Oxidative Effects of Potassium Dichromate on Biochemical, Hematological Characteristics, and Hormonal Levels in Rabbit Doe (Oryctolagus cuniculus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Toxicological Assessment
2.4. Hematological and Biochemical Analysis
2.5. Weight and Volume of Organs
2.6. Oxidative Stress Markers
2.6.1. Estimation of Catalase Activity
2.6.2. Estimation of Glutathione
2.6.3. Estimation of Lipid Peroxidation
2.6.4. Estimation of Superoxide Dismutase
2.7. Statistical Analysis
3. Results
3.1. Effects of Potassium Dichromate on Indicators of Toxicity in Rabbit Does
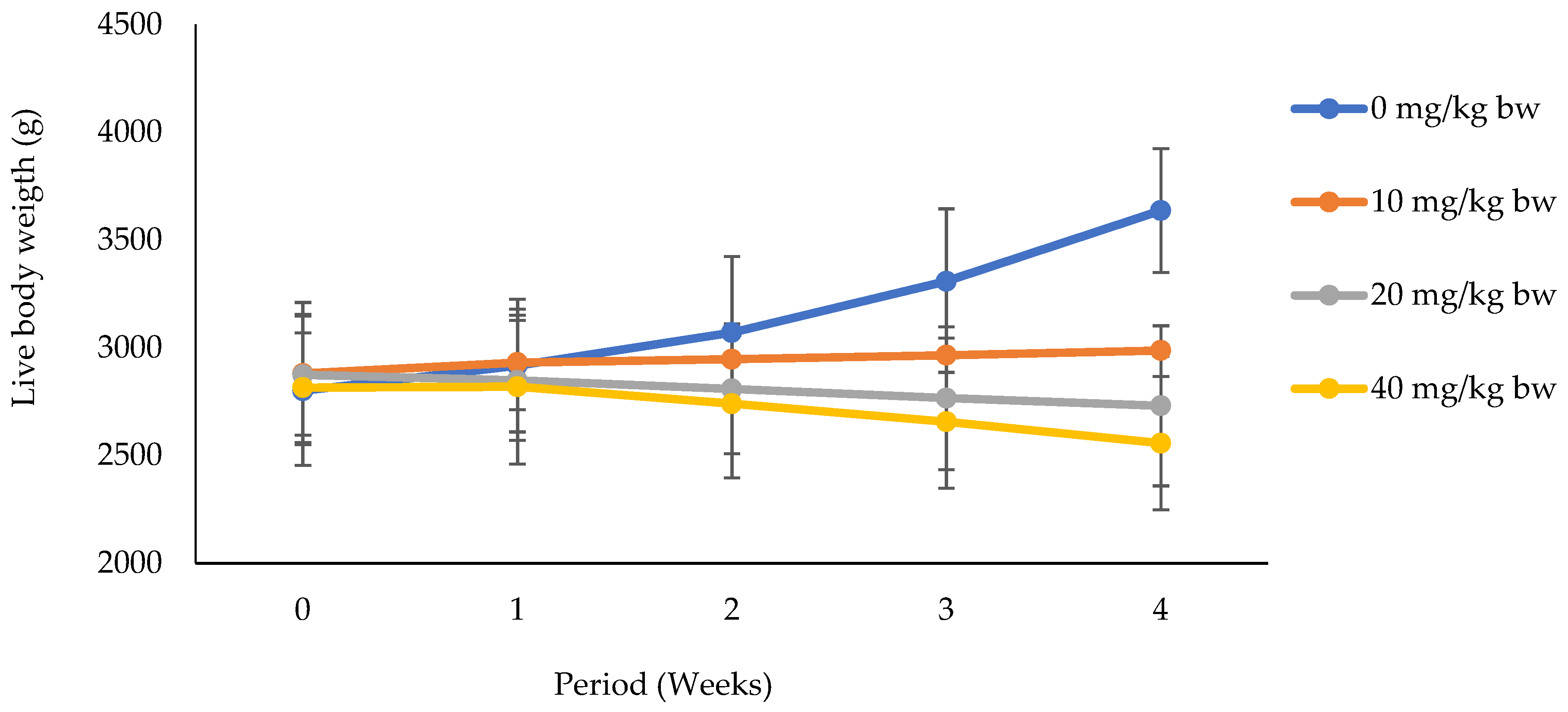
3.1.1. Effects of Potassium Dichromate on Live Body Weight in Rabbit Does
3.1.2. Kidney Weight and Volume and Biochemical Markers of Nephrotoxicity
3.1.3. Liver Weight and Volume and Biochemical Markers of Hepatotoxicity
3.2. Effects of Potassium Dichromate on Reproductive Toxicity Indicators in Rabbit Does
3.2.1. Ovary Weight and Tissue Proteins
3.2.2. Reproductive Hormones
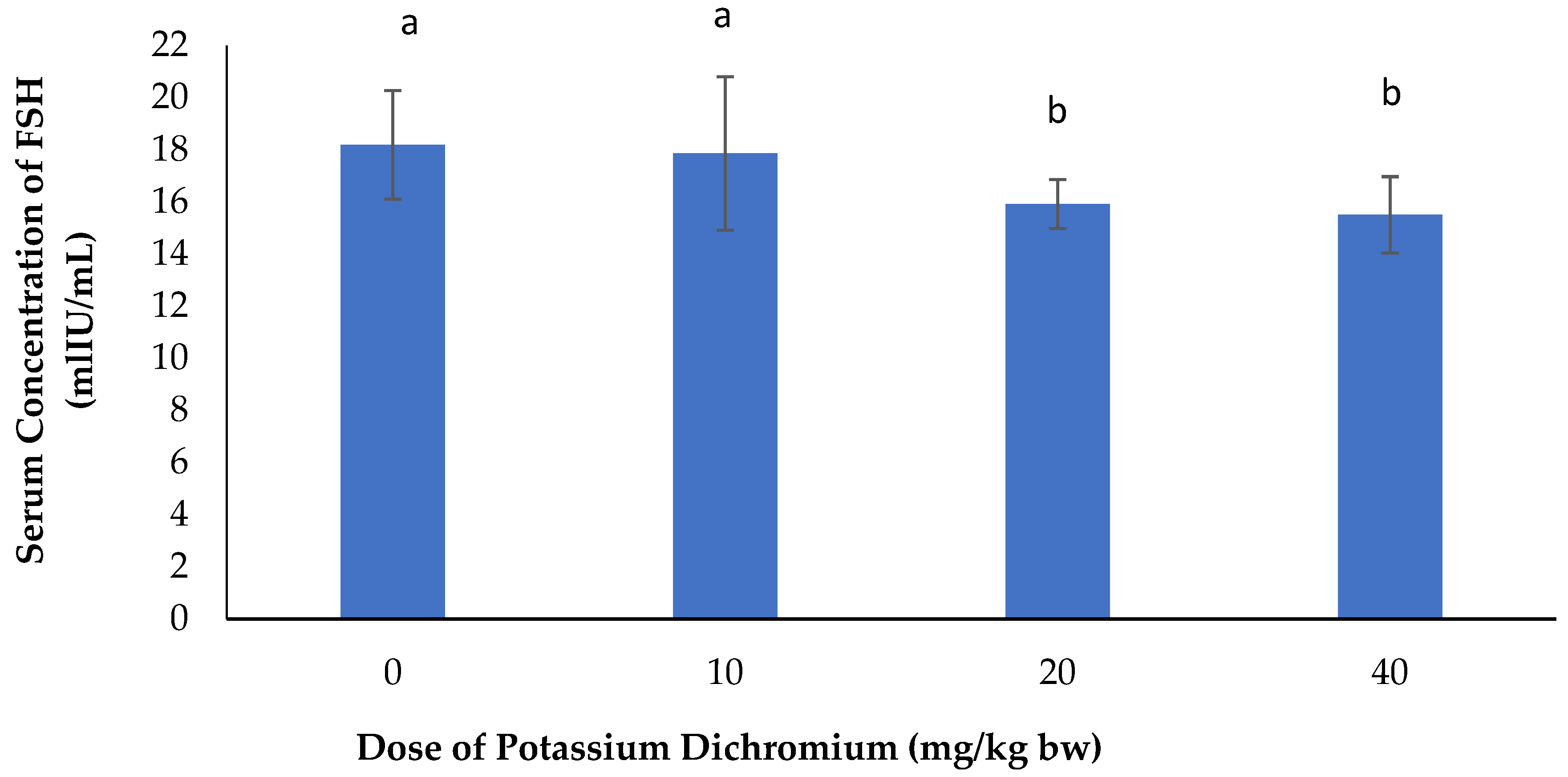
Serum Concentration of FSH
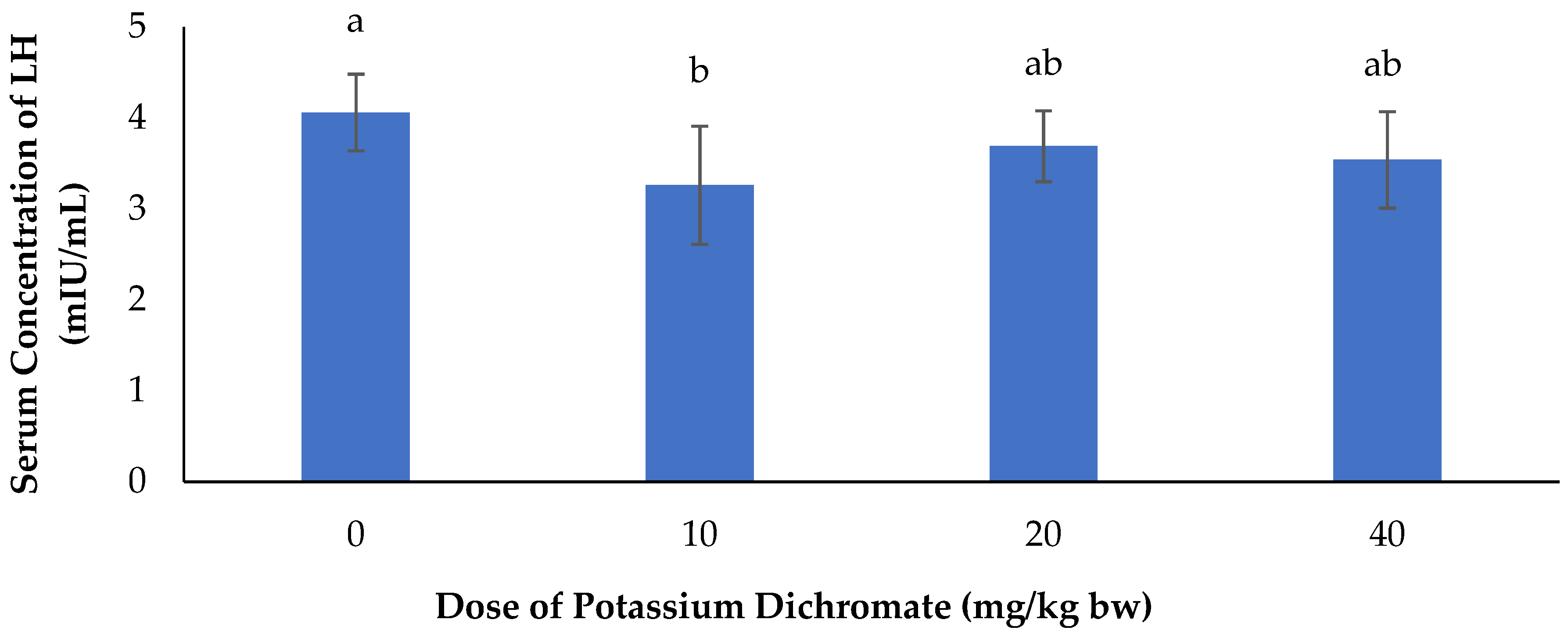
Serum Concentration of LH
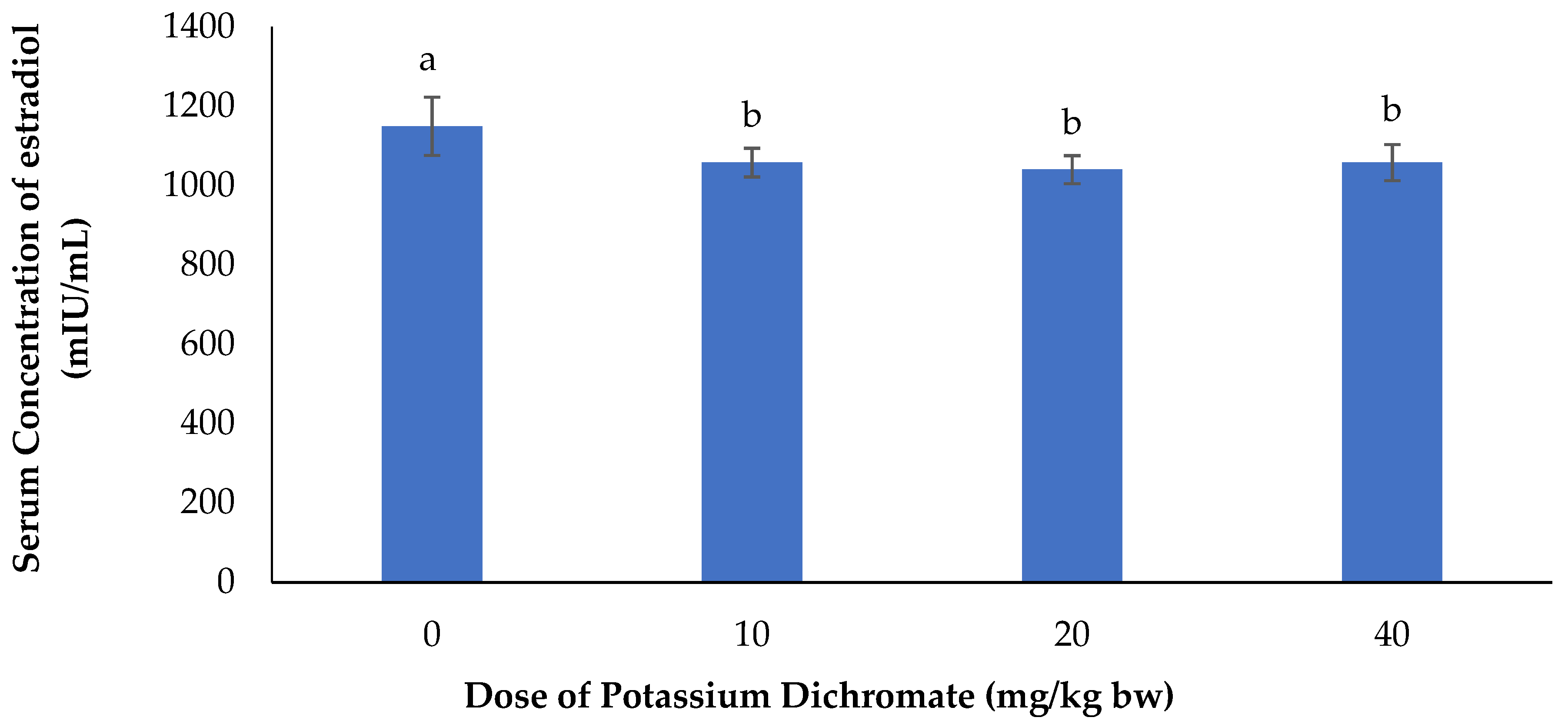
Serum Concentration of Estradiol
3.3. Effects of Potassium Dichromate on Hematological Parameters in Rabbit Does
3.4. Effects of Potassium Dichromate on Oxidative Stress Markers in Rabbit Does
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Holmes, A.L.; Wise, S.S.; Wise, J.P. Carcinogenicity of hexavalent chromium. Indian J. Med. Res. 2008, 128, 353–372. [Google Scholar]
- Mukesh, K.; Raikwar, P.; Manoj, S.; Anand, S. Toxic effect of heavy metals in livestock health. Indian Vet. Res. Inst. Izatnagar. 2016, 1, 28–30. [Google Scholar]
- Govind1, P.; Madhuri, S. Heavy Metals Causing Toxicity in Animals and Fishes. Res. J. Anim. Vet. Fish. Sci. 2014, 2, 17–23. [Google Scholar]
- Mahipal, S.S.; Mayuri, K.; Manisha, N.; Rajeev, K.; Prashant, A. Heavy metal contamination in soil and their toxic effect on human health: A review study. Int. J. Res. Educ. Sci. Methods 2016, 4, 2455–6211. [Google Scholar]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, Toxicity and Oxidative Stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Kader, A.F.; Kalapuram, S.P. Ameliorative effect of Emblica officinalis in potassium dichromate induced toxicity in rats. Eur. J. Pharm. Med. Res. 2017, 4, 267–274. [Google Scholar]
- Patlolla, A.K.; Constance, B.; Diahanna, H.; Tchounwou, P.B. Potassium dichromate induced cytotoxicity, genotoxicity and oxidative stress in human liver carcinoma (HepG2) cells. Int. J. Environ. Res. Public Health 2009, 6, 643–653. [Google Scholar] [CrossRef] [PubMed]
- International Program on Chemical Safety (IPCS). Inorganic Chromium (VI) Compounds: Concise International Chemical Assessment Document 78; World Health Organization: Geneva, Switzerland, 2013; p. 114. [Google Scholar]
- Ball, E. National Toxicology Program; U.S. Department of Health and Human Services: Washington, DC, USA, 2013.
- Richelmi, P.; Baldi, C.; Minoia, C. Blood levels of hexavalent chromium in rats. “In vitro” and “in vivo” experiments. Int. J. Environ. Anal. Chem. 1984, 17, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kerger, B.D.; Finley, B.L.; Corbett, G.E.; Dodge, D.G.; Paustenbach, D.J. Ingestion of chromium (VI) in drinking water by human volunteers: Absorption, distribution, and excretion of single and repeated doses. J. Toxicol. Environ. Health 1997, 50, 67–95. [Google Scholar] [PubMed]
- Corbett, G.E.; Finley, B.L.; Paustenbach, D.J.; Kerger, B.D. Systemic uptake of chromium in human volunteers following dermal contact with hexavalent chromium (22 mg/L). J. Expo. Anal. Environ. Epidemiol. 1997, 7, 179–189. [Google Scholar]
- Antonini, J.M.; Stone, S.; Roberts, J.R.; Chen, B.; Schwegler-Berry, D.; Afshari, A.A.; Frazer, D.G. Effect of short-term stainless steel welding fume inhalation exposure on lung inflammation, injury, and defense responses in rats. Toxicol. Appl. Pharmacol. 2007, 223, 234–245. [Google Scholar] [CrossRef]
- O’Flaherty, E.J.; Kerger, B.D.; Hayes, S.M.; Paustenbach, D.J. A physiologically based model for the ingestion of chromium (III) and chromium (VI) by humans. Toxicol. Sci. 2001, 60, 196–213. [Google Scholar] [CrossRef]
- Cavalleri, A.; Minoia, C. Serum and erythrocyte chromium distribution and urinary elimination in persons occupationally exposed to chromium (VI) and chromium (III). Giornale italiano di medicina del lavoro ed ergonomia 1985, 7, 35–38. [Google Scholar]
- Manerikar, R.S.; Akshaya, A.A.; Vikram, S.G. In vitro and in vivo genotoxicity assessment of Cr (VI) using comet assay in earthworm coelomocytes. Environ. Toxicol. Pharmacol. 2008, 25, 63–68. [Google Scholar] [CrossRef]
- Trivedi, B.; Saxena, D.K.; Murthy, R.C.; Chandra, S.V. Embryotoxicity and fetotoxicity of orally administered hexavalent chromium in mice. Reprod. Toxicol. 1989, 3, 275–278. [Google Scholar] [CrossRef]
- Junaid, M.; Murthy, R.C.; Saxena, D.K. Chromium fetotoxicity in mice during late pregnancy. Vet. Hum. Toxicol. 1995, 37, 320–323. [Google Scholar] [PubMed]
- Junaid, M.; Murthy, R.C.; Saxena, D.K. Embryotoxicity of orally administered chromium in mice: Exposure during the period of organogenesis. Toxicol. Lett. 1996, 84, 143–148. [Google Scholar] [CrossRef]
- Elsaieed, E.M.; Nada, S.A. Teratogenicity of hexavalent chromium in rats and the beneficial role of ginseng. Bull. Environ. Contam. Toxicol. 2002, 68, 361–368. [Google Scholar] [CrossRef] [PubMed]
- De Flora, S.; Iltcheva, M.; Balansky, R.M. Oral chromium (VI) does not affect the frequency of micronuclei in hematopoietic cells of adult mice and of transplacentally exposed fetuses. Mutat. Res. 2006, 610, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G.; Barceloux, D. Chromium. J. Toxicol. Clin. Toxicol. 1999, 37, 173–194. [Google Scholar] [CrossRef]
- Aebi, H. Catalase “in vitro”. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione—S-transferase activities in rat lung and liver. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The generation of superoxide radical during the autoxidation of haemoglobin. J. Biol. Chem. 1972, 247, 6960–6962. [Google Scholar]
- Oloyede, A.; Okpuzor, J.; Omidiji, O.; Odeigah, P. Evaluation of sub-chronic oral toxicity of joloo: A traditional medicinal decoction. Pharm. Biol. 2011, 49, 936–941. [Google Scholar] [CrossRef]
- Mossa, A.H.; Heikal, T.M.; Belaiba, M.; Raoelison, E.G.; Ferhout, H.; Bouajila, J. Antioxidant activity and hepatoprotective potential of Cedrelopsisgrevei on cypermethrin induced oxidative stress and liver damage in male mice. Complement. Altern. Med. 2015, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Trif, A.; Petrovici, S.; Petrovici, M.; Dumitrescu, E.; Olariu, L.; Tulcan, C.; Ghise, A. Effect of potassium dichromate intake on feed intake and body weight, in female rats, Rattus norvegicus (exposure on three generations). Hum. Vet. Med. 2010, 2, 32–36. [Google Scholar]
- García-Niño, W.R.; Tapia, E.; Zazueta, C.; Zatarain-Barrón, Z.L.; Hernández-Pando, R.; Vega-García, C.C.; Pedraza-Chaverrí, J. Curcumin pretreatment prevents potassium dichromate-induced hepatotoxicity, oxidative stress, decreased respiratory complex I activity, and membrane permeability transition pore opening. Evid. Based Complement. Alternat. Med. 2013. [Google Scholar] [CrossRef]
- Saha, J.; Choudhuri, S.; Choudhuri, D. Effect of subchronic exposure to chromium on hematological and biochemical parameters of male albino rat. Asian J. Pharm. Clin. Res. 2017, 10, 2017. [Google Scholar] [CrossRef]
- Petrovici, S.; Trif, A.; Dumitrescu, E.; Rankov, J. The impact of potassium dichromate (VI) intake on exposure and morphological integrity biomarkers (chromium level and weight of sexual organs) in female rats. Lucr. Stiinłifice Med. Vet. 2008, 41, 109–113. [Google Scholar]
- Bengt, A. Regulation of body fluids. Annu. Rev. Physiol. 1977, 39, 185–200. [Google Scholar]
- Corbett, J.V. Laboratory Tests and Diagnostic Procedures: With Nursing Diagnoses; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2008; p. 7. [Google Scholar]
- Zuo, Y.; Wang, C.; Zhou, J.; Sachdeva, A.; Ruelos, V.C. Simultaneous determination of creatinine and uric acid in human urine by high performance liquid chromatography. Anal. Sci. 2008, 24, 1589–1592. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Kumar, C.S.; Rani, M.U.; Kavita, K.; Boobalan, G.; Reddy, A.G. Evaluation of protective action of a-tocopherol in chromium-induced oxidative stress in female reproductive system of rats. J. Nat. Sci. Biol. Med. 2013, 4, 87–93. [Google Scholar]
- Yousif, A.S.; Ahmed, A.A. Effects of cadmium (Cd) and lead (Pb) on the structure and function of thyroid gland. Afr. J. Environ. Sci. Technol. 2009, 3, 78–85. [Google Scholar]
- Yanardag, R.; Sacan, O.O. Combined effects of vitamin C, vitamin E, and sodium selenite supplementation on absolute ethanol induced injury in various organs of rats. Int. J. Toxicol. 2007, 26, 513–523. [Google Scholar] [CrossRef] [PubMed]
- El-Sharaky, A.S.; Newairy, A.A.; Badreldeen, M.M.; Eweda, S.M.; Sheweita, S.A. Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology 2007, 235, 185–193. [Google Scholar] [CrossRef]
- Williamson, E.M.; Okpako, D.T.; Evans, F.J. Selection, preparation and pharmacological evaluation of plant material. J. Med. Chem. 1997, 40, 1559. [Google Scholar]
- Soudani, N.; Bouaziz, H.; Sefi, M.; Chtourou, Y.; Boudawara, T.; Zeghal, N. Toxic effects of chromium (VI) by maternal ingestion on liver function of female rats and their suckling pups. Environ. Toxicol. 2013, 28, 11–20. [Google Scholar] [CrossRef]
- Abbas, H.H.; Ali, F.K. Study the effect of hexavalent chromium on some biochemical, citotoxicological and histopathological aspects of the Orechromis spp. Fish. Pak. J. Biol. Sci. 2007, 10, 3973–3982. [Google Scholar]
- Zhu, H.; Jia, Y.; Cao, H.; Meng, F.; Liu, X. Biochemical and histopathological effects of subchronic oral exposure of rats to a mixture of five toxic elements. Food Chem. Toxicol. 2014, 71, 166–175. [Google Scholar] [CrossRef]
- Mehany, H.A.; Abo-youssef, A.M.; Ahmed, L.A.; El- Shaimaa, A.A.; Abd El-Latif, H.A. Protective effect of vitamin E and atorvastatin against potassium dichromate-induced nephrotoxicity in rats. Beni-Suef Univ. J. Basic Appl. Sci. 2013, 2, 96–102. [Google Scholar] [CrossRef]
- Mohamed, N.E.; Saber, R.A. Effect of aqueous extract of damsissa (Ambrosia maritima) on the biochemical changes induced by potassium dichromate in rats. J. Am. Sci. 2011, 7, 234–242. [Google Scholar]
- Krim, M.; Messaadia, A.; Maidi, I.; Aouacheri1, O.; Saka, S. Protective effect of ginger against toxicity induced by chromate in rats. Ann. Biol. Clin. 2013, 71, 165–173. [Google Scholar]
- Olafedehan, C.O.; Obun, A.M.; Yusuf, M.K.; Adewumi, O.O.; Oladefedehan, A.O.; Awofolaji, A.O.; Adeniji, A. Effects of residual cyanide in processed cassava peal meals on hematological and biochemical indices of growing rabbits. In Proceedings of the 35th Annual Conference of Nigerian Society for Animal Production, Ibadan, Nigeria, 14–17 March 2010; pp. 212–213. [Google Scholar]
- Shrivastava, R.; Srivastava, S.; Upreti, D.; Chaturvedi, U.C. Effects of dengue virus infection on peripheral blood cells of mice exposed to hexavalent chromium with drinking water. Indian J. Med Res. 2005, 122, 111–119. [Google Scholar]
- Stana, L.; Trif, A.; Stana, L.G.; Petrovici, S.; Gravila, C. Comparative study of the potassium dichromate effect on the osmotic resistance of rat erythrocyte membrane. Sci. Pap. Anim. Sci. Biotechnol. 2010, 43, 425–428. [Google Scholar]
- Ounassa, A. Protective effects of selenium against potassium dichromate-induced hematotoxicity in female and male Wistar albino rats. Ann. Toxicol. Anal. 2010, 22, 165–172. [Google Scholar]
- Vihol, P.D.; Patel, J.; Varia, R.D.; Patel, J.M.; Ghodasara, D.J.; Joshi, B.P.; Prajapati, K.S. Effects of sodium dichromate on haemato-biochemical parameters in Wistar rats. J. Pharmacol. Toxicol. 2012, 7, 58–63. [Google Scholar] [CrossRef]
- Assasa, M.F.; Farahat, M.M.I. Toxic effect of potassium dichromate on sex hormones and possible protective effect of rice bran oil in female albino rats. J. Pharmacol. Toxicol. 2014, 9, 90–96. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, G.H.; Li, Y.J.; Cai, M.Y.; Guo, H.Y.; Qin, K.L. Superovulation and expression of follicle-stimulating hormone receptor in young rabbit females. World Rabbit Sci. 2017, 25, 167–172. [Google Scholar] [CrossRef]
- Labib, F.; El Azab, E.A.; Soliman, F.A. Hormonal changes during formation and involution of corpus luteum in rabbits. Acta Vet. Hrno. 1978, 47, 23–31. [Google Scholar] [CrossRef]
- Mansour, S.A.; Mossa, A.T.; Heikal, T.M. Effects of methomyl on lipid peroxidation and antioxidant enzymes in rat erythrocytes: In vitro studies. Toxicol. Ind. Health 2009, 25, 557–563. [Google Scholar] [CrossRef]
- Rao, A.V.; Shaha, C. Multiple glutathione S-transferase isoforms are present on male germ cell plasma membrane. FEBS Lett. 2001, 507, 174–180. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of hepatic glutathione synthesis: Current concepts and controversies. FASEB J. 1999, 13, 1169–1183. [Google Scholar] [CrossRef]
- Srinivasan, K.; Narayanan, S.; Ananthasadagopan, S.; Ganapasam, S. Chromium (VI)-induced oxidative stress and apoptosis is reduced by garlic and its derivative S-allylcysteine through the activation of Nrf2 in the hepatocytes of Wistar rats. J. Appl. Toxicol. 2008, 28, 908–919. [Google Scholar]
- Pedraza-Chaverri, J.; Barrera, D.; Medina-Campos, O.N.; Carvajal, R.C.; Hernandez-Pando, R.; Macias-Ruvalcaba, N.A. Time course study of oxidative and nitrosative stress and antioxidant enzymes in K2Cr2O7-induced nephrotoxicity. BMC Nephrol. 2005, 6, 4. [Google Scholar] [CrossRef]
- Shati, A.A. Ameliorative effect of vitamin E on potassium dichromate-induced hepatotoxicity in rats. J. King Saud Univ. Sci. 2014, 26, 181–189. [Google Scholar] [CrossRef]
| Ingredients | Quantities (%) |
|---|---|
| Maize | 27.00 |
| Wheat bran | 14.00 |
| Kernel cake | 18.00 |
| Soy beans cake | 5.00 |
| Cotton cake | 4.00 |
| Premix10% * | 5.00 |
| Fish meal | 3.00 |
| Palm oil | 2.00 |
| Sea shells | 1.50 |
| Salt | 0.50 |
| Rice bran | 20.00 |
| Total (kg) | 100.00 |
| Chemical Characteristics | |
| Metabolizable energy (kcal/kg) | 2435.23 |
| Crude proteins (% DM) | 16.47 |
| Crude cellulose (% DM) | 13.65 |
| Calcium (% DM) | 1.26 |
| Phosphorus (% DM) | 0.55 |
| Sodium (% DM) | 0.28 |
| Lysine (% DM) | 0.83 |
| Methionine (% DM) | 0.36 |
| Toxicity Parameters | Doses of Potassium Dichromate (mg/kg bw) | p | |||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | ||
| Weight of the Kidney (g/100 g of bw) | 0.51 ± 0.04 | 0.47 ± 0.02 | 0.48 ± 0.06 | 0.51 ± 0.01 | 0.52 |
| Volume of the kidney (mL) | 12.50 ± 0.87 | 12.33 ± 0.58 | 13.00 ± 1.73 | 12.67 ± 1.15 | 0.91 |
| Creatinine (mg/dL) | 1.72 ± 0.17 b | 2.25 ± 0.30 a | 2.01 ± 0.27 ab | 2.18 ± 0.17 a | 0.02 |
| Urea (mg/dL) | 24.65 ± 7.86 b | 37.76 ± 7.34 a | 43.59 ± 8.09 a | 38.26 ± 3.74 a | 0.00 |
| Renal Tissue Protein (g/dL) | 1.52 ± 0.26 a | 0.63 ± 0.16 b | 0.53 ± 0.14 b | 0.70 ± 0.13 b | 0.00 |
| Toxicity Parameters | Doses of Potassium Dichromate (mg/kg bw) | p | |||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | ||
| Liver weight (g/100 g of bw) | 2.30 ± 0.37 | 2.10 ± 0.27 | 2.08 ± 0.28 | 2.74 ± 0.63 | 0.25 |
| Volume of the liver (mL) | 55.67 ± 10.07 | 51.67 ± 7.64 | 56.67 ± 11.55 | 68.33 ± 12.58 | 0.32 |
| ALT (IU) | 40.03 ± 8.88 c | 46.81 ± 7.68 c | 62.02 ± 9.96 b | 74.38 ± 8.53 a | 0.00 |
| AST (IU) | 54.69 ± 10.24 | 69.13 ± 14.94 | 66.21 ± 12.39 | 69.34 ± 10.26 | 0.31 |
| Total cholesterol (mg/dL) | 35.87 ± 7.55 b | 45.65 ± 6.00 ab | 54.45 ± 11.48 a | 44.69 ± 11.49 ab | 0.04 |
| Total protein (g/dL) | 4.68 ± 0.61 | 4.79 ± 0.65 | 4.60 ± 0.54 | 4.11 ± 0.63 | 0.42 |
| Albumin (g/dL) | 4.34 ± 0.38 a | 3.94 ± 0.54 ab | 3.71 ± 0.59 ab | 3.47 ± 0.46 b | 0.05 |
| hepatic tissue protein (g/dL) | 2.58 ± 0.53 a | 1.67 ± 0.27 b | 0.96 ± 0.27 c | 0.74 ± 0.27 c | 0.00 |
| Toxicity Parameters | Doses of Potassium Dichromate (mg/kg bw) | p | |||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | ||
| Ovary weight (g/100 g of bw) | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.87 |
| Ovarian tissue protein (g/dL) | 2.14 ± 0.30 a | 1.24 ± 0.27 b | 1.17 ± 0.33 b | 0.63 ± 0.13 c | 0.00 |
| Uterine tissue protein (g/dL) | 0.42 ± 0.08 a | 0.33 ± 0.03 ab | 0.27 ± 0.08 bc | 0.21 ± 0.05 c | 0.01 |
| Blood Parameters | Doses of Potassium Dichromate (mg/kg bw) | p | |||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | ||
| WBC (× 103/µL) | 12.43 ± 1.61 b | 12.83± 1.69 b | 14.17 ± 1.25 ab | 16.37 ± 2.14 a | 0.04 |
| Lymphocytes (× 103/µL) | 6.40 ± 0.69 b | 5.67 ± 0.47 b | 7.97 ± 0.91 a | 9.50 ± 1.11 a | 0.00 |
| Monocytes (× 103/µL) | 1.97 ± 0.38 | 1.77 ± 0.31 | 2.07 ± 0.49 | 2.27 ± 0.49 | 0.57 |
| Granulocytes (× 103/µL) | 4.07 ± 0.21 b | 5.40 ± 0.46 a | 4.47 ± 0.55 ab | 4.93 ± 1.01 ab | 0.05 |
| RBC (× 106/µL) | 5.28 ± 0.39 | 5.21 ± 0.30 | 4.99 ± 0.35 | 4.56 ± 0.61 | 0.23 |
| Haematocrit (%) | 12.47 ± 0.65 | 12.43 ± 0.70 | 12.33 ± 0.51 | 11.27 ± 1.17 | 0.27 |
| Haemoglobin (g/dL) | 38.20 ± 2.86 a | 38.47 ± 3.72 a | 37.03 ± 2.91 a | 30.17 ± 4.61 b | 0.04 |
| Platelets (× 104/µL) | 31.83 ± 3.67 a | 19.47 ± 2.22 b | 22.80 ± 3.48 b | 23.77 ± 4.14 b | 0.01 |
| Plaquetocrit (%) | 0.32 ± 0.08 | 0.21 ± 0.07 | 0.24 ± 0.07 | 0.25 ± 0.08 | 0.36 |
| Organs | Oxidative Stress Markers | Dose of Potassium Dichromate (mg/kg bw) | p | |||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | |||
| Liver | SOD (µM/min/g of tissue protein) | 3.79 ± 0.78 a | 1.79 ± 0.36 b | 1.58 ± 0.46 b | 1.31 ± 0.26 b | 0.00 |
| CAT (µM/min/g of tissue) | 10.25 ± 1.91 | 10.04 ± 1.45 | 9.35 ± 1.39 | 9.58 ± 1.07 | 0.73 | |
| GSH (µM/g of tissue) | 127.37 ± 28.25 | 114.32 ± 36.32 | 106.62 ± 32.64 | 100.44 ± 14.21 | 0.60 | |
| MDA (µM/g of tissue) | 0.98 ± 0.24 b | 1.29 ± 0.32 ab | 1.41 ± 0.28 ab | 1.56 ± 0.48 a | 0.05 | |
| Kidney | SOD (µM/min/g of tissue protein) | 1.94 ± 0.25 a | 1.98 ± 0.24 a | 1.61 ± 0.65 ab | 1.08 ± 0.47 b | 0.04 |
| CAT (µM/min/g of tissue) | 10.63 ± 1.38 a | 9.31 ± 1.00 ab | 9.38 ± 1.54 ab | 8.51 ± 1.18 b | 0.05 | |
| GSH (µM/g of tissue) | 87.75 ± 17.04 | 80.24 ± 17.23 | 72.19 ± 27.41 | 68.47 ± 16.49 | 0.51 | |
| MDA (µM/g of tissue) | 1.61 ± 0.32 c | 2.41 ± 0.53 ab | 2.01 ± 0.30 bc | 2.66 ± 0.24 a | 0.01 | |
| Ovary | SOD (µM/min/g of tissue protein) | 6.82 ± 0.69 a | 4.65 ± 0.93 b | 3.31 ± 0.72 c | 2.15 ± 0.55 d | 0.00 |
| CAT (µM/min/g of tissue) | 13.44 ± 1.77 a | 10.97 ± 1.51 b | 9.73 ± 1.95b c | 7.83 ± 0.66 c | 0.00 | |
| GSH (µM/g of tissue) | 111.76 ± 39.10 a | 59.94 ± 13.60 b | 79.67 ± 24.23 ab | 67.70 ± 17.13 b | 0.04 | |
| MDA (µM/g of tissue) | 1.40 ± 0.40 b | 2.40 ± 0.65 a | 2.09 ± 0.81ab | 2.35 ± 0.51 a | 0.04 | |
| Uterus | SOD (µM/min/g of tissue protein) | 5.81 ± 0.42 a | 5.20 ± 0.70a | 3.96 ± 0.75 b | 3.79 ± 0.44 b | 0.00 |
| CAT (µM/min/g of tissue) | 12.06 ± 2.39 a | 9.64 ± 0.37b | 10.70 ± 1.68 ab | 10.06 ± 0.52 ab | 0.05 | |
| GSH (µM/g of tissue) | 79.72 ± 14.26 | 56.93 ± 18.99 | 67.96 ± 12.94 | 57.18 ± 11.81 | 0.14 | |
| MDA (µM/g of tissue) | 1.24 ± 0.24 b | 1.47 ± 0.18 b | 1.63 ± 0.25 b | 2.31 ± 0.59 a | 0.00 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mary Momo, C.M.; Ferdinand, N.; Omer Bebe, N.K.; Alexane Marquise, M.N.; Augustave, K.; Bertin Narcisse, V.; Herve, T.; Joseph, T. Oxidative Effects of Potassium Dichromate on Biochemical, Hematological Characteristics, and Hormonal Levels in Rabbit Doe (Oryctolagus cuniculus). Vet. Sci. 2019, 6, 30. https://doi.org/10.3390/vetsci6010030
Mary Momo CM, Ferdinand N, Omer Bebe NK, Alexane Marquise MN, Augustave K, Bertin Narcisse V, Herve T, Joseph T. Oxidative Effects of Potassium Dichromate on Biochemical, Hematological Characteristics, and Hormonal Levels in Rabbit Doe (Oryctolagus cuniculus). Veterinary Sciences. 2019; 6(1):30. https://doi.org/10.3390/vetsci6010030
Chicago/Turabian StyleMary Momo, Chongsi Margaret, Ngoula Ferdinand, Ngouateu Kenfack Omer Bebe, Makona Ndekeng Alexane Marquise, Kenfack Augustave, Vemo Bertin Narcisse, Tchoffo Herve, and Tchoumboue Joseph. 2019. "Oxidative Effects of Potassium Dichromate on Biochemical, Hematological Characteristics, and Hormonal Levels in Rabbit Doe (Oryctolagus cuniculus)" Veterinary Sciences 6, no. 1: 30. https://doi.org/10.3390/vetsci6010030
APA StyleMary Momo, C. M., Ferdinand, N., Omer Bebe, N. K., Alexane Marquise, M. N., Augustave, K., Bertin Narcisse, V., Herve, T., & Joseph, T. (2019). Oxidative Effects of Potassium Dichromate on Biochemical, Hematological Characteristics, and Hormonal Levels in Rabbit Doe (Oryctolagus cuniculus). Veterinary Sciences, 6(1), 30. https://doi.org/10.3390/vetsci6010030





