Correlation of BRAF Variant V595E, Breed, Histological Grade and Cyclooxygenase-2 Expression in Canine Transitional Cell Carcinomas
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Histology
2.3. Immunohistochemistry
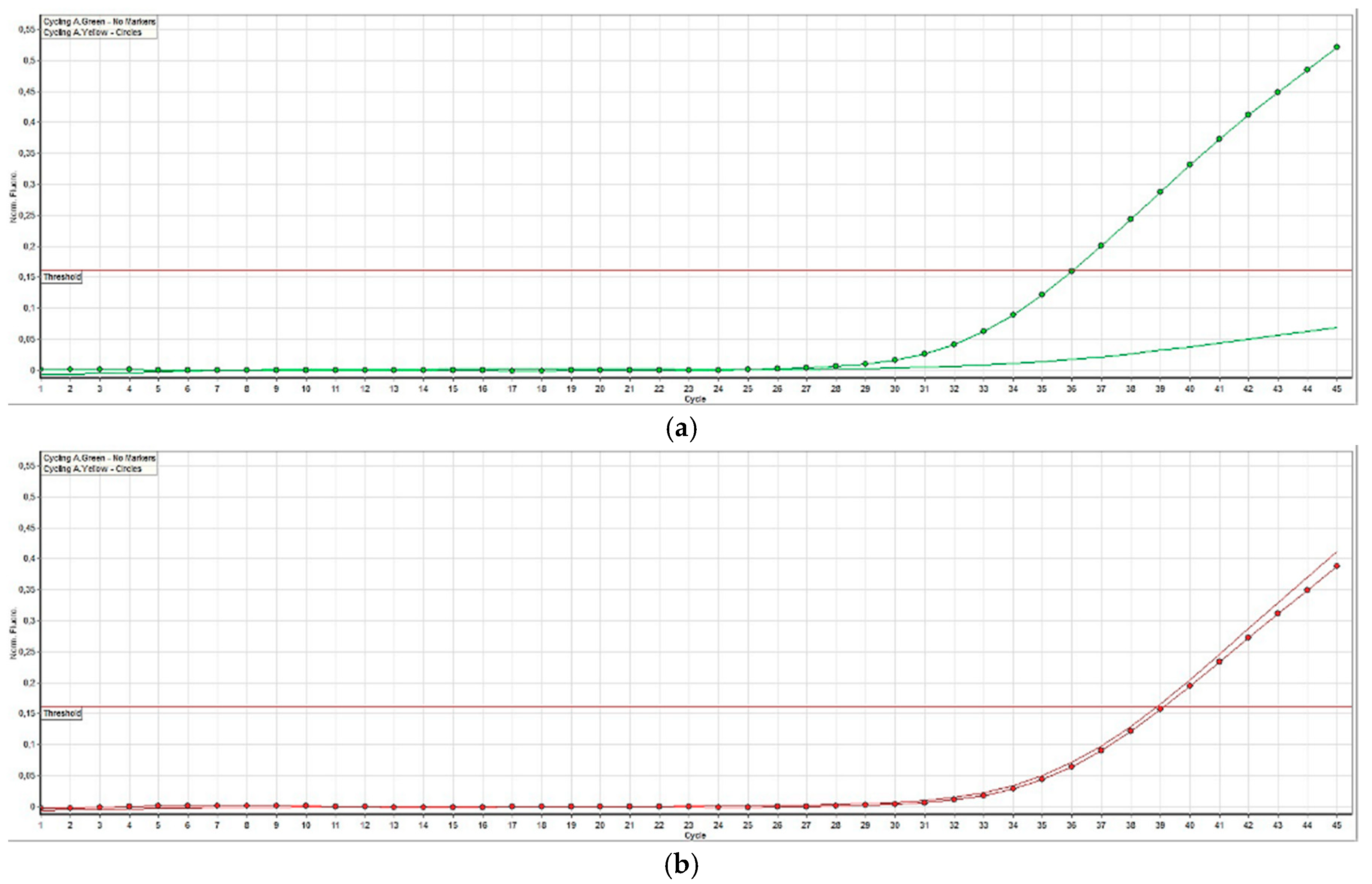
2.4. BRAF Mutation Analysis
2.5. Statistical Analyses
3. Results
3.1. Histology
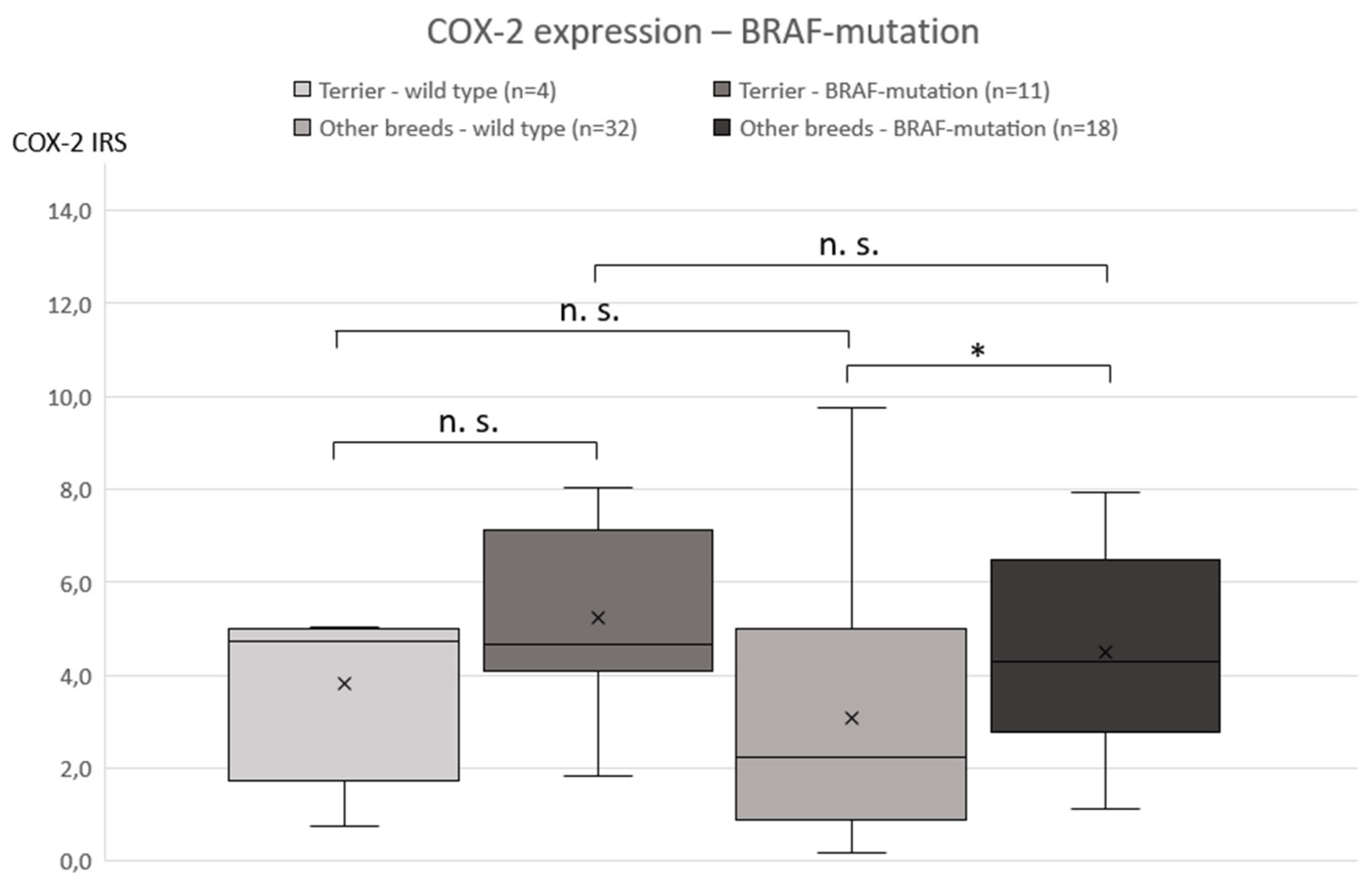
3.2. COX-2 Immunohistochemistry
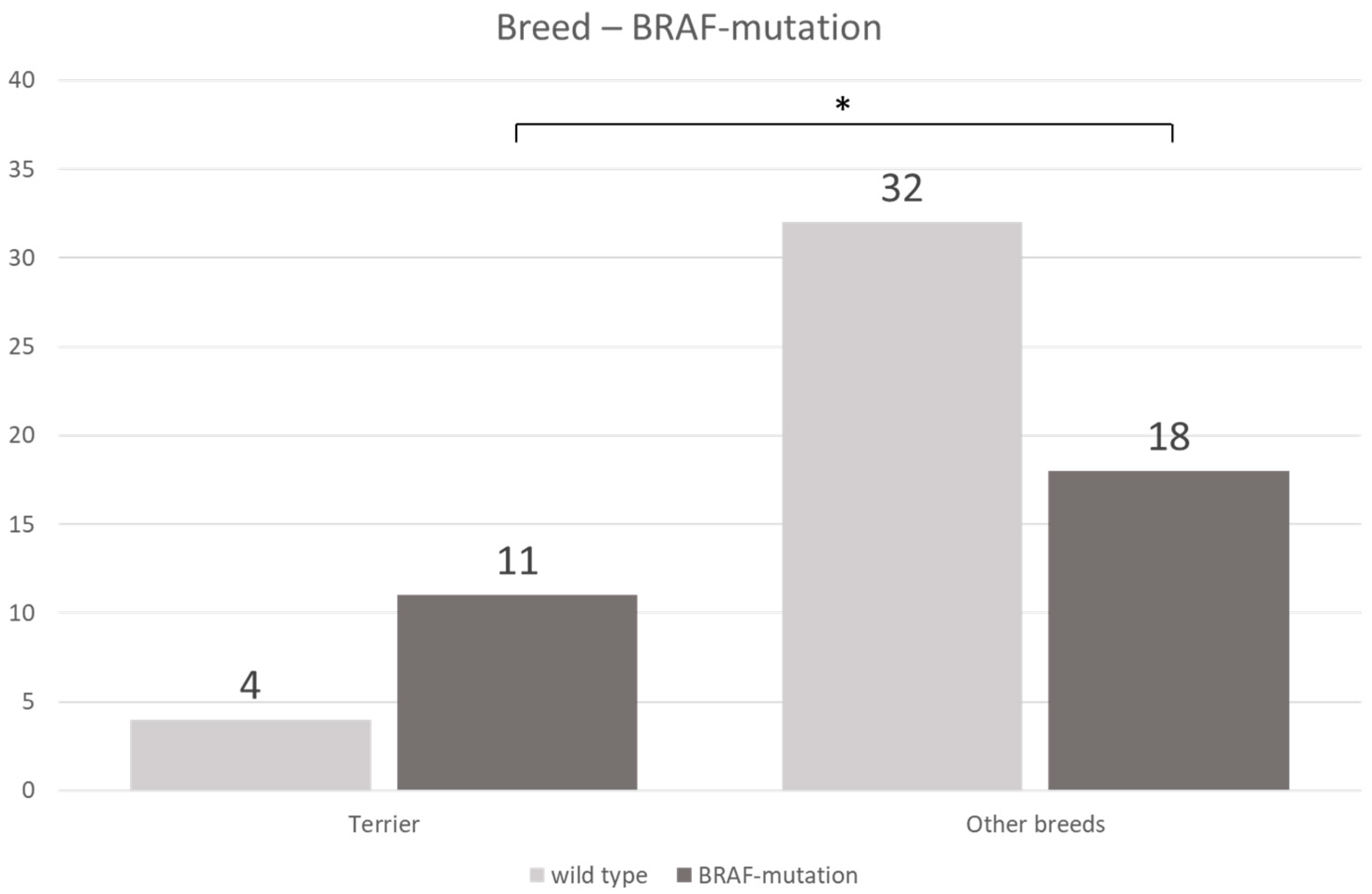
3.3. BRAF Mutation
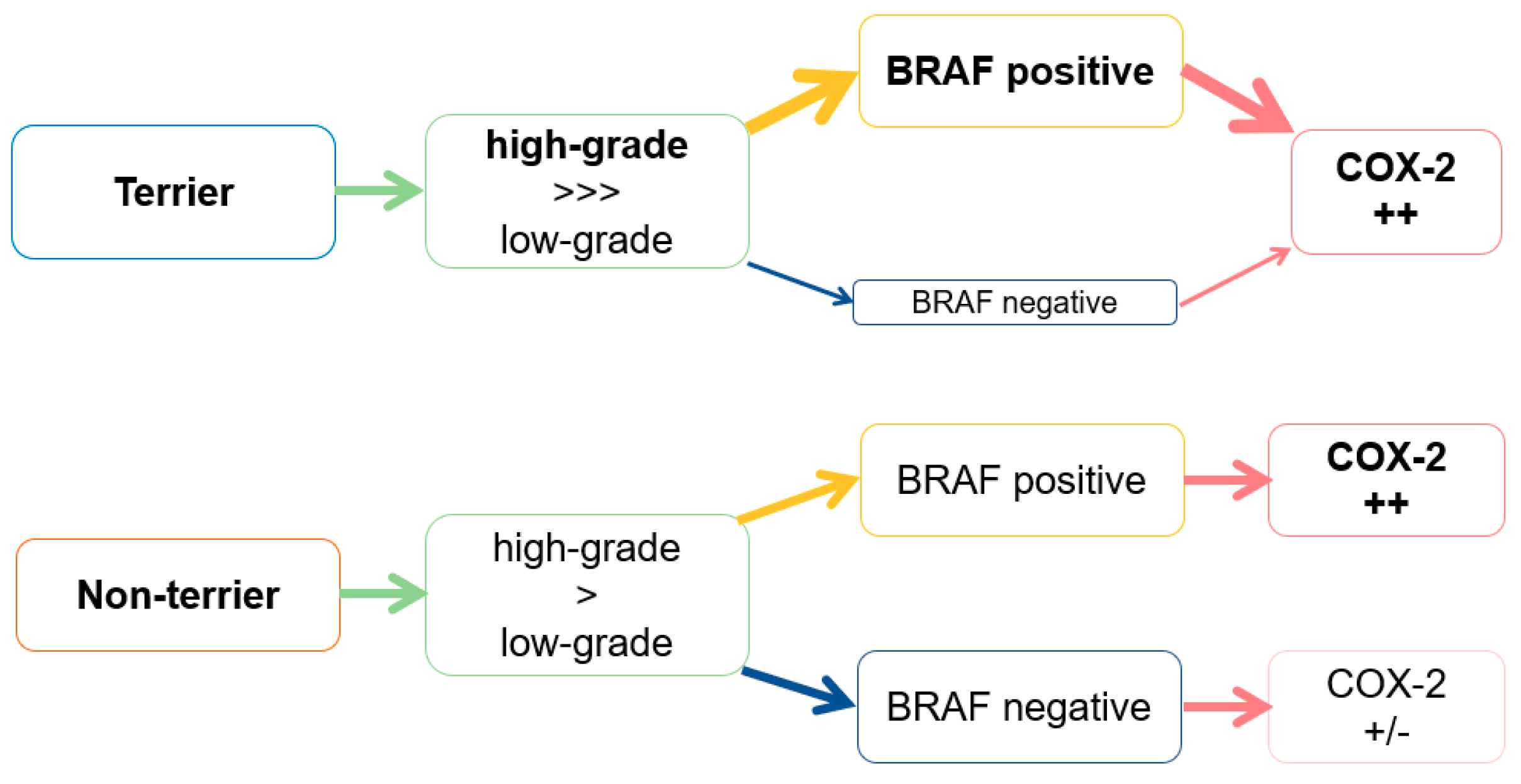
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fulkerson, C.M.; Knapp, D.W. Management of transitional cell carcinoma of the urinary bladder in dogs: A review. Vet. J. 2015, 205, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, A.J.; Widmer, W.R.; Deborah, W.; Knapp, D.W. Canine Transitional Cell Carcinoma. J. Vet. Intern Med. 2003, 17, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.W.; Glickman, N.W.; DeNicola, D.B.; Bonney, P.L.; Lin, T.L.; Glickman, L.T. Naturally-occurring canine transitional cell carcinoma of the urinary bladder. A relevant model of human invasive bladder cancer. Urol. Oncol. 2000, 5, 47–59. [Google Scholar] [CrossRef]
- Knapp, D.W.; Ramos-Vara, J.A.; Moore, G.E.; Dhawan, D.; Bonney, P.L.; Young, K.E. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. Ilar J. 2014, 55, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Norris, A.M.; Laing, E.J.; Valli, V.E.; Withrow, S.J.; Macy, D.W.; Ogilvie, G.K.; Tomlinson, J.; McCaw, D.; Pidgeon, G.; Jacobs, R.M. Canine bladder and urethral tumors: A retrospective study of 115 cases (1980–1985). J. Vet. Intern. Med. 1992, 6, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Pantke, P. Diagnosis and treatment of transitional cell carcinoma of the lower urinary tract in the dog. Kleintierprax 2018, 63, 76–92. [Google Scholar]
- Knapp, D.W.; Ruple-Czerniak, A.; Ramos-Vara, J.A.; Naughton, J.F.; Fulkerson, C.M.; Honkisz, S.I. A nonselective cyclooxygenase inhibitor enhances the activity of vinblastine in a naturally-occurring canine model of invasive urothelial carcinoma. Bladder Cancer 2016, 2, 241–250. [Google Scholar] [CrossRef]
- Knottenbelt, C.; Mellor, D.; Nixon, C.; Thompson, H.; Argyle, D.J. Cohort study of COX-1 and COX-2 expression in canine rectal and bladder tumours. J. Small Anim. Pract. 2006, 47, 196–200. [Google Scholar] [CrossRef]
- Mohammed, S.I.; Bennett, P.F.; Craig, B.A.; Glickman, N.W.; Mutsaers, A.J.; Snyder, P.W.; Widmer, W.R.; DeGortari, A.E.; Bonney, P.L.; Knapp, D.W. Effects of the cyclooxygenase inhibitor, piroxicam, on tumor response, apoptosis, and angiogenesis in a canine model of human invasive urinary bladder cancer. Cancer Res. 2002, 62, 56–58. [Google Scholar]
- Carvalho, S.; Stoll, A.L.; Priestnall, S.L.; Suarez-Bonnet, A.; Rassnick, K.; Lynch, S.; Schoepper, I.; Romanelli, G.; Buracco, P.; Atherton, M.; et al. Retrospective evaluation of COX-2 expression, histological and clinical factors as prognostic indicators in dogs with renal cell carcinomas undergoing nephrectomy. Vet. Comp. Oncol. 2017, 15, 1280–1294. [Google Scholar] [CrossRef]
- Mohammed, S.I.; Khan, K.N.; Sellers, R.S.; Hayek, M.G.; DeNicola, D.B.; Wu, L.; Bonney, P.L.; Knapp, D.W. Expression of cyclooxygenase-1 and 2 in naturally-occurring canine cancer. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 479–483. [Google Scholar] [CrossRef]
- Khan, K.N.; Knapp, D.W.; Denicola, D.B.; Harris, R.K. Expression of cyclooxygenase-2 in transitional cell carcinoma of the urinary bladder in dogs. Am. J. Vet. Res. 2000, 61, 478–481. [Google Scholar] [CrossRef]
- Lee, J.Y.; Tanabe, S.; Shimohira, H.; Kobayashi, Y.; Oomachi, T.; Azuma, S.; Ogihara, K.; Inokuma, H. Expression of cyclooxygenase-2, P-glycoprotein and multi-drug resistance-associated protein in canine transitional cell carcinoma. Res. Vet. Sci. 2007, 83, 210–216. [Google Scholar] [CrossRef]
- Mutsaers, A.J.; Mohammed, S.I.; DeNicola, D.B.; Snyder, P.W.; Glickman, N.W.; Bennett, P.F.; de Gortari, A.E.; Bonney, P.L.; Knapp, D.W. Pretreatment tumor prostaglandin E2 concentration and cyclooxygenase-2 expression are not associated with the response of canine naturally occurring invasive urinary bladder cancer to cyclooxygenase inhibitor therapy. Prostaglandins Leukot. Essent. Fat. Acids 2005, 72, 181–186. [Google Scholar] [CrossRef]
- Hurst, E.A.; Pang, L.Y.; Argyle, D.J. The selective cyclooxygenase-2 inhibitor mavacoxib (Trocoxcil™) exerts anti-tumour effects in-vitro independent of cyclooxygenase-2 expression levels. Vet. Comp. Oncol. 2019. [Google Scholar] [CrossRef]
- Herr, H.W.; Shipley, W.U.; Bajorin, D.F. Cancer of the bladder. In DeVita VT, 6th ed.; Hellman, S., Rosenberg, S.A., Eds.; Principles and Practice of Oncology; Lippincott-Raven Publishers: Philadelphia, PA, USA, 2001; pp. 139–141. [Google Scholar]
- Shariat, S.F.; Matsumoto, K.; Kim, J.; Ayala, G.E.; Zhou, J.H.; Jian, W.; Benedict, W.F.; Lerner, S. Correlation of cyclooxygenase-2 expression with molecular markers, pathological features and clinical outcome of transitional cell carcinoma of the bladder. J. Urol. 2003, 170, 985–989. [Google Scholar] [CrossRef]
- Agrawal, U.; Kumari, N.; Vasudeva, P.; Mohanty, N.K.; Saxena, S. Overexpression of COX2 indicates poor survival in urothelial bladder cancer. Ann. Diagn. Pathol. 2018, 34, 50–55. [Google Scholar] [CrossRef]
- Kömhoff, M.; Guan, Y.; Shappell, H.W.; Davis, L.; Jack, G.; Shyr, Y.; Koch, M.O.; Shappell, S.B.; Breyer, M.D. Enhanced expression of cyclooxygenase-2 in high grade human transitional cell bladder carcinomas. Am. J. Pathol. 2000, 157, 29–35. [Google Scholar] [CrossRef]
- Tabriz, H.M.; Olfati, G.; Ahmadi, S.A.; Yusefnia, S. Cyclooxygenase-2 expression in urinary bladder transitional cell carcinoma and its association with clinicopathological characteristics. Asian Pac. J. Cancer Prev. 2013, 14, 4539–4543. [Google Scholar] [CrossRef]
- Manoharan, M.; Soloway, M.S. Optimal management of the T1G3 bladder cancer. Urol. Clin. N. Am. 2005, 32, 133–145. [Google Scholar] [CrossRef]
- Reisz, P.A.; Laviana, A.A.; Chang, S.S. Management of High-grade T1 Urothelial Carcinoma. Curr. Urol. Rep. 2018, 19, 103. [Google Scholar] [CrossRef]
- Chou, R.; Buckley, D.; Fu, R.; Gore, J.L.; Gustafson, K.; Griffin, J.; Grusing, S.; Selph, S. AHRQ comparative effectiveness reviews. Emerging Approaches Diagnose and Treatment Non-Muscle-Invasive Bladder Cancer [Internet] 2015; Report No.: 15(16)-EHC017-EF; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2015.
- Burkhari, N.; Al-Shamsi, H.O.; Azam, F. Update on the Treatment of Metastatic Urothelial Carcinoma. Sci. World J. 2018, 2018, 5682078. [Google Scholar]
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Aupperle-Lellbach, H.; Grassinger, J.; Hohloch, C.; Kehl, A.; Pantke, P. Diagnostic value of the BRAF variant V595E in urine samples, smears and biopsies from canine transitional cell carcinoma. Tierarztl. Prax. K. 2018, 46, 289–295. [Google Scholar]
- Decker, B.; Parker, H.G.; Dhawan, D.; Kwon, E.M.; Karlins, E.; Davis, B.W.; Ramos-Vara, J.A.; Bonney, P.L.; McNiel, E.A.; Knapp, D.W.; et al. Homologous mutation to human BRAF V600E is common in naturally occurring canine bladder cancer—Evidence for a relevant model system and urine-based diagnostic test. Mol. Cancer Res. 2015, 13, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Kennedy, K.; Shapiro, S.G.; Breen, M. BRAF Mutations in canine cancers. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Shapiro, S.G.; Breen, M. Detection of BRAF Mutation in urine DNA as a molecular diagnostic for canine urothelial and prostatic carcinoma. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Dhillon, A.; Hagan, S.; Rath, O.; Kolch, W. Map kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Breen, M. Comparative aspects of BRAF mutations in canine cancers. Vet. Sci. 2015, 2, 231–245. [Google Scholar] [CrossRef]
- Boulalas, I.; Zaravinos, A.; Delakas, D.; Spandidos, D.A. Mutational analysis of the BRAF gene in transitional cell carcinoma of the bladder. Int. J. Biol. Markers 2009, 24, 17–21. [Google Scholar] [CrossRef]
- Marranci, A.; Jiang, Z.; Vitiello, M.; Guzzolino, E.; Comelli, L.; Sarti, S.; Lubrano, S.; Franchin, C.; Echevarría-Vargas, I.; Tuccoli, A.; et al. The landscape of BRAF transcript and protein variants in human cancer. Mol. Cancer 2017, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.T.; Nikiforova, M.N.; Zhu, Z.; Knauf, J.A.; Nikiforov, Y.E.; Fagin, J.A. High prevalence of BRAF mutations in thyroid cancer: Genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003, 63, 1454–1457. [Google Scholar] [PubMed]
- Rajagopalan, H.; Bardelli, A.; Lengauer, C.; Kinzler, K.W.; Vogelstein, B.; Velculescu, V.E. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature 2002, 418, 934. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Long, Z.W.; Yang, J.; Lin, X. Correlations of IGF-1R and COX-2 Expressions with Ras and BRAF genetic mutations, Clinicopathological Features and Prognosis of Colorectal Cancer Patients. Pathol. Oncol. Res. 2018, 24, 45–57. [Google Scholar] [CrossRef]
- Mochizuki, H.; Breen, M. Sequence analysis of RAS and RAF mutation hot spots in canine carcinoma. Vet. Comp. Oncol. 2017, 15, 1598–1605. [Google Scholar] [CrossRef]
- Bourn, J.; Cekanova, M. Cyclooxygenase inhibitors potentiate receptor tyrosine kinase therapies in bladder cancer cells in vitro. Drug Des. Devel. Ther. 2018, 13, 1727–1742. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Csh Protoc. 2008. [Google Scholar] [CrossRef]
- Meuten, D.J.; Meuten, T.L.K. Tumors of the Urinary System. In Tumors of Domestic Animals, 5th ed.; Meuten, D.J., Ed.; Wiley-Blackwell: Ames, IN, USA, 2017; Volume 1, pp. 632–688. [Google Scholar]
- Ramos-Vara, J.A.; Miller, M.A. When tissue antigens and antibodies get along: Revisiting the technical aspects of immunohistochemistry—The red, brown, and blue technique. Vet. Pathol. 2013, 51, 42–87. [Google Scholar] [CrossRef]
- Hoffmann, C.; Bazer, F.W.; Klug, J.; Aupperle, H.; Ellenberger, C.; Schoon, H.A. Immunohistochemical and histochemical identification of proteins and carbohydrates in the equine endometrium Expression patterns for mares suffering from endometrosis. Theriogenology 2009, 71, 264–274. [Google Scholar] [CrossRef] [PubMed]
- De Brot, S.; Robinson, B.D.; Scase, T.; Grau-Roma, L.; Wilkinson, E.; Boorjian, S.A.; Gardner, D.; Mongan, N.P. The dog as an animal model for bladder and urethral urothelial carcinoma: Comparative epidemiology and histology. Oncol. Lett. 2018, 16, 1641–1649. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Stoeltzing, O.; Liu, W.; Fan, F.; Wagner, C.; Stengel, K.; Somcio, R.J.; Reinmuth, N.; Parikh, A.A.; Hicklin, D.J.; Ellis, L.M. Regulation of cyclooxygenase-2 (COX-2) expression in human pancreatic carcinoma cells by the insulin-like growth factor-I receptor (IGF-IR) system. Cancer Lett. 2007, 258, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Doré, M. Cyclooxygenase-2 expression in animal cancers. Vet. Pathol. 2011, 48, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Allstadt, S.D.; Rodriguez, C.O., Jr.; Boostrom, B.; Rebhun, R.B.; Skorupski, K.A. Randomized phase III trial of piroxicam in combination with mitoxantrone or carboplatin for first-line treatment of urogenital tract transitional cell carcinoma in dogs. J. Vet. Intern. Med. 2015, 29, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.W.; Richardson, R.C.; Chan, T.C.; Bottoms, G.D.; Widmer, W.R.; DeNicola, D.B.; Teclaw, R.; Bonney, P.L.; Kuczek, T. Piroxicam therapy in 34 dogs with transitional cell carcinoma of the urinary bladder. J. Vet. Intern. Med. 1994, 8, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Arantes-Rodrigues, R.; Pinto-Leite, R.; Ferreira, R.; Neuparth, M.J.; Pires, M.J.; Gaivão, I.; Palmeira, C.; Santos, L.; Colaço, A.; Oliveira, P. Meloxicam in the treatment of in vitro and in vivo models of urinary bladder cancer. Biomed. Pharmacother. 2013, 67, 277–284. [Google Scholar] [CrossRef]
- Meyer, P.; Sergi, C.; Garbe, C. Polymorphisms of the BRAF gene predispose males to malignant melanoma. J. Carcinog. 2003, 2, 7. [Google Scholar] [CrossRef]
- Maeda, S.; Tomiyasu, H.; Tsuboi, M.; Inoue, A.; Ishihara, G.; Uchikai, T.; Chambers, J.K.; Uchida, K.; Yonezawa, T.; Matsuki, N. Comprehensive gene expression analysis of canine invasive urothelial bladder carcinoma by RNA-Seq. BMC Cancer 2018, 18, 472. [Google Scholar] [CrossRef]
- Breen, M.; Wiley, C. Flüssigbiopsie—Die Zukunft der Tumordiagnostik. Vet. Focus 2018, 28, 39–45. [Google Scholar]
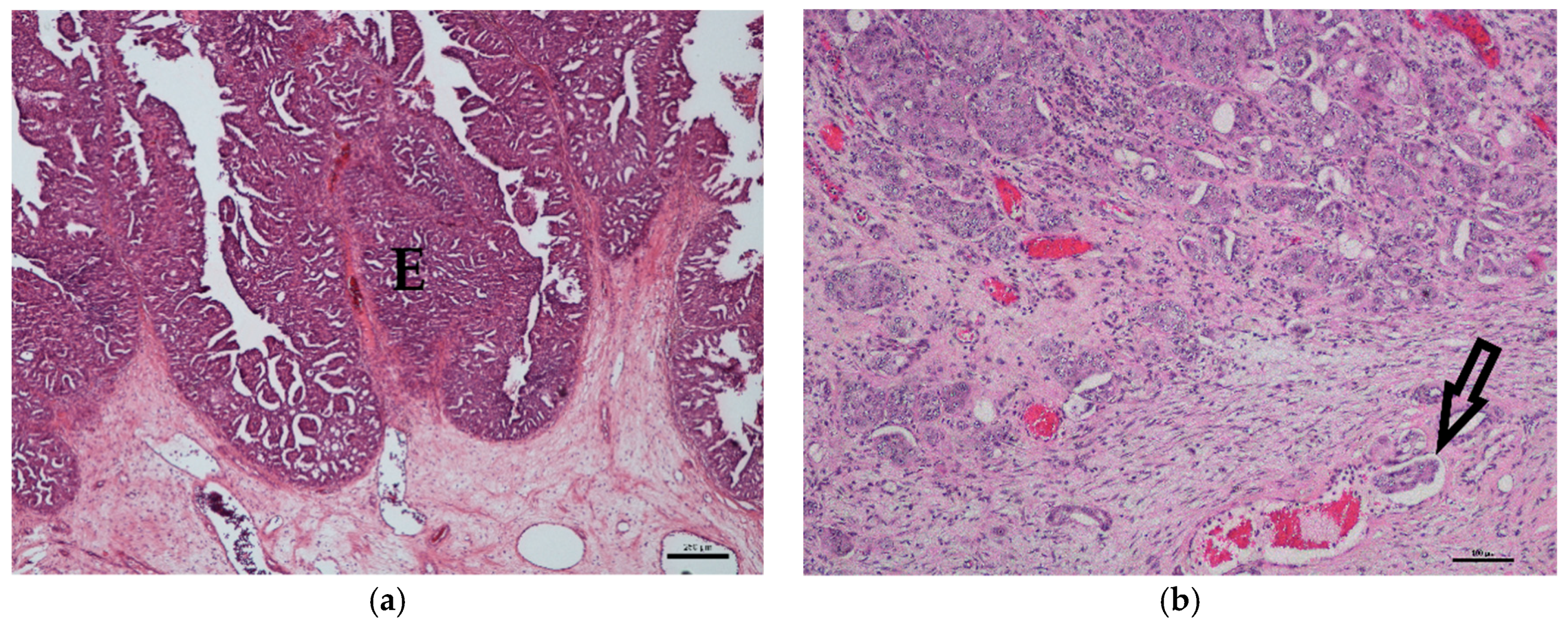
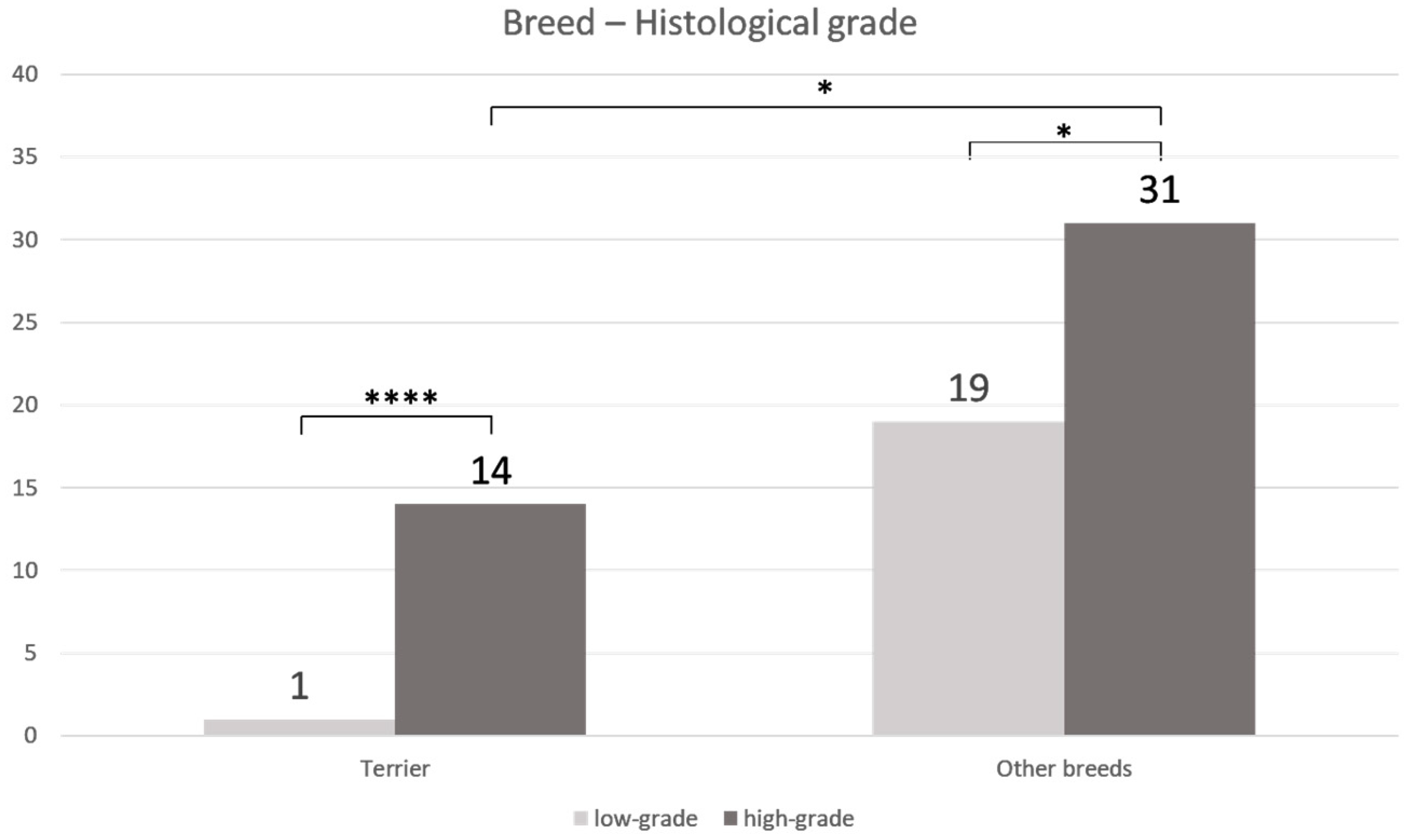
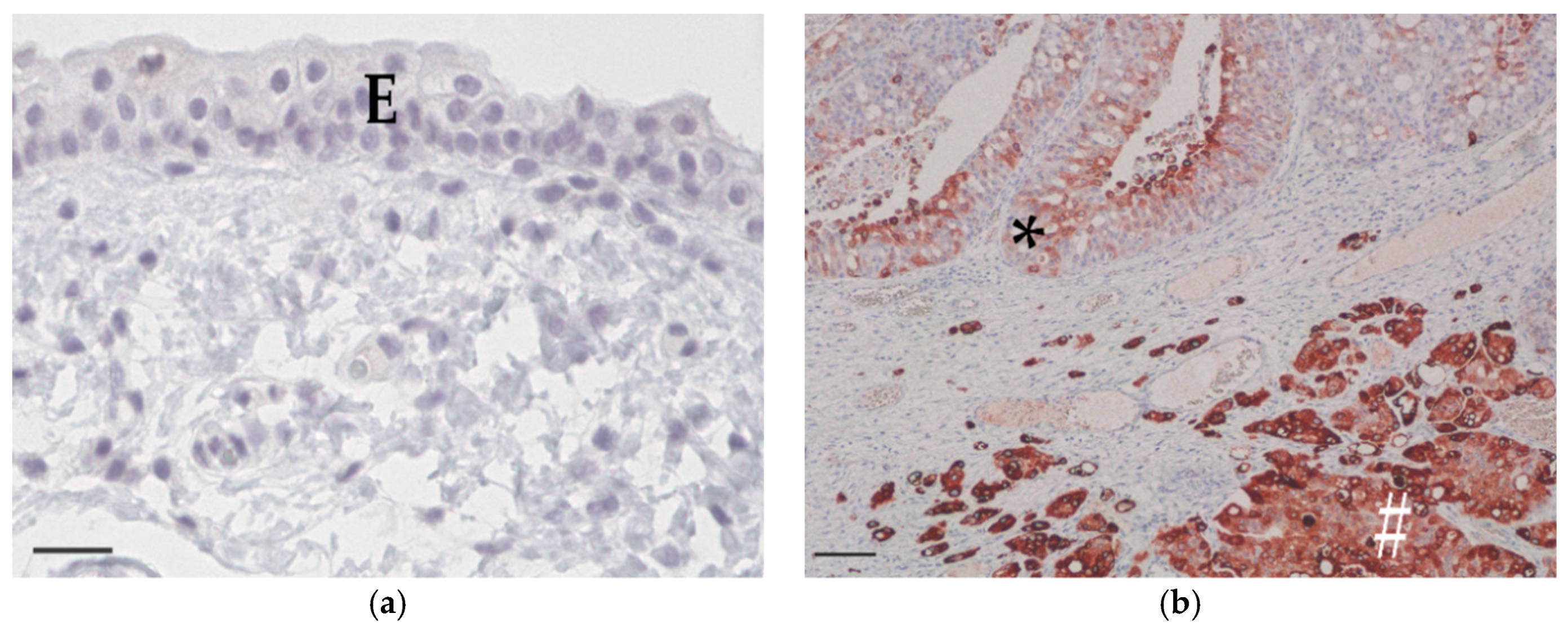
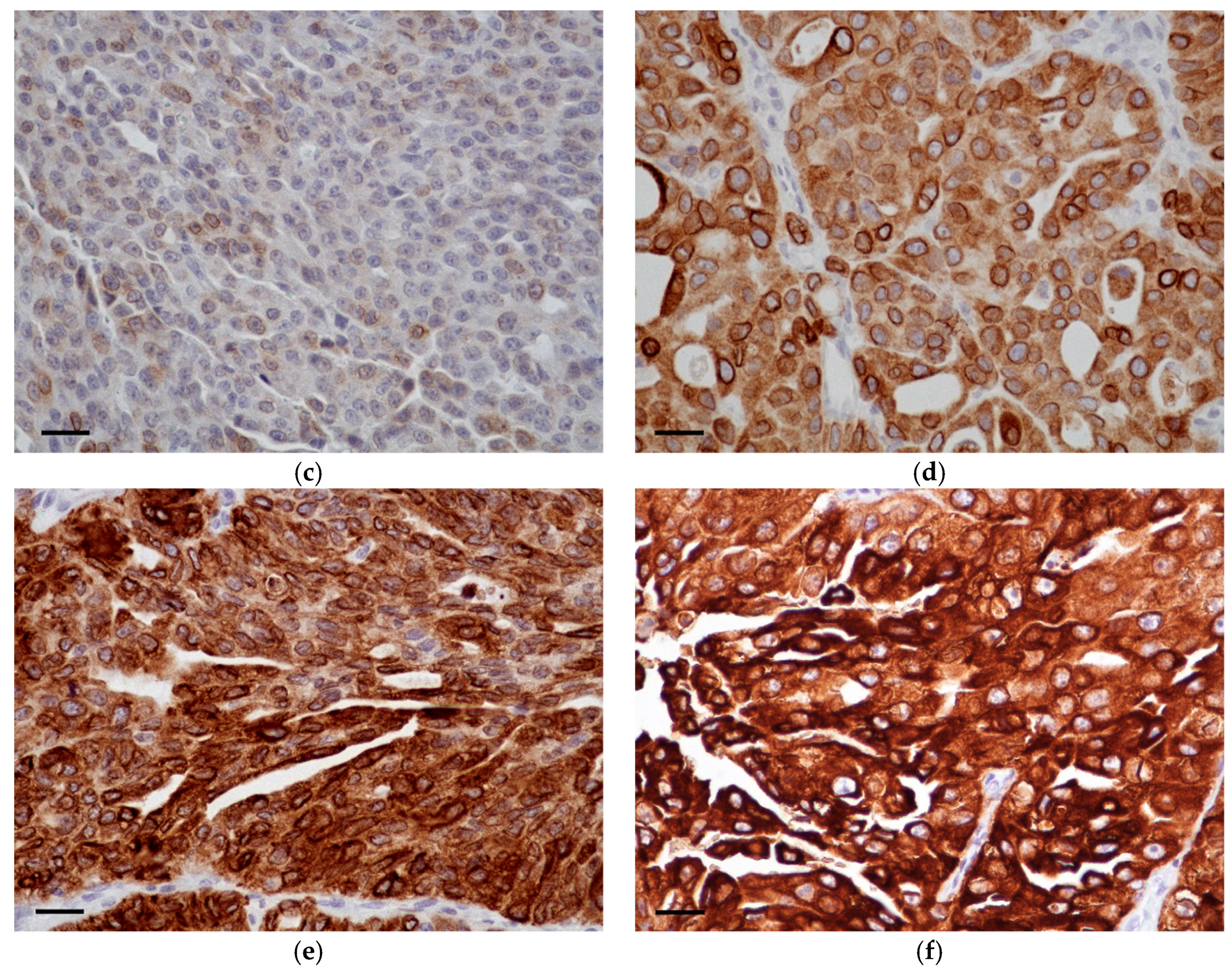
| Breed | Mean Age (Years) | Sex | Histological Grade | Median COX-2 IRS | BRAF Mutation |
|---|---|---|---|---|---|
| Scottish terrier (n = 5) | 10 ± 2 | 3 F, 1 FN, 1 M | 5 high | 4.5 (range: 0.8–7.6) | 4+ 1− |
| Jack Russel terrier (n = 4) | 12 ± 1 | 3 F, 1 FN | 4 high | 4.8 (range: 4.5–5.1) | 3+ 1− |
| West Highland white terrier (n = 2) | 11 ± 2 | 1 FN, 1 M | 1 high 1 low | 5.8 (range: 4.8–6.7) | 1+ 1− |
| Airedale terrier (n = 1) | 10 | FN | high | 4.1 | + |
| Fox terrier (n = 1) | 12 | FN | high | 3.8 | + |
| Welsh terrier (n = 1) | 12 | FN | high | 4.7 | − |
| Yorkshire terrier (n = 1) | 11 | F | high | 7.1 | + |
| Breed | Mean Age (Years) | Sex | Histological Grade | Median COX-2 IRS | BRAF Mutation |
|---|---|---|---|---|---|
| Mongrel (n = 21) | 11 ± 2 | 7 F, 6 FN, 4 M, 4MN | 13 high 8 low | 4.0 (range: 1.4–8.1) | 9+ 12− |
| Beagle (n = 4) | 10 ± 2 | 1 F, 3 FN | 3 high 1 low | 1.9 (range: 1.3–7.9) | 3+ 1− |
| Bernese mountain dog (n = 3) | 9 ± 2 | 1 FN, 2 M | 2 high 1 low | 5.2 (range: 0.5–6.9) | 1+ 2− |
| Cocker spaniel (n = 3) | 10 ± 3 | 1 F, 1 FN, 1 MN | 2 high 1 low | 0.7 (range: 0.4–1.2) | 3− |
| Poodle (n = 3) | 11 ± 1 | 1 F, 1 FN, 1 M | 2 high 1 low | 3.3 (range: 3.0–7.8) | 2+ 1− |
| Shetland sheepdog (n = 3) | 10 ± 2 | 3 F | 1 high 2 low | 1.9 (range: 0.3–2.3) | 1+ 2− |
| Australianshepherd (n = 2) | 10 ± 1 | 1 F, 1 FN | 1 high 1 low | 4.4 (range: 3.8–5.0) | 2− |
| Small Münsterländer (n = 1) | 11 | F | low | 5.0 | − |
| Rottweiler (n = 1) | 10 | M | low | 1.0 | − |
| Podenco (n = 1) | 11 | MN | low | 7.8 | + |
| Siberian husky (n = 1) | 12 | MN | low | 1.1 | + |
| German wirehaired pointer (n = 1) | 8 | F | high | 0.8 | − |
| Great dane (n = 1) | 7 | M | high | 0.9 | − |
| Bracke (n = 1) | 11 | FN | high | 0.2 | − |
| French bulldog (n = 1) | 10 | F | high | 4.1 | − |
| Basset (n = 1) | 12 | FN | high | 9.8 | − |
| Bichon frise (n = 1) | 11 | MN | high | 0.3 | − |
| Border collie (n = 1) | 12 | M | high | 2.0 | − |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassinger, J.M.; Merz, S.; Aupperle-Lellbach, H.; Erhard, H.; Klopfleisch, R. Correlation of BRAF Variant V595E, Breed, Histological Grade and Cyclooxygenase-2 Expression in Canine Transitional Cell Carcinomas. Vet. Sci. 2019, 6, 31. https://doi.org/10.3390/vetsci6010031
Grassinger JM, Merz S, Aupperle-Lellbach H, Erhard H, Klopfleisch R. Correlation of BRAF Variant V595E, Breed, Histological Grade and Cyclooxygenase-2 Expression in Canine Transitional Cell Carcinomas. Veterinary Sciences. 2019; 6(1):31. https://doi.org/10.3390/vetsci6010031
Chicago/Turabian StyleGrassinger, Julia M., Sophie Merz, Heike Aupperle-Lellbach, Hanna Erhard, and Robert Klopfleisch. 2019. "Correlation of BRAF Variant V595E, Breed, Histological Grade and Cyclooxygenase-2 Expression in Canine Transitional Cell Carcinomas" Veterinary Sciences 6, no. 1: 31. https://doi.org/10.3390/vetsci6010031
APA StyleGrassinger, J. M., Merz, S., Aupperle-Lellbach, H., Erhard, H., & Klopfleisch, R. (2019). Correlation of BRAF Variant V595E, Breed, Histological Grade and Cyclooxygenase-2 Expression in Canine Transitional Cell Carcinomas. Veterinary Sciences, 6(1), 31. https://doi.org/10.3390/vetsci6010031






