Detection and Molecular Characterization of a Natural Coinfection of Marek’s Disease Virus and Reticuloendotheliosis Virus in Brazilian Backyard Chicken Flock
Abstract
1. Introduction
2. Materials and Methods
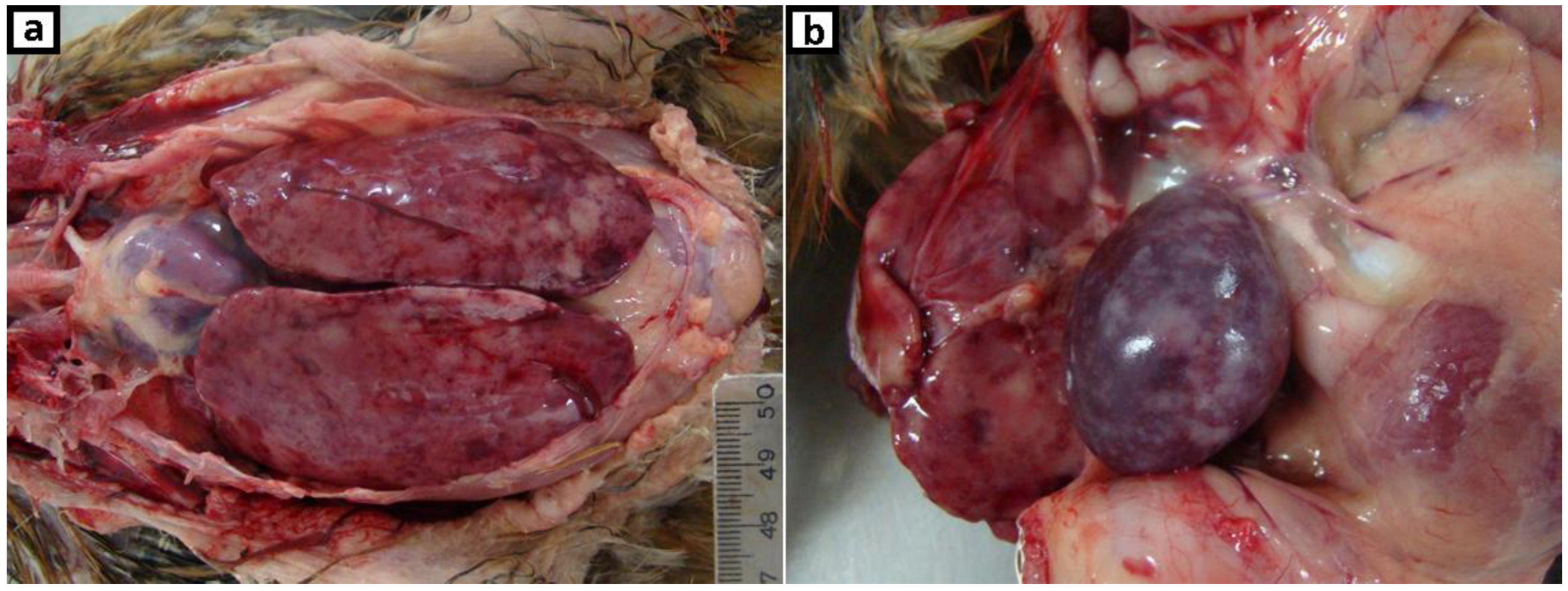
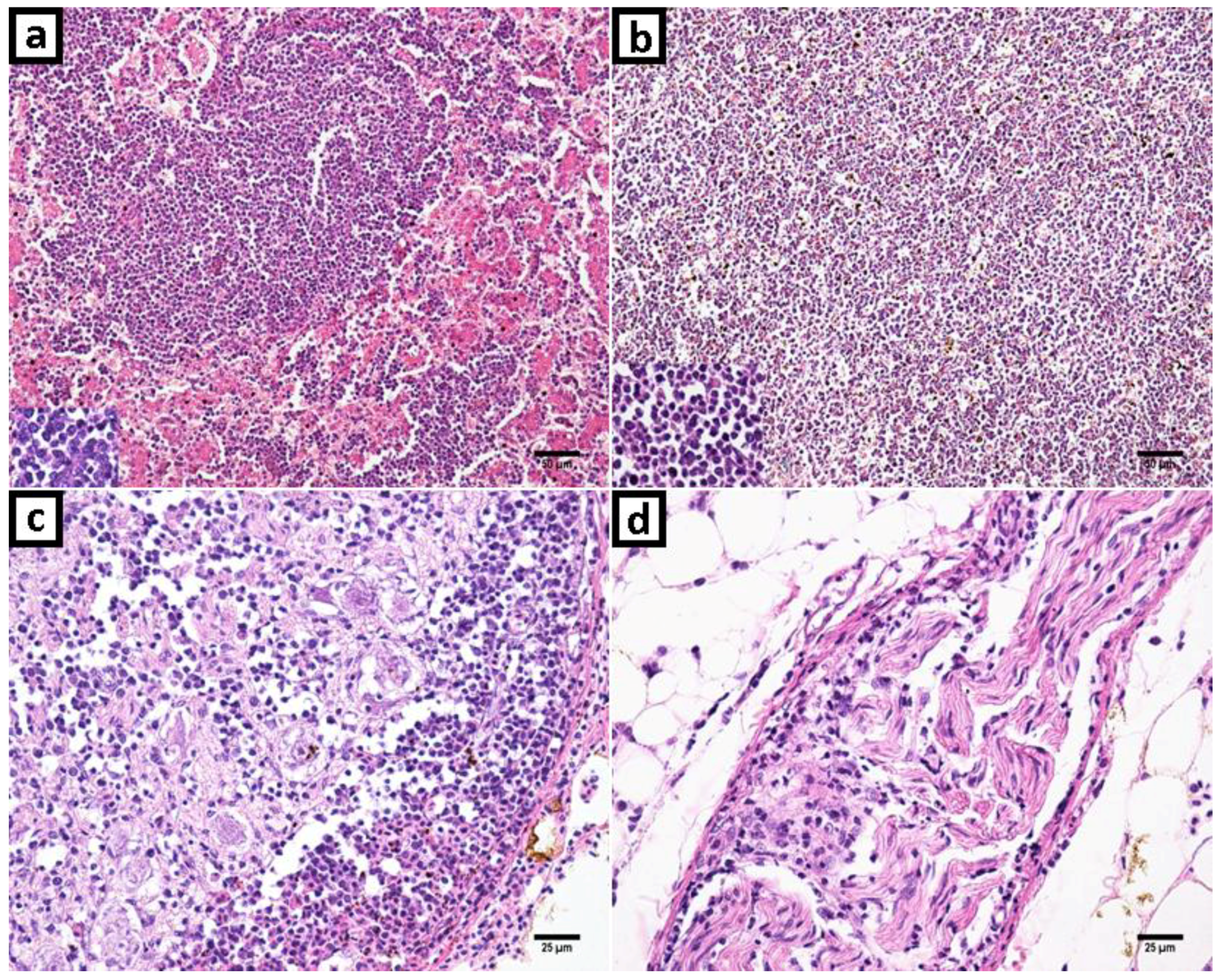
2.1. Clinical History, Gross Lesions, and Histopathologic Examination
2.2. Detection of MDV and REV Viruses through PCR Examination
2.3. Real-Time PCR to Detect and Differentiate CVI988 from the Field MDV Serotype
2.4. PCR Amplification of the Glycoprotein B Gene of the MDV Serotypes
2.5. PCR for the Gag, Polymerase, and Envelope Genes of REV
2.6. Sequencing and Phylogenetic Analysis
3. Results
3.1. Histopathologic Examination
3.2. Detection of MDV, REV and ALV through PCR examination
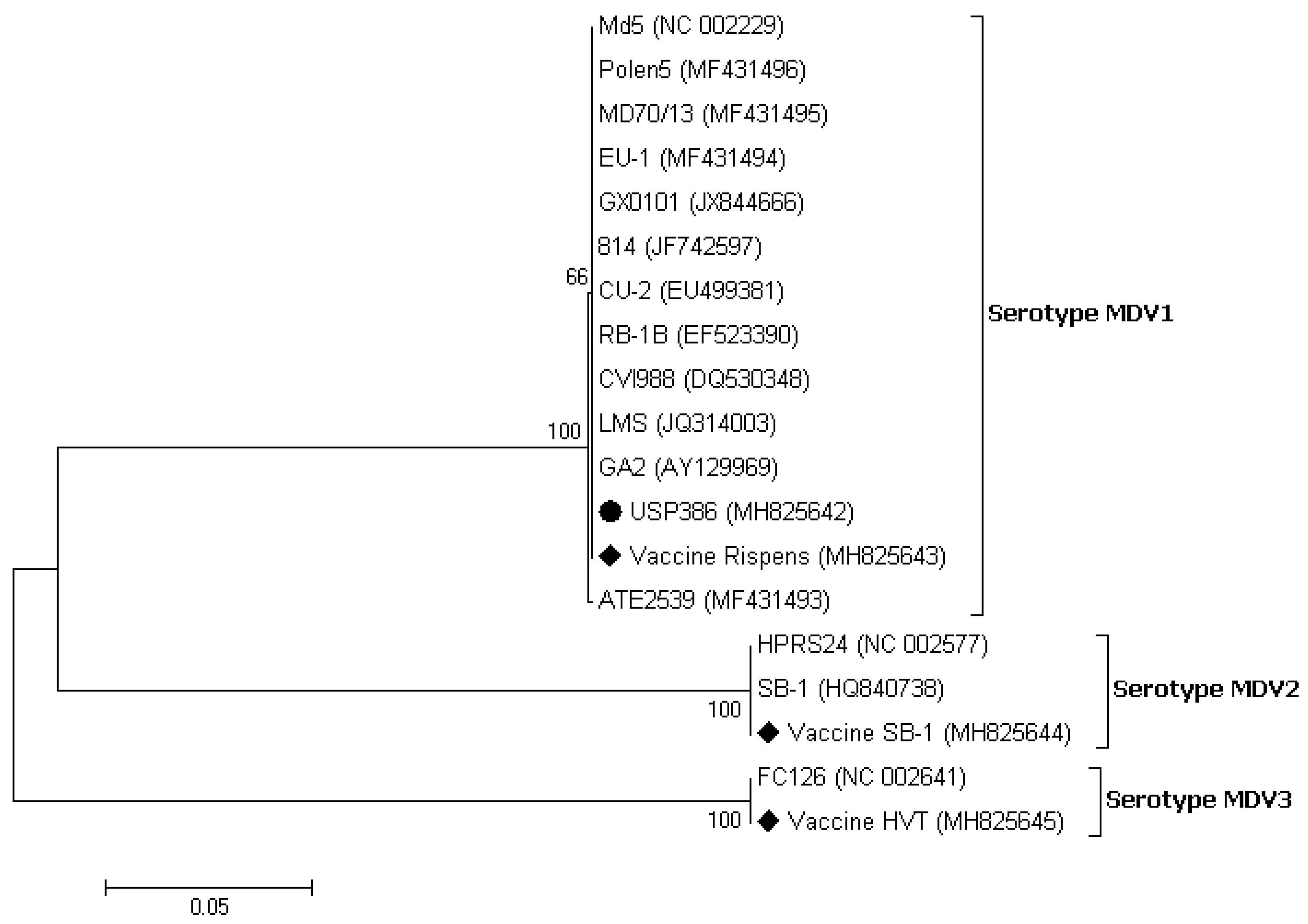
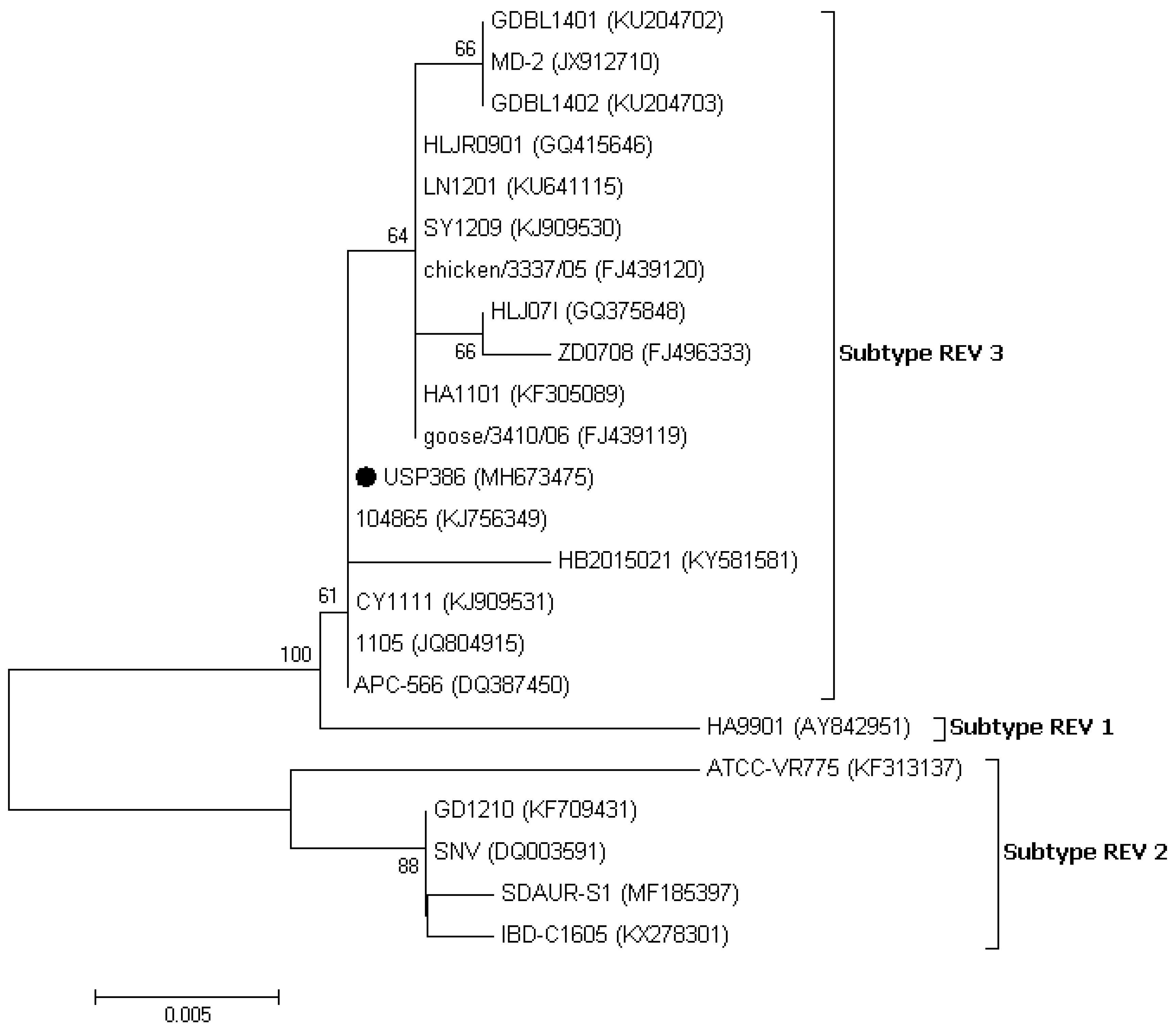
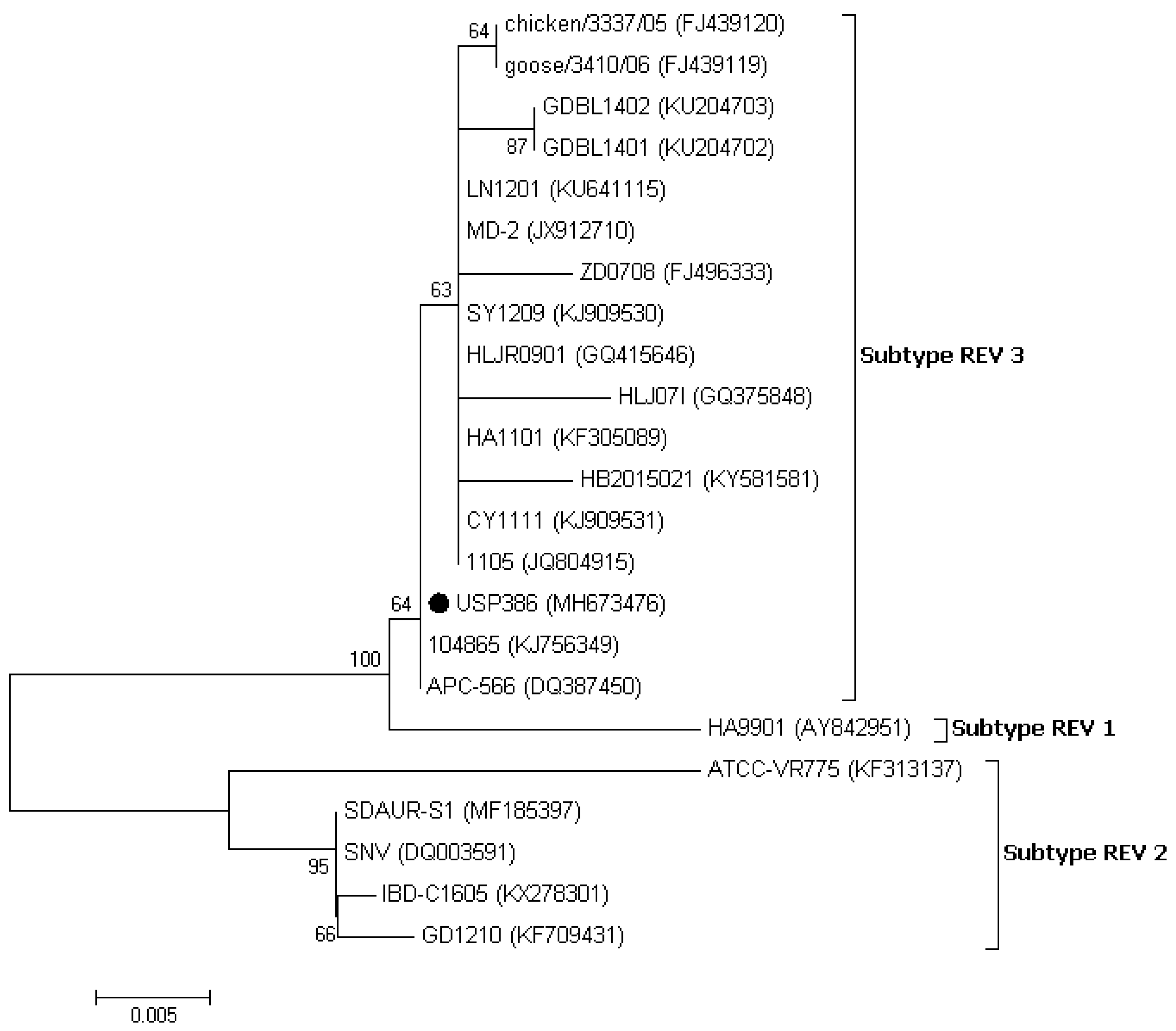
3.3. Sequence and Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schat, K.A.; Nair, V. Marek´s Disease. In Diseases of Poultry, 13th ed.; Swayne, D.E., Glisson, J.R., McDougald, L.R., Nolan, L.K., Suarez, D.L., Nair, V., Eds.; John Wiley & Sons, Inc.: Ames, IA, USA, 2013; pp. 515–552. [Google Scholar]
- Nair, V.; Fadly, A.M. Leukosis/Sarcoma Group. In Diseases of Poultry, 13th ed.; Swayne, D.E., Glisson, J.R., McDougald, L.R., Nolan, L.K., Suarez, D.L., Nair, V., Eds.; John Wiley & Sons, Inc.: Ames, IA, USA, 2013; pp. 553–592. [Google Scholar]
- Nair, V.; Zavala, G.; Fadly, A.M. Reticuloendotheliosis. In Diseases of Poultry, 13th ed.; Swayne, D.E., Glisson, J.R., McDougald, L.R., Nolan, L.K., Suarez, D.L., Nair, V., Eds.; John Wiley & Sons, Inc.: Ames, IA, USA, 2013; pp. 593–604. [Google Scholar]
- Adams, M.J.; Lefkowitz, E.J.; King, A.M.Q.; Harrach, B.; Harrison, R.L.; Knowles, N.J.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Mushegian, A.R.; et al. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses. Arch. Virol. 2016, 161, 2921–2949. [Google Scholar] [CrossRef] [PubMed]
- OIE (Office International des Epizooties). Marek´s disease. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 8th ed.; World Organization for Animal Health (WOAH): Paris, France, 2018; Volume 1–3, pp. 952–963. [Google Scholar]
- Chen, P.Y.; Cui, Z.; Lee, L.F.; Witter, R.L. Serologic differences among nondefective reticuloendotheliosis viruses. Arch. Virol. 1987, 93, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Mays, J.; Kiupel, M.; Dunn, J.R. Development of reliable techniques for the differential diagnosis of avian tumour viruses by immunohistochemistry and polymerase chain reaction from formalin-fixed paraffin-embedded tissue sections. Avian Pathol. 2018, 47, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Mays, J.; Dunn, J.; Fulton, R.; Silva, R.; Fadly, A. Use of polymerase chain reaction in detection of Marek’s disease and reticuloendotheliosis viruses in formalin-fixed, paraffin-embedded tumorous tissues. Avian Dis. 2013, 57, 785–789. [Google Scholar] [CrossRef]
- Bao, K.; Zhang, Y.; Zheng, H.; Lv, H.; Gao, Y.; Wang, J.; Gao, H.; Qi, X.; Cui, H.; Wang, Y.; et al. Isolation and full-genome sequence of two reticuloendotheliosis virus strains from mixed infections with Marek’s disease virus in China. Virus Genes 2015, 50, 418–424. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Bao, K.-Y.; Sun, G.-R.; Lv, H.-C.; Cui, H.-Y.; Gao, Y.-L.; Wang, X.-M.; Liu, C.-J. Characterization of a Gallid herpesvirus 2 strain with novel reticuloendotheliosis virus long terminal repeat inserts. Virus Genes 2017, 53, 386–391. [Google Scholar] [CrossRef]
- García, M.; Narang, N.; Reed, W.M.; Fadly, A.M. Molecular characterization of reticuloendotheliosis virus insertions in the genome of field and vaccine strains of fowl poxvirus. Avian Dis. 2003, 47, 343–354. [Google Scholar] [CrossRef]
- Hertig, C.; Coupar, B.E.; Gould, A.R.; Boyle, D.B. Field and vaccine strains of fowlpox virus carry integrated sequences from the avian retrovirus, reticuloendotheliosis virus. Virology 1997, 235, 367–376. [Google Scholar] [CrossRef]
- Cui, Z.; Zhuang, G.; Xu, X.; Sun, A.; Su, S. Molecular and biological characterization of a Marek’s disease virus field strain with reticuloendotheliosis virus LTR insert. Virus Genes 2010, 40, 236–243. [Google Scholar] [CrossRef]
- Sun, A.-J.; Xu, X.-Y.; Petherbridge, L.; Zhao, Y.-G.; Nair, V.; Cui, Z.-Z. Functional evaluation of the role of reticuloendotheliosis virus long terminal repeat (LTR) integrated into the genome of a field strain of Marek’s disease virus. Virology 2010, 397, 270–276. [Google Scholar] [CrossRef]
- Sun, G.-R.; Zhang, Y.-P.; Zhou, L.-Y.; Lv, H.-C.; Zhang, F.; Li, K.; Gao, Y.-L.; Qi, X.-L.; Cui, H.-Y.; Wang, Y.-Q.; et al. Co-Infection with Marek’s Disease Virus and Reticuloendotheliosis Virus Increases Illness Severity and Reduces Marek’s Disease Vaccine Efficacy. Viruses 2017, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Salas, E.; Icochea, E.; González, R.; Falcón, N. Evidencia serológica de anticuerpos contra el virus de la reticuloendotheliosis en gallinas reproductoras de Lima. Rev.de Investig. Vet. del Perú 2005, 16, 187–190. [Google Scholar]
- Buscaglia, C. Mixed infections of Marek’s disease and reticuloendotheliosis viruses in layer flocks in Argentina. Avian Dis. 2013, 57, 569–571. [Google Scholar] [CrossRef]
- García, M.; El-Attrache, J.; Riblet, S.M.; Lunge, V.R.; Fonseca, A.S.K.; Villegas, P.; Ikuta, N. Development and application of reverse transcriptase nested polymerase chain reaction test for the detection of exogenous avian leukosis virus. Avian Dis. 2003, 47, 41–53. [Google Scholar] [CrossRef]
- Gimeno, I.M.; Dunn, J.R.; Cortes, A.L.; El-Gohary, A.E.-G.; Silva, R.F. Detection and differentiation of CVI988 (Rispens vaccine) from other serotype 1 Marek’s disease viruses. Avian Dis. 2014, 58, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Miles, A.M.; Reddy, S.M.; Morgan, R.W. Coinfection of specific-pathogen-free chickens with Marek’s disease virus (MDV) and chicken infectious anemia virus: Effect of MDV pathotype. Avian Dis. 2001, 45, 9–18. [Google Scholar] [CrossRef]
- Wen, Y.; Huang, Q.; Yang, C.; Pan, L.; Wang, G.; Qi, K.; Liu, H. Characterizing the histopathology of natural co-infection with Marek’s disease virus and subgroup J avian leucosis virus in egg-laying hens. Avian Pathol. 2018, 47, 83–89. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L.; Sun, S. Natural Infection with Avian Hepatitis E Virus and Marek’s Disease Virus in Brown Layer Chickens in China. Avian Dis. 2016, 60, 698–704. [Google Scholar] [CrossRef]
- Afonso, C.L.; Tulman, E.R.; Lu, Z.; Zsak, L.; Rock, D.L.; Kutish, G.F. The genome of turkey herpesvirus. J. Virol. 2001, 75, 971–978. [Google Scholar] [CrossRef]
- Izumiya, Y.; Jang, H.K.; Ono, M.; Mikami, T. A complete genomic DNA sequence of Marek’s disease virus type 2, strain HPRS24. Curr. Top. Microbiol. Immunol. 2001, 255, 191–221. [Google Scholar]
- Spatz, S.J.; Petherbridge, L.; Zhao, Y.; Nair, V. Comparative full-length sequence analysis of oncogenic and vaccine (Rispens) strains of Marek’s disease virus. J. Gen. Virol. 2007, 88, 1080–1096. [Google Scholar] [CrossRef] [PubMed]
- Spatz, S.J.; Schat, K.A. Comparative genomic sequence analysis of the Marek’s disease vaccine strain SB-1. Virus Genes 2011, 42, 331–338. [Google Scholar] [CrossRef]
- Tulman, E.R.; Afonso, C.L.; Lu, Z.; Zsak, L.; Rock, D.L.; Kutish, G.F. The genome of a very virulent Marek’s disease virus. J. Virol. 2000, 74, 7980–7988. [Google Scholar] [CrossRef]
- Shamblin, C.E.; Greene, N.; Arumugaswami, V.; Dienglewicz, R.L.; Parcells, M.S. Comparative analysis of Marek’s disease virus (MDV) glycoprotein-, lytic antigen pp38- and transformation antigen Meq-encoding genes: Association of meq mutations with MDVs of high virulence. Vet. Microbiol. 2004, 102, 147–167. [Google Scholar] [CrossRef]
- Yoshida, S.; Lee, L.F.; Yanagida, N.; Nazerian, K. The glycoprotein B genes of Marek’s disease virus serotypes 2 and 3: Identification and expression by recombinant fowlpox viruses. Virology 1994, 200, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, T.; Zavala, G.; Cheng, S.; Villegas, P. Full genome sequence and some biological properties of reticuloendotheliosis virus strain APC-566 isolated from endangered Attwater’s prairie chickens. Virus Res. 2007, 124, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Bohls, R.L.; Linares, J.A.; Gross, S.L.; Ferro, P.J.; Silvy, N.J.; Collisson, E.W. Phylogenetic analyses indicate little variation among reticuloendotheliosis viruses infecting avian species, including the endangered Attwater’s prairie chicken. Virus Res. 2006, 119, 187–194. [Google Scholar] [CrossRef]
- Li, Y.; Cui, S.; Cui, Z.; Chang, S.; Zhao, P. Genome analysis and pathogenicity of reticuloendotheliosis virus isolated from a contaminated vaccine seed against infectious bursal disease virus: First report in China. J. Gen. Virol. 2016, 97, 2809–2815. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chen, C.-L.; Wang, C.-C.; Wang, C.-H. Isolation, identification, and complete genome sequence of an avian reticuloendotheliosis virus isolated from geese. Vet. Microbiol. 2009, 136, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, Z.; Jiang, S. Sequence analysis for the complete proviral genome of reticuloendotheliosis virus Chinese strain HA9901. Sci. China C Life Sci. 2006, 49, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Deng, X.; Gao, Y.; Li, K.; Chai, H.; Fan, Z.; Ren, X.; Wang, Q.; Zhang, L.; Yun, B.; et al. First isolation of reticuloendotheliosis virus from mallards in China. Arch. Virol. 2014, 159, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.-L.; Chen, S.-N.; Lin, T.; Wen, X.-H.; Wei, W.-K.; Lv, D.-H.; Chen, R.-A. Emergence of reticuloendotheliosis virus in pigeons in Guangdong Province, Southern China. Arch. Virol. 2016, 161, 2007–2011. [Google Scholar] [CrossRef]
- Jiang, L.; Qi, X.; Gao, Y.; Hua, Y.; Li, K.; Deng, X.; Wang, Q.; Zhang, L.; Chai, H.; Chen, Y.; et al. Molecular characterization and phylogenetic analysis of the reticuloendotheliosis virus isolated from wild birds in Northeast China. Vet. Microbiol. 2013, 166, 68–75. [Google Scholar] [CrossRef]
- Mays, J.K.; Silva, R.F.; Lee, L.F.; Fadly, A.M. Characterization of reticuloendotheliosis virus isolates obtained from broiler breeders, turkeys, and prairie chickens located in various geographical regions in the United States. Avian Pathol. 2010, 39, 383–389. [Google Scholar] [CrossRef]
- Thontiravong, A.; Wannaratana, S.; Sasipreeyajan, J. Genetic characterization of reticuloendotheliosis virus in chickens in Thailand. Poult. Sci. 2019, 98, 2432–2438. [Google Scholar] [CrossRef]
- Woźniakowski, G.; Mamczur, A.; Samorek-Salamonowicz, E. Common occurrence of Gallid herpesvirus-2 with reticuloendotheliosis virus in chickens caused by possible contamination of vaccine stocks. J. Appl. Microbiol. 2015, 118, 803–808. [Google Scholar] [CrossRef]
- Awad, A.M.; Abd El-Hamid, H.S.; Abou Rawash, A.A.; Ibrahim, H.H. Detection of reticuloendotheliosis virus as a contaminant of fowl pox vaccines. Poult. Sci. 2010, 89, 2389–2395. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, J.; Su, J.; Pu, J.; Zhang, G.; Liu, J. Full genome sequences of two reticuloendotheliosis viruses contaminating commercial vaccines. Avian Dis. 2009, 53, 341–346. [Google Scholar] [CrossRef]
- Ferro, P.J.; Morrow, M.E.; Flanagan, J.P.; Ortego, B.; Chester, R.E.; Mueller, J.M.; Lupiani, B. Wild Birds, a Source of Reticuloendotheliosis Virus Infection for the Endangered Attwater’s Prairie-Chicken (Tympanuchus cupido attwateri)? J. Wildl. Dis. 2017, 53, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.; Trautman, C.; Cox, F.; Spann, H.; Hardin, J.; Dittmar, R.; Edwards, D. Survey of Reticuloendotheliosis Virus in Wild Turkeys (Meleagris gallopavo) in Texas, USA. J. Wildl. Dis. 2019, 55, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Davidson, I.; Braverman, Y. Insect contribution to horizontal transmission of Reticuloendotheliosis virus. J. Med. Entomol. 2005, 42, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Woźniakowski, G.; Samorek-Salamonowicz, E.; Kozdruń, W. Molecular characteristics of Polish field strains of Marek’s disease herpesvirus isolated from vaccinated chickens. Acta Vet. Scand. 2011, 53, 10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Motha, M.X.; Egerton, J.R. Vertical transmission of reticuloendotheliosis virus in chickens. Avian Pathol. 1987, 16, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Bülow, V.V. Immunological effects of reticuloendotheliosis virus as potential contaminant of Marek’s disease vaccines. Avian Pathol. 1977, 6, 383–393. [Google Scholar] [CrossRef]
- Mete, A.; Gharpure, R.; Pitesky, M.E.; Famini, D.; Sverlow, K.; Dunn, J. Marek’s Disease in Backyard Chickens, A Study of Pathologic Findings and Viral Loads in Tumorous and Nontumorous Birds. Avian Dis. 2016, 60, 826–836. [Google Scholar] [CrossRef]
- Mescolini, G.; Lupini, C.; Felice, V.; Guerrini, A.; Silveira, F.; Cecchinato, M.; Catelli, E. Molecular characterization of the meq gene of Marek’s disease viruses detected in unvaccinated backyard chickens reveals the circulation of low- and high-virulence strains. Poult. Sci. 2019, 98, 3130–3137. [Google Scholar] [CrossRef]
- Bell, A.S.; Kennedy, D.A.; Jones, M.J.; Cairns, C.L.; Pandey, U.; Dunn, P.A.; Szpara, M.L.; Read, A.F. Molecular epidemiology of Marek’s disease virus in central Pennsylvania, USA. Virus Evol. 2019, 5, vey042. [Google Scholar] [CrossRef]
| Primer Designation | Primers Sequence | Target Gene | Location * | Size Product | Reference |
|---|---|---|---|---|---|
| MdCv-F MdCv-R | 5′-GTGATGGGAAGGCGATAGAA-3′ 5′-TCCGCATATGTTCCTCCTTC-3′ | pp38 | 127525–127506 A 127300–127319 A | 226 bp | [8] |
| SNV-LTR-F SNV-LTR-R | 5′-AATGGTTGTAAAGGGCAGAT-3’ 5’-CTCCTCTCACTGCCAATCT-3′ | LTR (REV) | 267-286/8012–8031 B 466–448/8211–8193 B | 201 bp | [8] |
| Leu3.2F Leu7R | 5′-GGAAATGTAGTGTTATRCRATACTCTTATG-3′ 5′-ATCCGCTTCATGCAGGTGCTC-3′ | LTR (ALV) | 7514–7543 C 7813–7834 C | 321 bp | [18] |
| Leu11F Leu12R | 5′-CGTCGATTGGTGGAAGTAAGGTGG-3′ 5′-TCA GGG AAT CGA CGG TCC GGC C-3′ | LTR (ALV) | 7594–7617 C 7785–7806 C | 213 bp | [18] |
| CVI988-F Non-CVI988-F MDV pp38-R | 5′-GAGGGAGAGTGGCTGTCAAG-3′ 5′-GAGGGAGAGTGGCTGTCAAA-3′ 5′-TCCGCATATGTTCCTCCTTC-3′ | pp38 | 127487–127468 A 127487–127468 A 127300–127319 A | 188 bp | [19] |
| MDVgB-gF1 MDVgB-gR1 | 5′-CCCATRCCGTTRAACAATTC-3′ 5′-GTYCAATTCGCCATGCTCCA-3′ | gB | 61554–61573 A 62281–62262 A | 728 bp | This study |
| REV-Pol1-F5 REV-Pol1-R5 | 5′-ACTCGCCCAGGAGAGTAGAG-3′ 5′-GAATAGTTTCGCGCAGGCTT-3′ | gag + pol | 2269–2288 B 3035–3016 B | 767 bp | This study |
| REV-Env3-F12 REV-Env3-R12 | 5′-GTGCATACTGGCATCAATCG-3′ 5′-CCACATTCCCCACYGCTCTT-3′ | env | 7050–7069 B 7752–7733 B | 703 bp | This study |
| Strain Designation | Isolation Year | Source | Country | GenBank Accession Numbers |
|---|---|---|---|---|
| SNV | 1959 | Duck | USA | DQ003591 |
| ATCC-VR775 | 1972 | Duck | USA | KF313137 |
| HA9901 | 1999 | Chicken | China | AY842951 |
| APC-566 | 2005 | Chicken | USA | DQ387450 |
| chicken/3337/05 | 2005 | Chicken | Taiwan | FJ439120 |
| goose/3410/06 | 2006 | Goose | Taiwan | FJ439119 |
| HLJ07I | 2007 | Chicken | China | GQ375848 |
| ZD0708 | 2007 | Chicken | China | FJ496333 |
| MD-2 | 2008 | HVT Vaccine | China | JX912710 |
| HLJR0901 | 2009 | Chicken | China | GQ415646 |
| 1105 | 2011 | Duck | China | JQ804915 |
| HA1101 | 2011 | Chicken | China | KF305089 |
| CY1111 | 2011 | Chicken | China | KJ909531 |
| GD1210 | 2012 | Chicken | China | KF709431 |
| SY1209 | 2012 | Chicken | China | KJ909530 |
| LN1201 | 2012 | Chicken | China | KU641115 |
| 104865 | 2014 | Turkey | USA | KJ756349 |
| GDBL1401 | 2014 | Pigeon | China | KU204702 |
| GDBL1402 | 2014 | Pigeon | China | KU204703 |
| HB2015021 | 2015 | Chicken | China | KY581581 |
| IBD-C1605 | 2016 | IBDV vaccine | China | KX278301 |
| SDAUR-S1 | 2017 | Chicken | China | MF185397 |
| Strain | Year | Source | Pathotype | Country | GenBank No. |
|---|---|---|---|---|---|
| Md5 | 1977 | Chicken | Very virulent | USA | NC_002229 |
| Polen5 | 2010 | Chicken | Very virulent plus | Poland | MF431496 |
| MD70/13 | 1970 | Chicken | Virulent | Hungary | MF431495 |
| EU-1 | 1992 | Chicken | Very virulent plus | Italy | MF431494 |
| GX0101 | 2001 | Chicken | Very virulent | China | JX844666 |
| 814 | 1986 | Chicken | Mild | China | JF742597 |
| CU-2 | 1968 | Chicken | Mild | USA | EU499381 |
| RB-1B | 1981 | Chicken | Very virulent | USA | EF523390 |
| CVI988 | 1969 | Chicken | Mild | The Netherlands | DQ530348 |
| ATE2539 | 2000 | Chicken | Very virulent plus | Hungary | MF431493 |
| LMS | 2007 | Chicken | Very virulent | China | JQ314003 |
| GA (att) | 1964 | Chicken | Virulent | USA | AY129969 |
| HPRS24 | 2001 | Chicken | vaccine | Japan | NC_002577 |
| SB-1 | 1978 | Chicken | vaccine | USA | HQ840738 |
| FC126 | 1970 | Turkey | vaccine | USA | NC_002641 |
| Serotype | Nucleotide Identity (%) | Aminoacid Identity (%) | ||||
|---|---|---|---|---|---|---|
| MDV1 | MDV2 | MDV3 | MDV1 | MDV2 | MDV3 | |
| USP386 (MH825642) | 99.8–100.0 | 77.3 | 75.5 | 100.0 | 91.0 | 89.6 |
| Subtype | Nucleotide Identity (%) | Aminoacid Identity (%) | ||||
|---|---|---|---|---|---|---|
| REV 1 | REV 2 | REV 3 | REV 1 | REV 2 | REV 3 | |
| USP386 Pol (MH673475) | 98.9 | 97.3–98.0 | 99.5–100.0 | 98.9 | 98.4–98.9 | 98.9–100.0 |
| USP386 Env (MH673476) | 98.5 | 95.40–96.9 | 99.2–100.0 | 98.1 | 96.6–97.6 | 98.5–100.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chacón, R.D.; Astolfi-Ferreira, C.S.; Guimarães, M.B.; Torres, L.N.; De la Torre, D.I.; Sá, L.R.M.d.; Piantino Ferreira, A.J. Detection and Molecular Characterization of a Natural Coinfection of Marek’s Disease Virus and Reticuloendotheliosis Virus in Brazilian Backyard Chicken Flock. Vet. Sci. 2019, 6, 92. https://doi.org/10.3390/vetsci6040092
Chacón RD, Astolfi-Ferreira CS, Guimarães MB, Torres LN, De la Torre DI, Sá LRMd, Piantino Ferreira AJ. Detection and Molecular Characterization of a Natural Coinfection of Marek’s Disease Virus and Reticuloendotheliosis Virus in Brazilian Backyard Chicken Flock. Veterinary Sciences. 2019; 6(4):92. https://doi.org/10.3390/vetsci6040092
Chicago/Turabian StyleChacón, Ruy D., Claudete S. Astolfi-Ferreira, Marta B. Guimarães, Luciana N. Torres, David I. De la Torre, Lilian R. M. de Sá, and Antonio J. Piantino Ferreira. 2019. "Detection and Molecular Characterization of a Natural Coinfection of Marek’s Disease Virus and Reticuloendotheliosis Virus in Brazilian Backyard Chicken Flock" Veterinary Sciences 6, no. 4: 92. https://doi.org/10.3390/vetsci6040092
APA StyleChacón, R. D., Astolfi-Ferreira, C. S., Guimarães, M. B., Torres, L. N., De la Torre, D. I., Sá, L. R. M. d., & Piantino Ferreira, A. J. (2019). Detection and Molecular Characterization of a Natural Coinfection of Marek’s Disease Virus and Reticuloendotheliosis Virus in Brazilian Backyard Chicken Flock. Veterinary Sciences, 6(4), 92. https://doi.org/10.3390/vetsci6040092






