Volatilome in Milk for Grana Padano and Parmigiano Reggiano Cheeses: A First Survey
Abstract
:1. Introduction
- -
- Carbohydrates.
- -
- Aminoacids.
- -
- Fatty acids.
- -
- Terpenes.
- -
- Other molecules
2. Materials and Methods
- 2-Methylbutanal.
- 2-Propanone.
- 2-Butanone.
- 2,3-Butanedione.
- 2,3-Heptanedione.
- 2-Heptanone.
- 3-Hydroxy-2-butanone.
- 2-Hydroxy-3-pentanone.
- 2-Nonanone.
- 5-Hydroxydimehyl-4-octanone.
- Methanol.
- 2-Propanol.
- Ethanol.
- 2-Methyl-1-propanol.
- 4-Methyl-2-pentanol.
- 1-Butanol.
- 2-Hexanol.
- 3-Methyl-1-butanol.
- 3-Methylbut-3-ene-1-ol (isoprenol).
- 1-Pentanol.
- 3-Methyl-2-butenol.
- 3-Pentanol.
- 1-Hexanol.
- 2-Hydroxy-3-pentanone 2.
- 1-Octen-3-ol.
- 1-Octen-4-ol.
- 2-Heptanol.
- 2,3-Butanediol.
- 2-Butanol.
- Benzene ethanol.
- 4-Methyl-2-oxovaleric acid.
- Ethanoic acid.
- 2-Methylpropanoic acid.
- Butanoic acid.
- Pentanoic acid.
- Hexanoic acid.
- Acetic acid methylester.
- Ethylacetate.
- Butyric acid methyl ester.
- Methyl-3-metilbutanoate.
- Acetic acid ethylenester.
- Isoamylacetate.
- Hexanoic acid ethyl ester.
- Hexanoic acid methyl ester.
- Isoamyl-n-butyrate.
- Octanoic acid methyl ester.
- Methyldecanoate.
- Butyric acid-3-methyl ester.
- Hexanoic acid methylester.
- o-Xylene.
- p-Xylene.
- Dimethylsulfide.
- Dimethylsulfoxide.
- Dimethylsulfone.
- Aldehydes.
- Ketones.
- Alcohols.
- Acids.
- Esters.
- Aromatic hydrocarbons.
- Solforates.
2.1. HS-SPME Extraction
2.2. Gas Chromatography-Mass Spectrometry Analysis of Volatile Organic Compounds (VOCs)
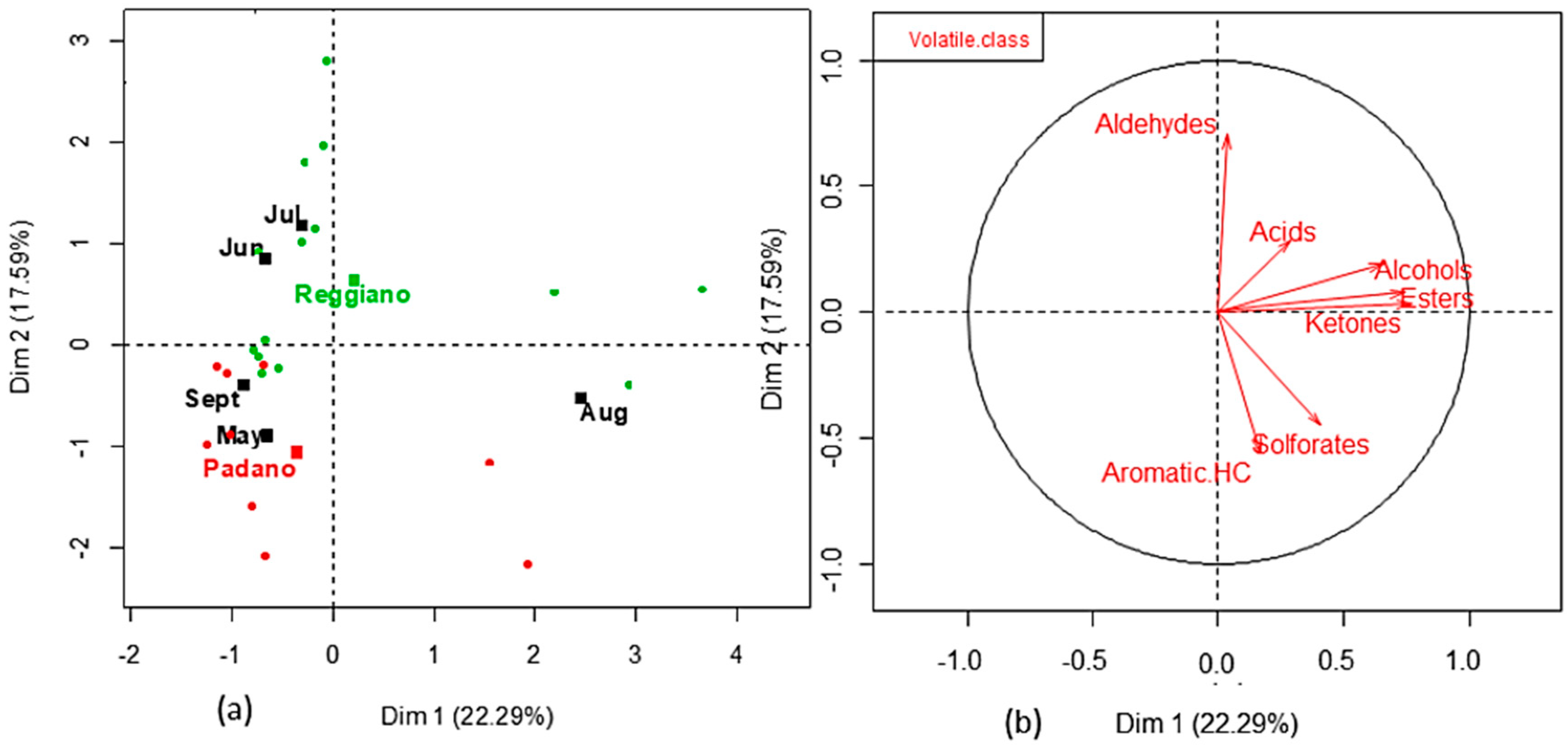
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Park, Y.W.; Haenlein, G.F.W. Handbook of Milk of Non-bovine Mammals, 2nd ed.; Blackwell Publishing Professional: Ames, IA, USA, 2017; pp. 595–654. [Google Scholar]
- Kim, Y.; Morr, C. Dynamic headspace analysis of light activated flavor in milk. Int. Dairy. J. 1996, 6, 185–193. [Google Scholar] [CrossRef]
- Toso, B.; Procida, G.; Stefanon, B. Determination of volatile compounds in cows’ milk using headspace GC-MS. J. Dairy Res. 2002, 69, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Moio, L.; Langlois, D.; Etievant, P.; Addeo, F. Powerful odorants in bovine, ovine, caprine and water buffalo milk determined by means of gas-chromatography-olfactometry. J. Dairy Res. 1993, 60, 215–222. [Google Scholar] [CrossRef]
- Moio, L.; Dekimpe, J.; Etievant, P.; Addeo, F. Neutral volatile compounds in the raw milks from different species. J. Dairy Res. 1993, 60, 199–213. [Google Scholar] [CrossRef]
- Moio, L.; Etievant, P.; Langlois, D.; Dekimpe, J.; Addeo, F. Detection of powerful odorants in heated milk by use of extract dilution sniffing analysis. J. Dairy Res. 1994, 61, 385–394. [Google Scholar]
- Moio, L.; Addeo, F. Grana Padano cheese aroma. J. Dairy Res. 1998, 65, 317–333. [Google Scholar] [CrossRef]
- Panseri, S.; Chiesa, L.M.; Biondi, P.A.; Cantoni, C. Head Space-Solid Phase Microextraction for characterization of volatile compounds and microbiological parameters in milk tainted with off-flavour. Milchwissenschaft 2009, 64, 372–375. [Google Scholar]
- Vanbergue, E.; Delaby, L.; Peyraud, J.L.; Colette, S.; Gallard, Y.; Hurtaud, C. Effects of breed, feeding system, and lactation stage on milk fat characteristics and spontaneous lipolysis in dairy cows. J. Dairy Sci. 2017, 100, 4623–4636. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.C.; Dhiman, T.R.; Ure, A.L.; Brennand, C.P.; Boman, R.L.; McMahon, D.J. Consumer acceptability of conjugated linoleic acid-enriched milk and cheddar cheese from cows grazing on pasture. J. Dairy Sci. 2005, 88, 1837–1847. [Google Scholar] [CrossRef]
- Croissant, A.E.; Washburn, S.P.; Dean, L.L.; Drake, M.A. Chemical properties and consumer perception of fluid milk from conventional and pasture-based production systems. J. Dairy Sci. 2007, 90, 4942–4953. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, H.; O’Callaghan, T.F.; McAuliffe, S.; Hennessy, D.; Stanton, C.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Effect of different forage types on the volatile and sensory properties of bovine milk. J. Dairy Sci. 2018, 101, 1034–1047. [Google Scholar] [CrossRef] [PubMed]
- Falchero, L.; Lombardi, G.; Gorlier, A.; Lonati, M.; Odoardi, M.; Cavallero, A. Variation in fatty acid composition of milk and cheese from cows grazed on two alpine pastures. Dairy Sci. Technol. 2010, 90, 657–672. [Google Scholar] [CrossRef]
- Kilcawley, K.N.; Faulkner, H.; Clarke, H.J.; O’Sullivan, M.G.; Kerry, J.P. Factors Influencing the Flavour of Bovine Milk and Cheese from Grass Based versus Non-Grass Based Milk Production Systems. Foods 2018, 7, 37. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Grana Padano (%) | Parmigiano Reggiano (%) |
|---|---|---|
| Crude protein | 15.36 | 15.94 |
| Ethereal extracts | 4.10 | 3.44 |
| Ash | 7.05 | 8.60 |
| Nitrogen disappearance rate | 54.75 | 66.68 |
| Fiber | 16.80 | 4.62 |
| Ca | 0.75 | 0.9 |
| P | 0.56 | 1.02 |
| Na | 0.50 | 1.75 |
| Alfalfa hay | Alfalfa hay | |
| Soy meal | Polyphyte hay | |
| Maize silage | Maize grain | |
| Sunflower meal | Soy meal | |
| Corn flakes | Sunflower meal | |
| Barley flakes | Wheat bran | |
| Wheat bran | Soybean hulls | |
| Molasses | Molasses | |
| Minerals | Minerals | |
| Hydrogenated fat |
| Volatile | Month | ANOVA Month | Destination | ANOVA Destination | |||||
|---|---|---|---|---|---|---|---|---|---|
| May | Jun | Jul | Aug | Sept | Padano | Reggiano | |||
| Aldehydes | 36.56 | 433.76 | 418.96 | 79.89 | 48.16 | <0.05 | 24.09 | 169.55 | <0.05 |
| SD | 34.57 | 664.44 | 264.89 | 73.52 | 27.65 | 45.23 | 310.76 | ||
| Ketones | 459.62 | 644.99 | 791.98 | 1774.09 | 276.54 | <0.001 | 441.55 | 658.01 | - |
| SD | 255.00 | 557.30 | 371.18 | 1571.43 | 184.59 | 749.95 | 828.72 | ||
| Alcohols | 325.15 | 1385.07 | 1843.71 | 12,133.02 | 211.11 | <0.001 | 308.04 | 2649.70 | <0.05 |
| SD | 342.34 | 1629.67 | 1010.86 | 7893.76 | 71.54 | 439.30 | 5223.71 | ||
| Acids | 91.72 | 979.42 | 426.48 | 342.64 | 42.63 | <0.001 | 205.64 | 313.82 | <0.05 |
| SD | 126.65 | 1329.30 | 243.26 | 205.97 | 24.63 | 624.56 | 584.32 | ||
| Esters | 55.20 | 129.78 | 91.33 | 10,006.10 | 3.44 | <0.001 | 129.88 | 1714.44 | - |
| SD | 10.89 | 207.39 | 65.74 | 9291.23 | 1.00 | 166.50 | 4972.23 | ||
| Aromatic hydrocarbons | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 | <0.01 | 1.26 | 0.02 | <0.01 |
| SD | 0.00 | 0.20 | 0.00 | 0.00 | 0.00 | 2.59 | 0.08 | ||
| Solforates | 16.57 | 1.13 | 8.99 | 40.33 | 29.50 | <0.01 | 21.94 | 16.09 | - |
| SD | 2.51 | 1.01 | 12.28 | 69.83 | 6.83 | 27.17 | 28.76 | ||
| Dimension 1 (27.13%) | Dimension 2 (17.06%) | Dimension 3 (10.79%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Correlation | p-Value | Correlation | p-Value | Correlation | p-Value | |||
| 3-methyl-1-butanol | 0.64 | 0.001 | 3-methyl-2-butenol | 0.78 | <0.0001 | 1-octen-3-ol | 0.9356 | <0.0001 |
| 2,3-eptanedione | 0.58 | 0.003 | 2,3-butanediol | 0.77 | <0.0001 | 2-hydroxy-3-pentanone | 0.9353 | <0.0001 |
| 3-methylbut-3-ene-1-ol | 0.5 | 0.003 | ethylacetate | 0.73 | <0.0001 | acetic acid methylester | 0.9106 | <0.0001 |
| ethylacetate | 0.57 | 0.003 | 3-hydroxy-2-butanone | 0.73 | 0.0001 | ethanol | 0.9083 | <0.0001 |
| 3-methyl-2-butenol | 0.57 | 0.003 | butyric acid methylester | 0.69 | 0.0002 | pentanoic acid | 0.8991 | <0.0001 |
| butyric acid methylester | 0.57 | 0.003 | 3-methylbut-3-ene-1-ol | 0.68 | 0.0003 | benzenethanol | 0.7657 | <0.0001 |
| 2,3-butanedione | 0.56 | 0.003 | hexanoic acid methylester | 0.66 | 0.0004 | 2-methyl-propanol | 0.752 | <0.0001 |
| hexanoic-acid-ethyl-ester | 0.54 | 0.006 | methyldecanoate | 0.65 | 0.0006 | hexanoic acid methylester | 0.5628 | 0.0042 |
| 1-hexanol | 0.53 | 0.007 | hexanoic acid ethylester | 0.64 | 0.0008 | 3-methyl-1-butanol | 0.537 | 0.0068 |
| isoamylacetate | 0.53 | 0.007 | isoamylacetate | 0.63 | 0.001 | |||
| 2-methyl-1-propanol | 0.52 | 0.007 | acetic acid ethylenester | 0.61 | 0.0015 | |||
| methyldecanoate | 0.51 | 0.01 | methyl 3 methylbutanoate | 0.61 | 0.0016 | |||
| acetic acid ethylenester | 0.50 | 0.01 | hexanoic acid methyl ester | 0.60 | 0.0021 | |||
| octanoic acid methylester | 0.50 | 0.01 | 2,3- heptanedione | 0.5772 | 0.0031 | |||
| 3-pentanol | 0.49 | 0.01 | 3-pentanol | 0.5597 | 0.0045 | |||
| 2-hydroxy-3-pentanone | 0.48 | 0.01 | 2-hydroxy-3-pentanone | 0.5352 | 0.007 | |||
| hexanoic acid methylester | 0.47 | 0.02 | octanoic acid methylester | 0.4815 | 0.0172 | |||
| 3-hydroxy-2-butanone | 0.44 | 0.03 | dimethylsulfide | 0.4244 | 0.0387 | |||
| hexanoic acid methylester | 0.43 | 0.033 | methanol | –0.4503 | 0.0272 | |||
| 1-octen-4-ol | 0.4318 | 0.03 | ||||||
| p-xylene | −0.4199 | 0.04 | ||||||
| Destination | R2 | p-value | Destination | R2 | p-value | Destination | R2 | p-value |
| 0.77 | <0.0001 | 0.1845 | 0.0362 | n.s. | n.s. | |||
| Reggiano | 10.5 | <0.0001 | Padano | 0.4079 | 0.0362 | Padano | n.s. | n.s. |
| Padano | –10.5 | <0.0001 | Reggiano | –0.4079 | 0.0362 | Reggiano | n.s. | n.s. |
| Dimension 4 (9.03%) | Dimension 5 (5.86%) | |||||||
| Correlation | p-value | Correlation | p-value | |||||
| 2-eptanone | 0.76 | <0.0001 | acetic acid | 0.8772 | <0.0001 | |||
| 2-methylpropanoic acid | 0.75 | <0.0001 | o-xylene | 0.847 | <0.0001 | |||
| dimethylsulfide | 0.71 | 0.0001 | 1-butanol | 0.8295 | <0.0001 | |||
| butyric acid-3-methylester | 0.53 | 0.0076 | methyl-3-methylbutanoate | 0.5721 | 0.0035 | |||
| octanoic acid methylester | 0.51 | 0.0094 | ||||||
| 1-octen-4-ol | 0.50 | 0.0121 | ||||||
| 2-hydroxy-3-pentanone | 0.4 | 0.0133 | ||||||
| 3-methyl-1-butanol | 0.4054 | 0.0493 | ||||||
| hexanoic acid ethylester | –0.43 | 0.0334 | ||||||
| 2-3-eptanedione | –0.45 | 0.0251 | ||||||
| isoamylacetate | –0.49 | 0.0143 | ||||||
| methyldecanoate | –0.50 | 0.0119 | ||||||
| 1-hexanol | –0.54 | 0.0057 | ||||||
| hexanoic acid methylester | –0.54 | 0.0056 | ||||||
| acetic acid etilenester | –0.55 | 0.0053 | ||||||
| Destination | R2 | p-value | Destination | R2 | p-value | |||
| n.s. | n.s. | n.s. | n.s. | |||||
| Padano | n.s. | n.s. | Padano | n.s. | n.s. | |||
| Reggiano | n.s. | n.s. | Reggiano | n.s. | n.s. | |||
| Padano | Reggiano | Mean Volatiles | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volatile | Statistics | May | Jun | Jul | Aug | Sept | May | Jun | Jul | Aug | Sept | ANOVA Month | Padano | Reggiano | ANOVA Milk |
| 2-methylbutanal | Mean | 8.53 | 3.12 | 12.83 | 48.81 | 65.63 | 36.56 | 433.76 | 418.96 | 79.89 | 48.16 | - | 24.09 | 16.,55 | <0.05 |
| Std Dev | 0.00 | 4.42 | 69.03 | 91.35 | 34.57 | 664.44 | 264.89 | 73.52 | 27.65 | 45.23 | 310.76 | ||||
| 2-propanone | Mean | 30.43 | 10.94 | 20.88 | 12.36 | 28.16 | 222.51 | 21.88 | 77.05 | 56.22 | 52.56 | <0.01 | 16.82 | 71.74 | - |
| Std Dev | 6.85 | 0.90 | 17.48 | 39.83 | 280.64 | 19.02 | 59.43 | 97.38 | 19.70 | 18.02 | 127.86 | ||||
| 2-butanone | Mean | 10.87 | 11.85 | 36.95 | 0.00 | 0.00 | 9.08 | 0.00 | 3.45 | 0.00 | 17.82 | - | 7.54 | 5.06 | - |
| Std Dev | 12.22 | 0.85 | 0.00 | 0.00 | 11.38 | 0.00 | 3.00 | 0.00 | 15.90 | 11.86 | 9.57 | ||||
| 2,3-butanedione | Mean | 12.85 | 0.40 | 10.44 | 4.90 | 10.56 | 2.82 | 46.32 | 27.36 | 37.57 | 13.47 | <0.05 | 6.17 | 21.26 | <0.05 |
| Std Dev | 13.23 | 0.56 | 6.93 | 12.63 | 2.67 | 32.39 | 12.54 | 24.88 | 14.05 | 8.21 | 23.53 | ||||
| 2,3-heptanedione | Mean | 0.00 | 0.00 | 0.00 | 1.44 | 0.00 | 7.54 | 6.91 | 4.85 | 31.16 | 0.00 | - | 0.26 | 8.41 | * |
| Std Dev | 0.00 | 0.00 | 2.04 | 0.00 | 13.07 | 10.76 | 4.31 | 47.39 | 0.00 | 0.87 | 20.47 | ||||
| 2-heptanone | Mean | 1.72 | 0.64 | 0.52 | 2.71 | 0.48 | 0.79 | 1.43 | 3.00 | 13.27 | 1.12 | - | 1.06 | 3.27 | - |
| Std Dev | 1.36 | 0.05 | 2.45 | 0.38 | 0.90 | 1.26 | 1.51 | 22.98 | 0.90 | 1.34 | 9.21 | ||||
| 3-hydroxy-2-butanone | Mean | 270.47 | 37.91 | 206.83 | 1671.07 | 46.19 | 216.65 | 557.59 | 670.74 | 1632.59 | 187.73 | <0.001 | 387.11 | 544.31 | - |
| Std Dev | 169.11 | 45.94 | 1211.05 | 56.70 | 185.95 | 541.34 | 373.89 | 1507.99 | 165.44 | 750.55 | 794.46 | ||||
| 2-hydroxy-3-pentanone | Mean | 1.70 | 0.32 | 1.80 | 4.60 | 0.23 | 0.00 | 3.71 | 3.80 | 8.45 | 0.00 | <0.05 | 1.41 | 2.66 | - |
| Std Dev | 1.18 | 0.45 | 2.27 | 0.32 | 0.00 | 2.71 | 4.01 | 8.42 | 0.00 | 1.91 | 4.60 | ||||
| 2-nonanone | Mean | 8.74 | 0.33 | 0.00 | 0.00 | 0.00 | 0.22 | 0.00 | 1.72 | 0.00 | 3.84 | - | 1.65 | 0.96 | - |
| Std Dev | 0.14 | 0.47 | 0.00 | 0.00 | 0.39 | 0.00 | 2.98 | 0.00 | 6.66 | 3.51 | 2.90 | ||||
| 5-idrossidimetil-4-ottanone | Mean | 107.41 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 7.14 | 0.00 | 0.00 | 0.00 | - | 19.53 | 1,19 | - |
| Std Dev | 142.99 | 0.00 | 0.00 | 0.00 | 0.00 | 12.37 | 0.00 | 0.00 | 0.00 | 62.71 | 5,05 | ||||
| Methanol | Mean | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 38.06 | 11.32 | 0.00 | 18.53 | <0.05 | 0.00 | 11.32 | * |
| Std Dev | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 9.86 | 10.33 | 0.00 | 24.37 | 0.00 | 17.23 | ||||
| 2-propanol | Mean | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 5.13 | 19.93 | 54.48 | 0.00 | 1.47 | - | 0.00 | 13.50 | <0.01 |
| Std Dev | 0.00 | 0.00 | 0.00 | 0.00 | 8.88 | 34.52 | 94.36 | 0.00 | 2.54 | 0.00 | 40.04 | ||||
| Ethanol | Mean | 170.06 | 18.85 | 39.42 | 377.55 | 0.00 | 18.93 | 37.99 | 31.89 | 5175.42 | 44.00 | <0.001 | 106.58 | 884.71 | - |
| Std Dev | 166.68 | 17.29 | 305.12 | 0.00 | 32.79 | 54.80 | 30.21 | 5687.78 | 42.97 | 184.78 | 2775.90 | ||||
| 2-methyl-1-propanol | Mean | 11.91 | 0.49 | 1.79 | 26.87 | 0.22 | 21.03 | 19.53 | 42.34 | 250.06 | 0.88 | <0.001 | 7.35 | 55.64 | - |
| Std Dev | 5.33 | 0.33 | 4.13 | 0.31 | 14.47 | 32.31 | 27.74 | 161.83 | 0.88 | 10.88 | 107.42 | ||||
| 4-methyl-2-pentanol | Mean | 0.33 | 0.02 | 0.00 | 5.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | - | 0..8 | 0.00 | - |
| Std Dev | 0.46 | 0.02 | 7.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3..1 | 0.00 | ||||
| 1-butanol | Mean | 0.00 | 0.00 | 0.00 | 0.56 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | - | 0..0 | 0.02 | - |
| Std Dev | 0.00 | 0.00 | 0.80 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.25 | 0.34 | 0.10 | ||||
| 2-hexanol | Mean | 0.63 | 0.00 | 0.00 | 0.00 | 0.00 | 0.18 | 0.00 | 0.00 | 0.00 | 0.00 | <0.5 | 0.12 | 0.03 | - |
| Std Dev | 0.60 | 0.00 | 0.00 | 0.00 | 0.31 | 0.00 | 0.00 | 0.00 | 0.00 | 0.31 | 0.13 | ||||
| 3-methyl-1-butanol | Mean | 190.67 | 59.84 | 107.83 | 568.10 | 25.31 | 269.91 | 884.81 | 1451.82 | 6065.24 | 86.11 | <0.001 | 163.25 | 1459.66 | ° |
| Std Dev | 167.41 | 82.53 | 217.28 | 5.07 | 301.79 | 1037.11 | 918.35 | 2069.45 | 35.84 | 229.60 | 2345.35 | ||||
| 3-methylbut-3-ene-1-ol | Mean | 0.33 | 0.06 | 0.31 | 0.95 | 0.33 | 1.47 | 0.00 | 0.11 | 4.76 | 0.58 | <0.05 | 0.33 | 1.16 | * |
| Std Dev | 0.04 | 0.08 | 1.34 | 0.03 | 0.23 | 0.00 | 0.20 | 4.13 | 0.55 | 0.54 | 2.26 | ||||
| 1-pentanol | Mean | 0.30 | 0.06 | 0.31 | 0.00 | 0.55 | 0.00 | 0.19 | 0.45 | 0.00 | 0.60 | <0.05 | 0.19 | 0.21 | - |
| Std Dev | 0.18 | 0.08 | 0.00 | 0.33 | 0.00 | 0.33 | 0.78 | 0.00 | 0.26 | 0.25 | 0.39 | ||||
| 3-methyl-2-butenol | Mean | 0.00 | 0.00 | 0.25 | 1.81 | 0.06 | 0.00 | 0.49 | 0.29 | 5.62 | 0.27 | <0.01 | 0.36 | 1.11 | - |
| Std Dev | 0.00 | 0.00 | 0.05 | 0.08 | 0.00 | 0.42 | 0.50 | 5.37 | 0.24 | 0.72 | 2.79 | ||||
| 3-pentanol | Mean | 1.62 | 0.07 | 3.51 | 6.41 | 0.60 | 1.35 | 4.76 | 4.08 | 10.47 | 0.20 | <0.001 | 1.90 | 3.48 | - |
| Std Dev | 1.35 | 0.10 | 3.17 | 0.65 | 1.13 | 7.42 | 2.33 | 6.92 | 0.35 | 2.70 | 5.17 | ||||
| 1-hexanol | Mean | 0.00 | 0.13 | 0.35 | 0.00 | 0.22 | 0.90 | 1.21 | 1.91 | 1.63 | 0.71 | - | 0.10 | 1.06 | - |
| Std Dev | 0.00 | 0.10 | 0.00 | 0.02 | 1.56 | 1.62 | 1.73 | 2.82 | 0.62 | 0.13 | 1.53 | ||||
| 2-hydroxy-3-pentanone 2 | Mean | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.74 | 0.00 | 0.00 | 10.36 | 0.00 | - | 0.00 | 1.85 | - |
| Std Dev | 0.00 | 0.00 | 0.00 | 0.00 | 1.28 | 0.00 | 0.00 | 17.94 | 0.00 | 0.00 | 7.31 | ||||
| 1-octen-3-ol | Mean | 0.00 | 0.06 | 5.84 | 0.00 | 0.00 | 2.27 | 0.00 | 0.00 | 257.85 | 0.00 | - | 0.54 | 43.35 | - |
| Std Dev | 0.00 | 0.01 | 0.00 | 0.00 | 3.93 | 0.00 | 0.00 | 446.61 | 0.00 | 1.76 | 182.24 | ||||
| 1-octen-4-ol | Mean | 0.00 | 1.21 | 1.14 | 41.68 | 0.24 | 0.00 | 331.66 | 227.37 | 241.98 | 0.38 | <0.01 | 7.95 | 133.56 | - |
| Std Dev | 0.00 | 1.58 | 55.38 | 0.34 | 0.00 | 525.86 | 207.31 | 343.34 | 0.66 | 24.20 | 267.27 | ||||
| 2-heptanol | Mean | 0.00 | 0.29 | 0.00 | 0.00 | 4.87 | 0.23 | 6.86 | 0.00 | 2.54 | 1.28 | - | 0.94 | 1.82 | - |
| Std Dev | 0.00 | 0.29 | 0.00 | 6.89 | 0.40 | 11.88 | 0.00 | 4.40 | 2.22 | 2.92 | 5.07 | ||||
| 2,3-butanediol | Mean | 0.00 | 0.00 | 0.00 | 14.74 | 0.09 | 0.00 | 0.00 | 0.00 | 9.75 | 0.49 | <0.01 | 2.70 | 1.71 | - |
| Std Dev | 0.00 | 0.00 | 7.71 | 0.13 | 0.00 | 0.00 | 0.00 | 16.88 | 0.85 | 6.43 | 6.88 | ||||
| 2-butanol | Mean | 0.10 | 0.00 | 0.00 | 53.21 | 0.00 | 0.00 | 2.87 | 2.86 | 3.32 | 0.00 | - | 9.69 | 1.51 | - |
| Std Dev | 0.14 | 0.00 | 75.25 | 0.00 | 0.00 | 3.26 | 4.95 | 5.75 | 0.00 | 32.08 | 3.23 | ||||
| Benzene ehtanol | Mean | 0.50 | 0.77 | 1.39 | 25.37 | 0.00 | 3.01 | 36.72 | 14.80 | 94.01 | 55.48 | - | 4.97 | 34.00 | - |
| Std Dev | 0.71 | 1.09 | 35.88 | 0.00 | 3.86 | 34.55 | 15.31 | 162.81 | 83.03 | 15.19 | 72.50 | ||||
| 4-methyl-2-oxovaleric acid | Mean | 0.71 | 0.37 | 1.90 | 18.83 | 0.24 | 3.14 | 104.72 | 55.93 | 69.18 | 0.47 | <0.001 | 3.84 | 38.91 | - |
| Std Dev | 0.71 | 0.52 | 9.57 | 0.34 | 5.44 | 163.45 | 47.20 | 55.42 | 0.41 | 8.03 | 74.17 | ||||
| Ethanoic acid | Mean | 1.77 | 0.00 | 0.00 | 929.79 | 0.20 | 58.89 | 23.64 | 189.16 | 28.77 | 0.00 | - | 169.41 | 50.08 | - |
| Std Dev | 0.68 | 0.00 | 1314.92 | 0.28 | 79.68 | 34.57 | 167.19 | 49.83 | 0.00 | 560.56 | 94.78 | ||||
| 2-methylpropanoic acid | Mean | 0.00 | 0.00 | 9.36 | 0.00 | 0.00 | 2.56 | 5.82 | 0.00 | 84.84 | 0.51 | - | 0.85 | 15.62 | - |
| Std Dev | 0.00 | 0.00 | 0.00 | 0.00 | 4.43 | 10.08 | 0.00 | 146.95 | 0.45 | 2.82 | 59.78 | ||||
| Butanoic acid | Mean | 16.18 | 7.10 | 0.00 | 74.35 | 7.24 | 4.22 | 827.63 | 23.03 | 52.46 | 18.60 | - | 19.07 | 154.33 | - |
| Std Dev | 22.88 | 5.53 | 105.14 | 0.42 | 4.09 | 1153.83 | 27.35 | 90.87 | 19.82 | 44.04 | 504.02 | ||||
| Pentanoic acid | Mean | 0.00 | 0.00 | 0.00 | 7.82 | 38.36 | 22.91 | 17.60 | 16.62 | 89.90 | 3.74 | - | 8.40 | 25.13 | - |
| Std Dev | 0.00 | 0.00 | 11.06 | 50.77 | 34.36 | 30.49 | 28.79 | 155.71 | 0.72 | 22.34 | 64.45 | ||||
| Hexanoic acid | Mean | 0.00 | 0.00 | 0.00 | 31.67 | 3.52 | 0.00 | 0.00 | 141.74 | 17.49 | 19.31 | <0.05 | 6.40 | 29.76 | - |
| Std Dev | 0.00 | 0.00 | 44.79 | 2.26 | 0.00 | 0.00 | 160.94 | 30.29 | 21.35 | 18.95 | 77.05 | ||||
| acetic acid methylester | Mean | 18.20 | 8.53 | 0.00 | 21.35 | 25.08 | 36.80 | 5.93 | 11.59 | 829.83 | 0.00 | <0.01 | 13.36 | 147.42 | - |
| Std Dev | 8.55 | 12.06 | 16.10 | 29.52 | 20.97 | 10.27 | 13.87 | 1014.77 | 0.00 | 15.37 | 469.05 | ||||
| Ethylacetate | Mean | 196.72 | 22.03 | 0.00 | 45.53 | 0.00 | 9.13 | 6.76 | 52.51 | 6654.61 | 0.00 | <0.05 | 48.06 | 1120.56 | - |
| Std Dev | 111.47 | 31.16 | 64.39 | 0.00 | 15.81 | 11.71 | 37.52 | 7413.99 | 0.00 | 86.40 | 3598.99 | ||||
| Butyric acid methyl ester | Mean | 0.00 | 0.91 | 0.00 | 5.61 | 0.43 | 0.00 | 0.39 | 2.41 | 53.09 | 0.00 | <0.05 | 1.27 | 9.32 | - |
| Std Dev | 0.00 | 1.29 | 7.93 | 0.61 | 0.00 | 0.68 | 4.17 | 46.36 | 0.00 | 3.35 | 25.72 | ||||
| Methyl-3-metilbutanoate | Mean | 0.00 | 0.00 | 0.00 | 63.44 | 28.82 | 4.49 | 0.00 | 0.00 | 57.67 | 0.00 | <0.01 | 16.77 | 10.36 | - |
| Std Dev | 0.00 | 0.00 | 89.72 | 40.75 | 7.77 | 0.00 | 0.00 | 25.77 | 0.00 | 40.40 | 23.71 | ||||
| acetic acid ethylenester | Mean | 0.01 | 0.00 | 0.00 | 2.43 | 0.00 | 2.13 | 0.00 | 6.48 | 26.42 | 1.14 | - | 0.44 | 6.03 | - |
| Std Dev | 0.01 | 0.00 | 3.44 | 0.00 | 3.70 | 0.00 | 11.22 | 45.75 | 1.98 | 1.46 | 18.87 | ||||
| Isoamylacetate | Mean | 7.28 | 0.26 | 0.28 | 26.88 | 0.12 | 0.99 | 0.00 | 13.54 | 1853.35 | 0.00 | <0.01 | 6.31 | 311.31 | - |
| Std Dev | 4.45 | 0.37 | 38.02 | 0.16 | 0.96 | 0.00 | 16.37 | 2378.68 | 0.00 | 16.06 | 1081.33 | ||||
| Hexanoic acid ethyl ester | Mean | 0.73 | 1.00 | 0.68 | 4.59 | 0.72 | 1.66 | 4.59 | 4.82 | 14.48 | 0.50 | <0.05 | 1.35 | 4.34 | - |
| Std Dev | 0.18 | 0.27 | 0.80 | 0.13 | 1.07 | 6.50 | 2.96 | 19.84 | 0.48 | 1.66 | 8.82 | ||||
| Hexanoic acid methyl ester | Mean | 0.50 | 0.00 | 0.00 | 30.71 | 1.07 | 0.00 | 0.00 | 0.00 | 61.78 | 1.52 | <0.001 | 5.87 | 10.55 | - |
| Std Dev | 0.71 | 0.00 | 36.17 | 0.83 | 0.00 | 0.00 | 0.00 | 43.59 | 0.48 | 16.79 | 27.92 | ||||
| isoamyl-n-butyrate | Mean | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | - | 0.01 | 0.00 | - |
| Std Dev | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | ||||
| Octanoic acid methyl ester | Mean | 0.00 | 0.22 | 0.57 | 0.00 | 0.00 | 0.00 | 2.16 | 0.00 | 18.31 | 0.28 | - | 0.09 | 3.46 | - |
| Std Dev | 0.00 | 0.31 | 0.00 | 0.00 | 0.00 | 3.27 | 0.00 | 20.67 | 0.49 | 0.21 | 9.94 | ||||
| Methyldecanoate | Mean | 1.16 | 0.09 | 0.00 | 12.88 | 0.00 | 0.00 | 0.00 | 0.00 | 180.01 | 0.00 | <0.01 | 2.57 | 30,00 | - |
| Std Dev | 1.64 | 0.01 | 18.22 | 0.00 | 0.00 | 0.00 | 0.00 | 243.36 | 0.00 | 7.72 | 108.32 | ||||
| Butyric acid-3-methyl ester | Mean | 0.00 | 0.00 | 0.00 | 172.33 | 0.00 | 0.00 | 109.94 | 0.00 | 145.61 | 0.00 | <0.05 | 31.33 | 42.59 | - |
| Std Dev | 0.00 | 0.00 | 243.71 | 0.00 | 0.00 | 190.43 | 0.00 | 211.31 | 0.00 | 103.92 | 116.07 | ||||
| Hexanoic acid methylester | Mean | 0.72 | 2.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 58.55 | 0.00 | - | 0.52 | 9.76 | - |
| Std Dev | 1.02 | 2.98 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 101.42 | 0.00 | 1.30 | 41.40 | ||||
| o-xylene | Mean | 0.13 | 0.00 | 0.00 | 2.58 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 | - | 0.49 | 0.02 | - |
| Std Dev | 0.18 | 0.00 | 3.65 | 0.00 | 0.00 | 0.20 | 0.00 | 0.00 | 0.00 | 1.55 | 0.08 | ||||
| p-xylene | Mean | 3.04 | 0.00 | 0.00 | 1.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | - | 0.77 | 0.00 | <0.001 |
| Std Dev | 3.06 | 0.00 | 1.66 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.64 | 0.00 | ||||
| Dimethylsulfide | Mean | 21.73 | 7.30 | 35.46 | 56.01 | 1.80 | 14.53 | 0.00 | 7.67 | 40.32 | 9.69 | <0.05 | 19.01 | 12.03 | - |
| Std Dev | 5.32 | 6.54 | 42.67 | 2.55 | 4.25 | 0.00 | 13.27 | 69.83 | 13.29 | 25.51 | 28.55 | ||||
| Dimethylsulfoxide | Mean | 0.00 | 0.21 | 0.00 | 2.49 | 0.00 | 0.00 | 1.13 | 0.00 | 0.00 | 6.32 | - | 0.49 | 1.24 | |
| Std Dev | 0.00 | 0.30 | 3.52 | 0.00 | 0.00 | 1.01 | 0.00 | 0.00 | 8.21 | 1.49 | 3.70 | ||||
| Dimethylsulfone | Mean | 0.81 | 2.11 | 0.87 | 2.23 | 7.80 | 2.04 | 0.00 | 1.32 | 0.01 | 13.49 | <0.001 | 2.44 | 2.81 | - |
| Std Dev | 0.26 | 1.00 | 3.16 | 5.96 | 1.83 | 0.00 | 2.29 | 0.01 | 5.63 | - | 3.52 | 5.43 | - | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faustini, M.; Quintavalle Pastorino, G.; Colombani, C.; Chiesa, L.M.; Panseri, S.; Vigo, D.; Curone, G. Volatilome in Milk for Grana Padano and Parmigiano Reggiano Cheeses: A First Survey. Vet. Sci. 2019, 6, 41. https://doi.org/10.3390/vetsci6020041
Faustini M, Quintavalle Pastorino G, Colombani C, Chiesa LM, Panseri S, Vigo D, Curone G. Volatilome in Milk for Grana Padano and Parmigiano Reggiano Cheeses: A First Survey. Veterinary Sciences. 2019; 6(2):41. https://doi.org/10.3390/vetsci6020041
Chicago/Turabian StyleFaustini, Massimo, Giovanni Quintavalle Pastorino, Carla Colombani, Luca Maria Chiesa, Sara Panseri, Daniele Vigo, and Giulio Curone. 2019. "Volatilome in Milk for Grana Padano and Parmigiano Reggiano Cheeses: A First Survey" Veterinary Sciences 6, no. 2: 41. https://doi.org/10.3390/vetsci6020041
APA StyleFaustini, M., Quintavalle Pastorino, G., Colombani, C., Chiesa, L. M., Panseri, S., Vigo, D., & Curone, G. (2019). Volatilome in Milk for Grana Padano and Parmigiano Reggiano Cheeses: A First Survey. Veterinary Sciences, 6(2), 41. https://doi.org/10.3390/vetsci6020041







