and categorisation of animal diseases within the framework of the animal health law (regulation (eu) no2016/429): Bovine tuberculosis. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
- Gormley, E.; Doyle, M.; Duignan, A.; Good, M.; More, S.J.; Clegg, T.A. Identification of risk factors associated with disclosure of false positive bovine tuberculosis reactors using the gamma-interferon (ifn gamma) assay. Vet. Res. 2013, 44, 117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Biet, F.; Boschiroli, M.L. Non-tuberculous mycobacterial infections of veterinary relevance. Res. Vet. Sci. 2014, 97, S69–S77. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, R.H.; Buergelt, C. Preclinical and clinical manifestations of paratuberculosis (including pathology). Vet. Clin. N. Am.-Food A 1996, 12, 345. [Google Scholar] [CrossRef]
- Bezos, J.; Casal, C.; Romero, B.; Schroeder, B.; Hardegger, R.; Raeber, A.J.; Lopez, L.; Rueda, P.; Dominguez, L. Current ante-mortem techniques for diagnosis of bovine tuberculosis. Res. Vet. Sci. 2014, 97, S44–S52. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.A. Review of the diagnosis and study of tuberculosis in non-bovine wildlife species using immunological methods. Transbound. Emerg. Dis. 2009, 56, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.O.; Gormley, E.; Gcebe, N.; Fosgate, G.T.; Conan, A.; Aagaard, C.; Michel, A.L.; Rutten, V.P.M.G. Cross reactive immune responses in cattle arising from exposure to mycobacterium bovis and non-tuberculous mycobacteria. Prev. Vet. Med. 2018, 152, 16–22. [Google Scholar] [CrossRef]
- Milner, A.R.; Mack, W.N.; Coates, K.J.; Hill, J.; Gill, I.; Sheldrick, P. The sensitivity and specificity of a modified elisa for the diagnosis of johnes disease from a field trial in cattle. Vet. Microbiol. 1990, 25, 193–198. [Google Scholar] [CrossRef]
- Bechnielsen, S.; Jorgensen, J.B.; Ahrens, P.; Feld, N.C. Diagnostic-accuracy of a mycobacterium-phlei-absorbed serum enzyme-linked-immunosorbent-assay for diagnosis of bovine paratuberculosis in dairy-cows. J. Clin. Microbiol. 1992, 30, 613–618. [Google Scholar]
- Scott, M.C.; Bannantine, J.P.; Kaneko, Y.; Branscum, A.J.; Whitlock, R.H.; Mori, Y.; Speer, C.A.; Eda, S. Absorbed evelisa: A diagnostic test with improved specificity for johne’s disease in cattle. Foodborne Pathog. Dis. 2010, 7, 1291–1296. [Google Scholar] [CrossRef]
- Nunez-Garcia, J.; Downs, S.H.; Parry, J.E.; Abernethy, D.A.; Broughan, J.M.; Cameron, A.R.; Cook, A.J.; de la Rua-Domenech, R.; Goodchild, A.V.; Gunn, J.; et al. Meta-analyses of the sensitivity and specificity of ante-mortem and post-mortem diagnostic tests for bovine tuberculosis in the uk and ireland. Prev. Vet. Med. 2018, 153, 94–107. [Google Scholar] [CrossRef] [PubMed]
- McKenna, S.L.B.; Keefe, G.P.; Barkema, H.W.; Sockett, D.C. Evaluation of three elisas for mycobacterium avium subsp paratuberculosis using tissue and fecal culture as comparison standards. Vet. Microbiol. 2005, 110, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Chiaradia, L.; Lefebvre, C.; Parra, J.; Marcoux, J.; Burlet-Schiltz, O.; Etienne, G.; Tropis, M.; Daffe, M. Dissecting the mycobacterial cell envelope and defining the composition of the native mycomembrane. Sci. Rep.-UK 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Daffe, M.; Crick, D.C.; Jackson, M. Genetics of capsular polysaccharides and cell envelope (glyco) lipids. Microbiol. Spectr. 2014, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Bonham, A.J.; Chan, E.D.; Honda, J.R. A paucity of knowledge regarding nontuberculous mycobacterial lipids compared to the tubercle bacillus. Tuberculosis 2019, 115, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.S.; Fukuda, T.; Sena, C.B.; Yamaryo-Botte, Y.; McConville, M.J.; Kinoshita, T. Inositol lipid metabolism in mycobacteria: Biosynthesis and regulatory mechanisms. Biochim. Biophys. Acta 2011, 1810, 630–641. [Google Scholar] [CrossRef]
- Goren, M.B. Biosynthesis and structure of phospholipids and sulfatides. In The Mycobacteria: A Sourcebook, Part A, 1st ed.; Kubica, G.P., Wayne, L.G., Eds.; Marcel Dekker: New York, NY, USA, 1984; pp. 379–416. [Google Scholar]
- Beatty, W.L.; Rhoades, E.R.; Ullrich, H.J.; Chatterjee, D.; Heuser, J.E.; Russell, D.G. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic (Cph. Den.) 2000, 1, 235–247. [Google Scholar] [CrossRef]
- Vergne, I.; Fratti, R.A.; Hill, P.J.; Chua, J.; Belisle, J.; Deretic, V. Mycobacterium tuberculosis phagosome maturation arrest: Mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol. Biol. Cell 2004, 15, 751–760. [Google Scholar] [CrossRef]
- Villeneuve, C.; Gilleron, M.; Maridonneau-Parini, I.; Daffe, M.; Astarie-Dequeker, C.; Etienne, G. Mycobacteria use their surface-exposed glycolipids to infect human macrophages through a receptor-dependent process. J. Lipid Res. 2005, 46, 475–483. [Google Scholar] [CrossRef]
- Sieling, P.A.; Chatterjee, D.; Porcelli, S.A.; Prigozy, T.I.; Mazzaccaro, R.J.; Soriano, T.; Bloom, B.R.; Brenner, M.B.; Kronenberg, M.; Brennan, P.J.; et al. Cd1-restricted t cell recognition of microbial lipoglycan antigens. Sci. (N.Y.) 1995, 269, 227–230. [Google Scholar] [CrossRef]
- Jones, B.W.; Means, T.K.; Heldwein, K.A.; Keen, M.A.; Hill, P.J.; Belisle, J.T.; Fenton, M.J. Different toll-like receptor agonists induce distinct macrophage responses. J. Leukoc. Biol. 2001, 69, 1036–1044. [Google Scholar] [PubMed]
- Driessen, N.N.; Ummels, R.; Maaskant, J.J.; Gurcha, S.S.; Besra, G.S.; Ainge, G.D.; Larsen, D.S.; Painter, G.F.; Vandenbroucke-Grauls, C.M.; Geurtsen, J.; et al. Role of phosphatidylinositol mannosides in the interaction between mycobacteria and dc-sign. Infect. Immun. 2009, 77, 4538–4547. [Google Scholar] [CrossRef] [PubMed]
- Toyonaga, K.; Torigoe, S.; Motomura, Y.; Kamichi, T.; Hayashi, J.M.; Morita, Y.S.; Noguchi, N.; Chuma, Y.; Kiyohara, H.; Matsuo, K.; et al. C-type lectin receptor dcar recognizes mycobacterial phosphatidyl-inositol mannosides to promote a th1 response during infection. Immunity 2016, 45, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Kallenius, G.; Correia-Neves, M.; Buteme, H.; Hamasur, B.; Svenson, S.B. Lipoarabinomannan, and its related glycolipids, induce divergent and opposing immune responses to mycobacterium tuberculosis depending on structural diversity and experimental variations. Tuberc. (Edinb. Scotl.) 2016, 96, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Doi, T.; Maekura, R.; Ito, M.; Yano, I. Differences in serological responses to specific glycopeptidolipid-core and common lipid antigens in patients with pulmonary disease due to mycobacterium tuberculosis and mycobacterium avium complex. J. Med. Microbiol. 2006, 55, 189–199. [Google Scholar] [CrossRef]
- Hur, Y.G.; Kim, A.; Kang, Y.A.; Kim, A.S.; Kim, D.Y.; Kim, Y.; Kim, Y.; Lee, H.; Cho, S.N. Evaluation of antigen-specific immunoglobulin g responses in pulmonary tuberculosis patients and contacts. J. Clin. Microbiol. 2015, 53, 904–909. [Google Scholar] [CrossRef]
- Sugden, E.A.; Corner, A.H.; Samagh, B.S.; Brooks, B.W.; Turcotte, C.; Nielsen, K.H.; Stewart, R.B.; Duncan, J.R. Serodiagnosis of ovine paratuberculosis, using lipoarabinomannan in an enzyme-linked immunosorbent assay. Am. J. Vet. Res. 1989, 50, 850–854. [Google Scholar]
- Koets, A.P.; Rutten, V.P.; de Boer, M.; Bakker, D.; Valentin-Weigand, P.; van Eden, W. Differential changes in heat shock protein-, lipoarabinomannan-, and purified protein derivative-specific immunoglobulin g1 and g2 isotype responses during bovine mycobacterium avium subsp. Paratuberculosis infection. Infect. Immun. 2001, 69, 1492–1498. [Google Scholar] [CrossRef]
- Ganusov, V.V.; Klinkenberg, D.; Bakker, D.; Koets, A.P. Evaluating contribution of the cellular and humoral immune responses to the control of shedding of mycobacterium avium spp. Paratuberculosis in cattle. Vet. Res. 2015, 46, 62. [Google Scholar] [CrossRef]
- Koets, A.; Hoek, A.; Langelaar, M.; Overdijk, M.; Santema, W.; Franken, P.; Eden, W.; Rutten, V. Mycobacterial 70 kd heat-shock protein is an effective subunit vaccine against bovine paratuberculosis. Vaccine 2006, 24, 2550–2559. [Google Scholar] [CrossRef]
- Merkal, R.S.; Curran, B.J. Growth and metabolic characteristics of mycobacterium paratuberculosis. Appl. Microbiol. 1974, 28, 276–279. [Google Scholar] [PubMed]
- Slayden, R.A.; Barry, C.E. Analysis of the lipids of mycobacterium tuberculosis. In Mycobacterium Tuberculosis Protocols; Parish, T., Stoker, G., Eds.; Humana Press: Totowa, NJ, USA, 2001; Volume 54, pp. 229–245. [Google Scholar]
- Van Zaane, D.; Ijzerman, J. Monoclonal antibodies against bovine immunoglobulins and their use in isotype-specific elisas for rotavirus antibody. J. Immunol. Methods 1984, 72, 427–441. [Google Scholar] [CrossRef]
- Willemsen, P.T.; Westerveen, J.; Dinkla, A.; Bakker, D.; van Zijderveld, F.G.; Thole, J.E. Secreted antigens of mycobacterium avium subspecies paratuberculosis as prominent immune targets. Vet. Microbiol. 2006, 114, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Van Rhijn, I.; Nguyen, T.K.; Michel, A.; Cooper, D.; Govaerts, M.; Cheng, T.Y.; van Eden, W.; Moody, D.B.; Coetzer, J.A.; Rutten, V.; et al. Low cross-reactivity of t-cell responses against lipids from mycobacterium bovis and m. Avium paratuberculosis during natural infection. Eur. J. Immunol. 2009, 39, 3031–3041. [Google Scholar] [CrossRef] [PubMed]
- Lyashchenko, K.; Whelan, A.O.; Greenwald, R.; Pollock, J.M.; Andersen, P.; Hewinson, R.G.; Vordermeier, H.M. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with mycobacterium bovis bcg and infected with m. Bovis. Infect. Immun. 2004, 72, 2462–2467. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.V.; Waters, W.R.; Thacker, T.C.; Greenwald, R.; Esfandiari, J.; Lyashchenko, K.P. Effects of different tuberculin skin-testing regimens on gamma interferon and antibody responses in cattle experimentally infected with mycobacterium bovis. Clin. Vaccine Immunol. CVI 2006, 13, 387–394. [Google Scholar] [CrossRef]
- Fournie, J.J.; Mullins, R.J.; Basten, A. Isolation and structural characteristics of a monoclonal antibody-defined cross-reactive phospholipid antigen from mycobacterium tuberculosis and mycobacterium leprae. J. Biol. Chem. 1991, 266, 1211–1219. [Google Scholar]
- Mullins, R.J.; Fournie, J.J.; Moloney, B.; Baumgart, K.; Jones, P.; Brown, P.; Basten, A. Serological response to purified mycobacterial phosphatidylinositol mannoside in healthy controls and in patients with tuberculosis and leprosy. Int. J. Lepr. Other Mycobact. Dis. Off. Organ Int. Lepr. Assoc. 1992, 60, 353–367. [Google Scholar]
- Correia-Neves, M.; Froberg, G.; Korshun, L.; Viegas, S.; Vaz, P.; Ramanlal, N.; Bruchfeld, J.; Hamasur, B.; Brennan, P.; Kallenius, G. Biomarkers for tuberculosis: The case for lipoarabinomannan. ERJ Open Res. 2019, 5, 115–2018. [Google Scholar] [CrossRef]
- Choudhary, A.; Patel, D.; Honnen, W.; Lai, Z.; Prattipati, R.S.; Zheng, R.B.; Hsueh, Y.C.; Gennaro, M.L.; Lardizabal, A.; Restrepo, B.I.; et al. Characterization of the antigenic heterogeneity of lipoarabinomannan, the major surface glycolipid of mycobacterium tuberculosis, and complexity of antibody specificities toward this antigen. J. Immunol. (Baltim. Md. 1950) 2018, 200, 3053–3066. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Toft, N. Effect of days in milk and milk yield on testing positive in milk antibody elisa to mycobacterium avium subsp. Paratuberculosis in dairy cattle. Vet. Immunol. Immunopathol. 2012, 149, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Zervens, L.M.; Nielsen, S.S.; Jungersen, G. Characterisation of an elisa detecting immunoglobulin g to mycobacterium avium subsp. Paratuberculosis in bovine colostrum. Vet. J. (Lond. Engl. 1997) 2013, 197, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Roperto, S.; Varano, M.; Russo, V.; Luca, R.; Cagiola, M.; Gaspari, M.; Ceccarelli, D.M.; Cuda, G.; Roperto, F. Proteomic analysis of protein purified derivative of mycobacterium bovis. J. Transl. Med. 2017, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Infantes-Lorenzo, J.A.; Moreno, I.; Risalde, M.L.A.; Roy, A.; Villar, M.; Romero, B.; Ibarrola, N.; de la Fuente, J.; Puentes, E.; de Juan, L.; et al. Proteomic characterisation of bovine and avian purified protein derivatives and identification of specific antigens for serodiagnosis of bovine tuberculosis. Clin. Proteom. 2017, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Osterstock, J.B.; Fosgate, G.T.; Norby, B.; Manning, E.J.; Collins, M.T.; Roussel, A.J. Contribution of environmental mycobacteria to false-positive serum elisa results for paratuberculosis. J. Am. Vet. Medical. Assoc. 2007, 230, 896–901. [Google Scholar] [CrossRef]
- Pirson, C.; Engel, R.; Jones, G.J.; Holder, T.; Holst, O.; Vordermeier, H.M. Highly purified mycobacterial phosphatidylinositol mannosides drive cell-mediated responses and activate nkt cells in cattle. Clin. Vaccine Immunol. CVI 2015, 22, 178–184. [Google Scholar] [CrossRef]
| Number | Infection Status (*) | Experimental or Natural Infection | Number of Cows | Serum Samples (n) | Duration of Study | Reference |
|---|---|---|---|---|---|---|
| 1 | MAP + MB − | experimental | 20 | 700 | 4 years | [30] |
| 2 | MAP + MB − | experimental | 10 + 10 # | 350 | 2 years | [31] |
| 3 | MAP – MB − | n/a (controls) | 10 + 10 # | 350 | 2 years | [31] |
| 4 | MAP − MB + | experimental | 9 | 120 | 20 weeks | unpublished |
| 5 | MAP − MB − | n/a (controls) | 5 | 120 | 20 weeks | unpublished |
| 6 | MAP − MB + | natural | 69 | 69 | Diagnostic | Serum biobank |
| 7 | MAP − MB − | n/a (controls) | 30 | 30 | Diagnostic % | Serum biobank |
| 8 | MAP + MB − | natural | 254 | 254 | Diagnostic | Serum biobank |
| 9 | MAP + MB + | natural | 147 | 147 | Diagnostic | Serum biobank |
| Number | Reagent | Species of Origin |
|---|---|---|
| NR-14846 | Purified Phosphatidylinositol mannosides (PIM) 1 and 2 H37Rv | M. tuberculosis |
| NR-14844 | Purified Trehalose Dimycolate H37Rv | M. tuberculosis |
| NR-14845 | Purified Sulfolipid-1 H37Rv | M. tuberculosis |
| NR-14847 | Purified Phosphatidylinositol mannosides (PIM) 6 H37Rv | M. tuberculosis |
| NR-14848 | Purified Lipoarabinomannan (LAM) H37Rv | M. tuberculosis |
| NR-14850 | Purified Lipomannan (LM) H37Rv | M. tuberculosis |
| NR-20328 | Purified dimycocerosate H37Rv | M. tuberculosis |
| NR-48784 | Purified trehalose monomycolate (TMM) H37Rv | M. tuberculosis |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koets, A.P.; van den Esker, M.H.; Riepema, K.; Bakker, D. The Role of Phosphatidylinositol Mannosides in the Serological Diagnosis of Mycobacterial Infections. Vet. Sci. 2019, 6, 91. https://doi.org/10.3390/vetsci6040091
Koets AP, van den Esker MH, Riepema K, Bakker D. The Role of Phosphatidylinositol Mannosides in the Serological Diagnosis of Mycobacterial Infections. Veterinary Sciences. 2019; 6(4):91. https://doi.org/10.3390/vetsci6040091
Chicago/Turabian StyleKoets, Ad P., Marielle H. van den Esker, Karel Riepema, and Douwe Bakker. 2019. "The Role of Phosphatidylinositol Mannosides in the Serological Diagnosis of Mycobacterial Infections" Veterinary Sciences 6, no. 4: 91. https://doi.org/10.3390/vetsci6040091
APA StyleKoets, A. P., van den Esker, M. H., Riepema, K., & Bakker, D. (2019). The Role of Phosphatidylinositol Mannosides in the Serological Diagnosis of Mycobacterial Infections. Veterinary Sciences, 6(4), 91. https://doi.org/10.3390/vetsci6040091





