Development and Evaluation of Epitope-Blocking ELISA for Detection of Antibodies against Contagious Caprine Pleuropneumonia in Goat Sera
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Approval
2.2. Preparation of Mycoplasma Capricolum Subsp. Capripneumoniae (Mccp) Antigen
2.3. Serum Samples
2.4. Monoclonal Antibody Production, Its Conjugation, and Evaluation in Epitope Blocking
2.5. CCPP Blocking-ELISA Development and Procedure
2.6. Evaluation of CCPP Blocking ELISA
2.6.1. Analytical Sensitivity
2.6.2. Cut-Off Value Determination
2.6.3. Assay Repeatability
2.6.4. Determination of Diagnostic Sensitivity and Specificity
3. Results
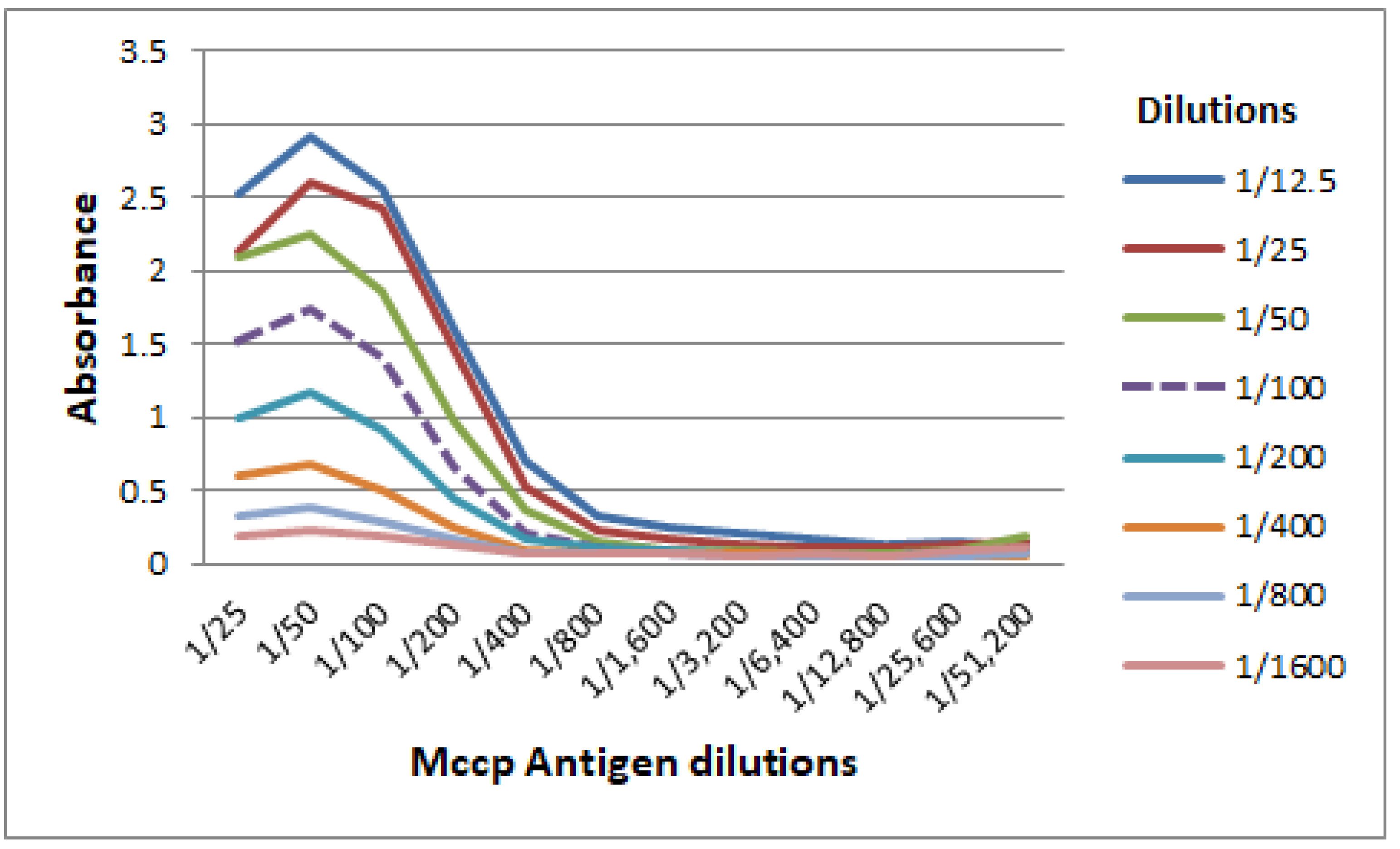
3.1. Titration of Mccp Antigen and Conjugate Used in the CCPP b-ELISA
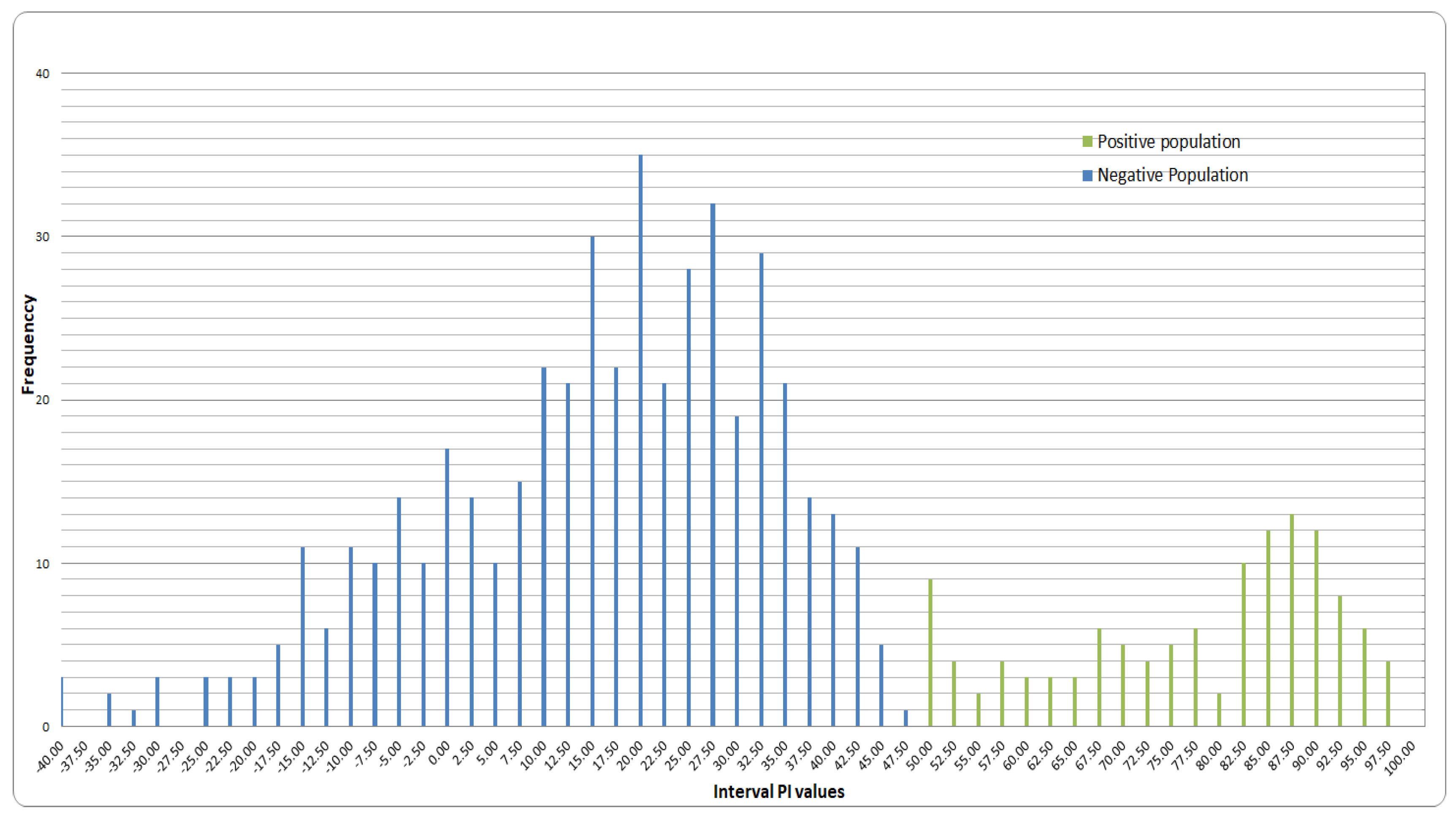
3.2. Cut-Off Value Determination
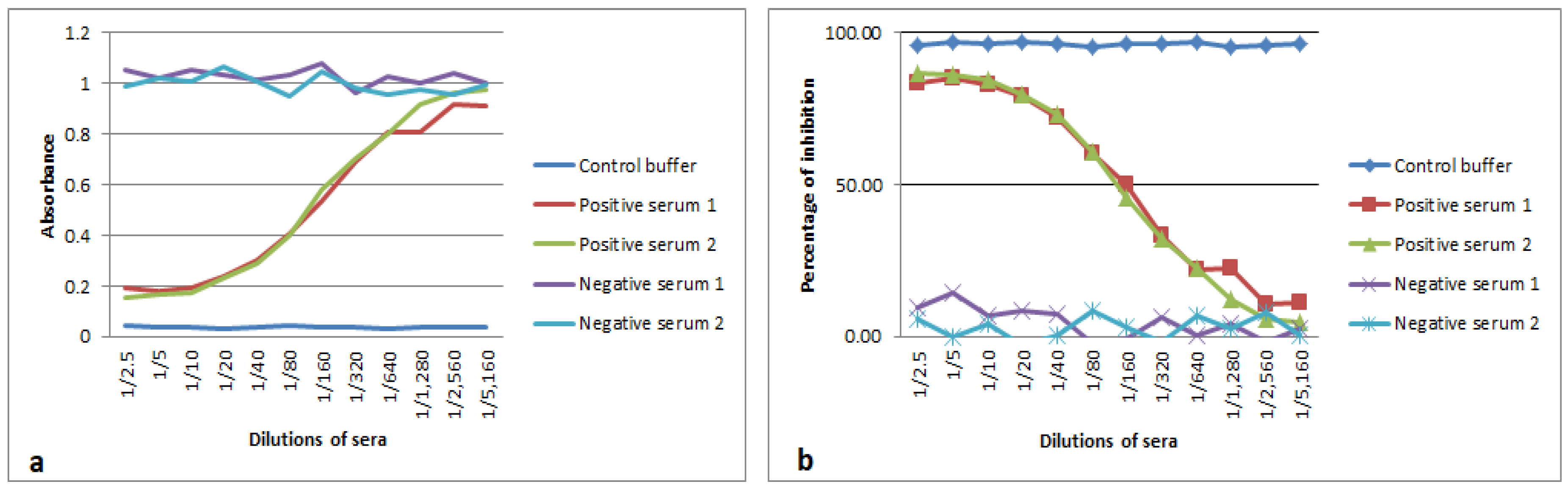
3.3. Analytical Sensitivity and Blocking Epitope by Serum Samples
3.4. Assay Repeatability
3.5. Comparison of the Newly Developed CCPP b-ELISA with the Available Commercial CCPP cELISA
3.6. Diagnostic Sensitivity and Specificity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dupuy, V.; Verdier, A.; Thiaucourt, F.; Manso-Silván, L. A large-scale genomic approach affords unprecedented resolution for the molecular epidemiology and evolutionary history of contagious caprine pleuropneumonia. Vet. Res. 2015, 46, 74. [Google Scholar] [CrossRef] [PubMed]
- Wesonga, H.O.; Bölske, G.; Thiaucourt, F.; Wanjohi, C.; Lindberg, R. Experimental contagious caprine pleuropneumonia: A long term study on the course of infection and pathology in a flock of goats infected with Mycoplasma capricolum subsp. capripneumoniae. Acta Vet. Scand. 2004, 45, 167. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, W.M. Contagious Caprine Pleuropneumonia: The Challenging Transboundary Disease of Goats. Int. J. Vet. Health Sci. Res. 2017, 59, 189–196. [Google Scholar]
- Nicholas, R.; Churchward, C. Contagious Caprine Pleuropneumonia: New Aspects of an Old Disease. Transbound. Emerg. Dis. 2012, 59, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Thiaucourt, F.; Bolske, G. Contagious caprine pleuropneumonia and other pulmonary mycoplasmoses of sheep and goats. Rev. Sci. Tech. 2016, 15, 1397–1414. [Google Scholar] [CrossRef] [PubMed]
- MacOwan, K.J.; Minette, J.E. A mycoplasma from acute contagious caprine pleuropneumonia in Kenya. Trop. Anim. Health Prod. 1976, 8, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Meenowa, D.; Barden, G.; Salguero, F.J.; Churchward, C.; Nicholas, R.A.J. Contagious caprine pleuropneumonia in Mauritius. Vet. Rec. 2010, 167, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Samiullah, S. Contagious Caprine Pleuropneumonia and Its Current Picture in Pakistan: Veterinarni Medicina, A Review; University of New England: Armidale, Australia, 2013; Volume 58. [Google Scholar]
- OIE World Organization for Animal Health Contagious caprine pleuropneumonia. OIE Terr. Man. 2008, 1000–1012.
- Cho, H.J.; Ruhnke, H.L.; Langford, E.V. The Indirect Hemagglutination Test for the Detection of Antibodies in Cattle Naturally Infected with Mycoplasmas. Can. J. Comp. Med. 1976, 40, 20. [Google Scholar] [PubMed]
- Thiaucourt, F.; Bölske, G.; Libeau, G.; Le Goff, C.; Lefèvre, P.C. The use of monoclonal antibodies in the diagnosis of contagious caprine pleuropneumonia (CCPP). Vet. Microbiol. 1994, 41, 191–203. [Google Scholar] [CrossRef]
- Rurangirwa, F.R.; Masiga, W.N.; Muthomi, E. Immunity to contagious caprine pleuropneumonia caused by F-38 strain of Mycoplasma. Vet. Rec. 1981, 109, 310. [Google Scholar] [CrossRef] [PubMed]
- Takele, T.; Hunderra, S.; Martha, Y.; Bedaso, M. Evaluation of safety and immunogenicity of inactivated whole culture contagious caprine pleuropneumonia trial vaccine in National Veterinary Institute, Ethiopia. Afr. J. Microbiol. Res. 2017, 11, 466–473. [Google Scholar] [CrossRef]
- Elisa, D. Chessboard Titrations in ELISA1. Available online: http://www-naweb.iaea.org/nafa/aph/public/ras-ai-elisaii.pdf (accessed on 8 July 2017).
- Crowther, J.R. The ELISA Guidebook. 2002. Available online: https://www.fws.gov/aah/PDF/PrinValDiagAssayforInfDis-JACOBSON.pdf (accessed on 1 June 2019).
- Albini, B.; Herzog, F.; Wick, G. Photometric Evaluation of Indirect Immunofluorescence Chessboard Titrations for the Characterization of FITC-Labelled Antibodies. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1407939/pdf/immunology00344-0079.pdf (accessed on 24 May 2019).
- How to Use Checkerboard Titration to Optimize Your ELISA Immunoassays. Available online: https://www.bosterbio.com/newsletter-archive/20170616-checkerboard-titration (accessed on 24 May 2019).
- Baziki, J.d.D.; Bodjo, S.C.; Nwankpa, N.; Maina, N.; Chitsungo, E.; Boukary, C.R.M.; Tefera, T.A.; Nwankpa, R.V. Development and evaluation of an Immuno-Capture Enzyme-Linked Immunosorbent Assay to quantify the Mycoplasma capricolum subsp. capripneumoniae (Mccp) protein in Contagious Caprine Pleuropneumonia (CCPP) vaccine. J. Vet. Med. submitted.
- Bodjo, S.C.; Baziki, J.d.D.; Nwankpa, N.; Chitsungo, E.; Koffi, Y.M.; Couacy-Hymann, E.; Diop, M.; Gizaw, D.; Tajelser, I.B.A.; Lelenta, M.; et al. Development and validation of an epitope-blocking ELISA using an anti-haemagglutinin monoclonal antibody for specific detection of antibodies in sheep and goat sera directed against peste des petits ruminants virus. Arch. Virol. 2018, 163, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Peyraud, A.; Poumarat, F.; Tardy, F.; Manso-Silván, L.; Hamroev, K.; Tilloev, T.; Amirbekov, M.; Tounkara, K.; Bodjo, C.; Wesonga, H.; et al. An international collaborative study to determine the prevalence of contagious caprine pleuropneumonia by monoclonal antibody-based CCPP cELISA. BMC Vet. Res. 2014, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, R.H. Principles of Validation of Diagnostic Assays for Infectious Diseases 1; Cornell University: New York, NY, USA, 1998. [Google Scholar]
- Noble, W.; Yu, H.; Wray, S.; Andreasson, U.; Perret-Liaudet, A.; Van Waalwijk Van Doorn, L.J.C.; Blennow, K.; Chiasserini, D.; Engelborghs, S.; Fladby, T.; et al. A practical guide to immunoassay method validation. Neurol 2015, 6, 179. [Google Scholar]
- OIE (2014). MEASUREMENT UNCERTAINTY. Available online: http://www.scahls.org.au/Guidelines/Pages/Measurement-of-Uncertainty.aspx (accessed on 1 June 2019).
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Fleiss, J.L. Statistical Methods for Rates and Proportions; John Wiley & Sons: Hoboken, NJ, USA, 1973. [Google Scholar]
- Fleiss, J.L.; Levin, B.; Paik, M.C. Statistical Methods for Rates and Proportions; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 9780471526292. [Google Scholar]
- Iqbal Yatoo, M.; Raffiq Parray, O.; Tauseef Bashir, S.; Ahmed Bhat, R.; Gopalakrishnan, A.; Karthik, K.; Dhama, K.; Vir Singh, S.; Singh, S.V. Contagious caprine pleuropneumonia—A comprehensive review. Vet. Q. 2019, 39, 1–25. [Google Scholar] [CrossRef] [PubMed]
| Type of Test | Tested (+) | Tested (−) |
|---|---|---|
| Test (+) | T+ | F− |
| Test (−) | F+ | T− |
| Sera | Commercial CCPP cELISA | CCPP b-ELISA | ||
|---|---|---|---|---|
| Positive ≥ 55 PI | Negative < 55 PI | Positive ≥ 50 PI | Negative < 50 PI | |
| 252 | 90 | 162 | 104 | 148 |
| Newly Developed Test | Commercial CCPP cELISA | Total | ||
|---|---|---|---|---|
| Positive | Negative | |||
| CCPP b-ELISA | Positive | 84 | 20 | 104 |
| Negative | 6 | 142 | 148 | |
| Total | 90 | 162 | 252 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jean de Dieu, B.; Charles, B.S.; Nwankpa, N.; Chitsungo, E.; Moustapha Boukary, C.R.; Maina, N.; Tefera, T.A.; Nwankpa, R.V.; Mwangi, N.; Mathurin Koffi, Y. Development and Evaluation of Epitope-Blocking ELISA for Detection of Antibodies against Contagious Caprine Pleuropneumonia in Goat Sera. Vet. Sci. 2019, 6, 82. https://doi.org/10.3390/vetsci6040082
Jean de Dieu B, Charles BS, Nwankpa N, Chitsungo E, Moustapha Boukary CR, Maina N, Tefera TA, Nwankpa RV, Mwangi N, Mathurin Koffi Y. Development and Evaluation of Epitope-Blocking ELISA for Detection of Antibodies against Contagious Caprine Pleuropneumonia in Goat Sera. Veterinary Sciences. 2019; 6(4):82. https://doi.org/10.3390/vetsci6040082
Chicago/Turabian StyleJean de Dieu, Baziki, Bodjo S. Charles, Nick Nwankpa, Ethel Chitsungo, Cisse Rahamatou Moustapha Boukary, Naomi Maina, Takele A. Tefera, Rume Veronica Nwankpa, Nduta Mwangi, and Yao Mathurin Koffi. 2019. "Development and Evaluation of Epitope-Blocking ELISA for Detection of Antibodies against Contagious Caprine Pleuropneumonia in Goat Sera" Veterinary Sciences 6, no. 4: 82. https://doi.org/10.3390/vetsci6040082
APA StyleJean de Dieu, B., Charles, B. S., Nwankpa, N., Chitsungo, E., Moustapha Boukary, C. R., Maina, N., Tefera, T. A., Nwankpa, R. V., Mwangi, N., & Mathurin Koffi, Y. (2019). Development and Evaluation of Epitope-Blocking ELISA for Detection of Antibodies against Contagious Caprine Pleuropneumonia in Goat Sera. Veterinary Sciences, 6(4), 82. https://doi.org/10.3390/vetsci6040082





