Intracoronary Gene Delivery of the Cytoprotective Factor Vascular Endothelial Growth Factor-B167 in Canine Patients with Dilated Cardiomyopathy: A Short-Term Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Selection and General Protocol
2.2. Echocardiographic Exams
2.3. Viral Vector Production
2.4. Anesthetic Protocol and Intra-Operative Monitoring
2.5. Intracoronary Gene Delivery Procedure
2.6. Post-Procedural Monitoring
2.7. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosamond, W.; Flegal, K.; Furie, K.; Go, A.; Greenlund, K.; Haase, N.; Hailpern, S.M.; Ho, M.; Howard, V.; Kissela, B.; et al. Heart Disease and Stroke Statistics—2008 Update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2010, 117, e25–e146. [Google Scholar] [CrossRef]
- Martin, M.W.; Stafford Johnson, M.J.; Celona, B. Canine dilated cardiomyopathy: A retrospective study of signalment, presentation and clinical findings in 369 cases. J. Small Anim. Pract. 2009, 50, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Dukes-McEwan, J.; Borgarelli, M.; Tidholm, A.; Vollmar, A.C.; Häggström, J. ESVC Taskforce for Canine Dilated Cardiomyopathy. Proposed guidelines for the diagnosis of canine idiopathic dilated cardiomyopathy. J. Vet. Cardiol. 2003, 5, 7–19. [Google Scholar] [CrossRef]
- Fuentes, V.; Corcoran, B.; French, A.; Schober, K.; Kleemann, R.; Justus, C. A Double-Blind, Randomized, Placebo-Controlled Study of Pimobendan in Dogs with Dilated Cardiomyopathy. J. Vet. Intern. Med. 2002, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, M.R.; Minors, S.L.; O’Sullivan, M.L.; Horne, R. Effect of pimobendan on case fatality rate in Doberman Pinschers with congestive heart failure caused by dilated cardiomyopathy. J. Vet. Intern. Med. 2008, 22, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, N.J.; Boswood, A.; O’Grady, M.R.; Gordon, S.G.; Dukes-McEwan, J.; Oyama, M.A.; Smith, S.; Patteson, M.; French, A.T.; Culshaw, G.J.; et al. Efficacy of Pimobendan in the Prevention of Congestive Heart Failure or Sudden Death in Doberman Pinschers with Preclinical Dilated Cardiomyopathy (The PROTECT Study). J. Vet. Intern. Med. 2012, 26, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, A.; Fox, P. Long-term Outcome of Irish Wolfhound Dogs with Preclinical Cardiomyopathy, Atrial Fibrillation, or Both Treated with Pimobendan, Benazepril Hydrochloride, or Methyldigoxin Monotherapy. J. Vet. Intern. Med. 2016, 30, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Woitek, F.; Zentilin, L.; Hoffman, N.; Powers, J.C.; Ottiger, I.; Parikh, S.; Kulczycki, A.M.; Hurst, M.; Ring, N.; Wang, T.; et al. Intracoronary Cytoprotective Gene Therapy. J. Am. Coll. Cardiol. 2015, 66, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.; Tang, J.; Woodruff, K.; Defrancesco, T.; Williams, C.; Breen, M.; Meurs, K.K.; Cheng, K. Intracoronary allogeneic cardiosphere- derived stem cells are safe for use in dogs with dilated cardiomyopathy. J. Cell. Mol. Med. 2017, 21, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Pogue, B.; Estrada, A.H.; Sosa-Samper, I.; Maisenbacher, H.W.; Lamb, K.E.; Mincey, B.D.; Erger, K.E.; Conlon, T.J. Stem-cell therapy for dilated cardiomyopathy: A pilot study evaluating retrograde coronary venous delivery. J. Small Anim. Pract. 2013, 54, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Hogarth, K.A.; O’Sullivan, M.L.; Regnier, M.; Pyle, W.G. 2-Deoxyadenosine triphosphate restores the contractile function of cardiac myofibril from adult dogs with naturally occurring dilated cardiomyopathy. Am. J. Physiol. Heart.Circ. Physiol. 2016, 310, H80–H91. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Fujita, T.; Kishimura, M.; Suita, K.; Hidaka, Y.; Cai, W.; Umemura, M.; Yokoyama, U.; Uechi, M.; Ishikawa, Y. Vidarabine, an Anti-Herpes Virus Agent, Protects Against the Development of Heart Failure With Relatively Mild Side-Effects on Cardiac Function in a Canine Model of Pacing-Induced Dilated Cardiomyopathy. Circ. J. 2016, 80, 2496–2505. [Google Scholar] [CrossRef] [PubMed]
- Shirasaka, T.; Miyagawa, S.; Fukushima, S.; Kawaguchi, N.; Nakatani, S.; Daimon, T.; Okita, Y.; Sawa, Y. Skeletal Myoblast Cell Sheet Implantation Ameliorates Both Systolic and Diastolic Cardiac Performance in Canine Dilated Cardiomyopathy Model. Transplantation 2016, 100, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Sosa, I.; Estrada, A.H.; Winter, B.D.; Erger, K.E.; Conlon, T.J. In vitro evaluation of mitochondrial dysfunction and treatment with adeno-associated virus vector in fibroblasts from Doberman Pinschers with dilated cardiomyopathy and a pyruvate dehydrogenase kinase 4 mutation. Am. J. Vet. Res. 2016, 77, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, F.; Nagai, N.; Tang, Z.; Zhang, S.; Scotney, P.; Lennartsson, J.; Zhu, C.; Qu, Y.; Fang, C.; et al. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J. Clin. Investig. 2008, 118, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Narula, J.; Haider, N.; Virmani, R.; DiSalvo, T.; Kolodgie, F.; Hajjar, R.; Schmidt, U.; Semigran, M.J.; Dec, G.W.; Khaw, B. Apoptosis in Myocytes in End-Stage Heart Failure. N. Engl. J. Med. 1996, 335, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Olivetti, G.; Abbi, R.; Quaini, F.; Kajstura, J.; Cheng, W.; Nitahara, J.A.; Quaini, E.; Di Loreto, C.; Beltrami, C.; Krajewski, S.; et al. Apoptosis in the failing human heart. N. Engl. J. Med. 1997, 336, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Saraste, A.; Pulkki, K.; Kallajoki, M.; Heikkila, P.; Laine, P.; Mattila, S.; Nieminen, M.S.; Parvinen, M.; Voipio-Pulkki, L.M. Cardiomyocyte apoptosis and progression of heart failure to transplantation. Eur. J. Clin. Investig. 1999, 29, 380–386. [Google Scholar] [CrossRef]
- Lähteenvuo, J.E.; Lähteenvuo, M.T.; Kivelä, A.; Rosenlew, C.; Falkevall, A.; Klar, J.; Heikura, T.; Rissanen, T.T.; Vähäkangas, E.; Korpisalo, P.; et al. Vascular endothelial growth factor-B induces myocardium- specific angiogenesis and arteriogenesis via vascular endothelial growth factor receptor-1- and neuropilin receptor-1-dependent mechanisms. Circulation 2009, 119, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.; Mamdani, M.; Zentilin, L.; Csiszar, A.; Qanud, K.; Zacchigna, S.; Ungvari, Z.; Puligadda, U.; Moimas, S.; Xu, X.; et al. Intramyocardial VEGF-B167 gene delivery delays the progression towards congestive failure in dogs with pacing-induced dilated cardiomyopathy. Circ. Res. 2010, 106, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- Zacchigna, S.; Zentilin, L.; Giacca, M. Adeno-associated virus vectors as therapeutic and investigational tools in the cardiovascular system. Circ. Res 2014, 114, 1827–1846. [Google Scholar] [CrossRef] [PubMed]
- Wess, G.; Domenech, O.; Dukes-McEwan, J.; Häggström, J.; Gordon, S. European Society of Veterinary Cardiology screening guidelines for dilated cardiomyopathy in Doberman Pinschers. J. Vet. Cardiol. 2017, 19, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Zacchigna, S.; Pattarini, L.; Zentilin, L.; Moimas, S.; Carrer, A.; Sinigaglia, M.; Arsic, N.; Tafuro, S.; Sinagra, G.; Giacca, M. Bone marrow cells recruited through the neuropilin-1 receptor promote arterial formation at the sites of adult neoangiogenesis in mice. J. Clin. Investig. 2008, 118, 2062–2075. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, E.A.; Pedersen, H.K.; Klow, N.E.; Refsum, H. Cardiac electrophysiology, arrhythmogenic mechanisms and roentgen contrast media. Acta Radiol. Suppl. 1995, 399, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Laynez, A.; Ben-Dor, I.; Barbash, I.M.; Hauville, C.; Sardi, G.; Maluenda, G.; Xue, Z.; Satler, L.F.; Pichard, A.D.; Lindsay, J.; Waksman, R. Frequency of conduction disturbances after Edwards SAPIEN percutaneous valve implantation. Am. J. Cardiol. 2012, 110, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.J.; Han, B.; Zhang, J.J.; Yi, Y.C.; Jiang, D.D.; Lyu, J.L. Postprocedural Outcomes and Risk Factors for Arrhythmias Following Transcatheter Closure of Congenital Perimembranous Ventricular Septal Defect: A Single-center Retrospective Study. Chin. Med. J. 2017, 130, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.M.; Rasmussen, H.; Eriksen, M.; Hvoslef, A.M.; Evans, P.; Newton, B.B.; Videm, S. Predicting cardiotoxicity propensity of the novel iodinated contrast medium GE-145: Ventricular fibrillation during left coronary arteriography in pigs. Acta Radiol. 2010, 51, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Sleeper, M.M.; Bish, L.T.; Sweeney, H.L. Status of therapeutic gene transfer to treat canine dilated cardiomyopathy in dogs. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 717–724. [Google Scholar] [CrossRef] [PubMed]
| Dog | Breed | Age (Years) | Sex | Clinical History | Clinical Signs and Symptoms at T0 | ECG Findings and Heart Rate at T0 | Ongoing Treatment (from at Least One Month before Procedure) |
|---|---|---|---|---|---|---|---|
| C 1 | Italian Mastiff | 3 | M | Disorexia, exercise intolerance, ascites, Afib | None. Afib DCM-like | Afib, HR = 134/min. | pimobendan 0.25 mg/kg q12 h, digossina 0.22 mg/m2 q12 h, benazepril 0.25 mg/kg q12 h, furosemide 2 mg/kg q12 h |
| C 2 | Dogue de Bordeau | 8 | M | Mild depression, ascites, Afib | None. Afib DCM-like | Afib, HR = 121/min. | pimobendan 0.25 mg/kg q12 h, digossina 0.22 mg/m2 q12 h, benazepril 0.25 mg/kg q12 h |
| C 3 | German Shepherd | 8 | M | Ascites, Afib | None. Afib DCM-like | Afib, HR = 147/min. | pimobendan 0.25 mg/kg q12 h, digossina 0.22 mg/m2 q12 h, benazepril 0.25 mg/kg q12 h, furosemide 2 mg/kg q24 h |
| C 4 | Great Dane | 6.5 | M | Depression, exercise intollerance, ascites | Exercise intollerance, ascites, weak pulse. Familiar DCM | Synus rhythm HR = 160/min. | pimobendan 0.25 mg/kg q12 h, torasemide 0.3 mg/kg q12 h, benazepril 0.25 mg/kg q12 h, spironolattone 2 mg/kg q24h |
| C 5 | Saint Bernard | 6 | M | Syncope, both thoracic and abdominal effusion, Afib | None. Afib Familiar DCM | Afib, HR = 95/min. | pimobendan 0.25 mg/kg q12 h, digossina 0.22 mg/m2 q12 h, benazepril 0.25 mg/kg q12 h, furosemide 2 mg/kg q12 h |
| C 6 | Dobermann Pincher | 5 | F | Syncope | None. Familiar DCM | Synus rhythm, WP, HR = 51/min, sporadic PVC (1/60”) | pimobendan 0.25 mg/kg q12 h, benazepril 0.25 mg/kg q12 h, furosemide 2 mg/kg q12 h sotalol 2 mg/ q12 h |
| C 7 | Italian Mastiff | 7.5 | M | Exercise intollerance, ascites, Afib | None. Afib DCM-like | Afib, HR = 88/min. | digossina 0.22 mg/m2 q12 h, benazepril 0.25 mg/Kg q12 h, furosemide 2 mg/kg q12 h |
| C 8 | Amstaff | 7 | M | Acute depression, ascites, Afib | None. Afib DCM-like | Afib, HR = 150/min | pimobendan 0.25 mg/kg q12 h, benazepril 0.25 mg/Kg q12 h, digossina 0.22 mg/m2 q12 h, furosemide 2 mg/kg q12 h |
| C 9 | Great Dane | 5.5 | M | Syncope, dyspnoea post-surgery, Afib | None. Afib Familiar DCM | Afib, HR = 150/min | pimobendan 0.25 mg/kg q12 h, benazepril 0.25 mg/Kg q12 h, digossina 0.22 mg/m2 q12 h, furosemide 2 mg/kg q12 h |
| C 10 | Giant Schnauzer | 8 | M | Exercise intollerance, Afib | None. Afib Familiar DCM | Afib, HR = 85/min | pimobendan 0.25 mg/kg q12 h, digossina 0.22 mg/m2 q12 h, benazepril 0.25 mg/Kg q12 h, furosemide 2 mg/kg q12 h |
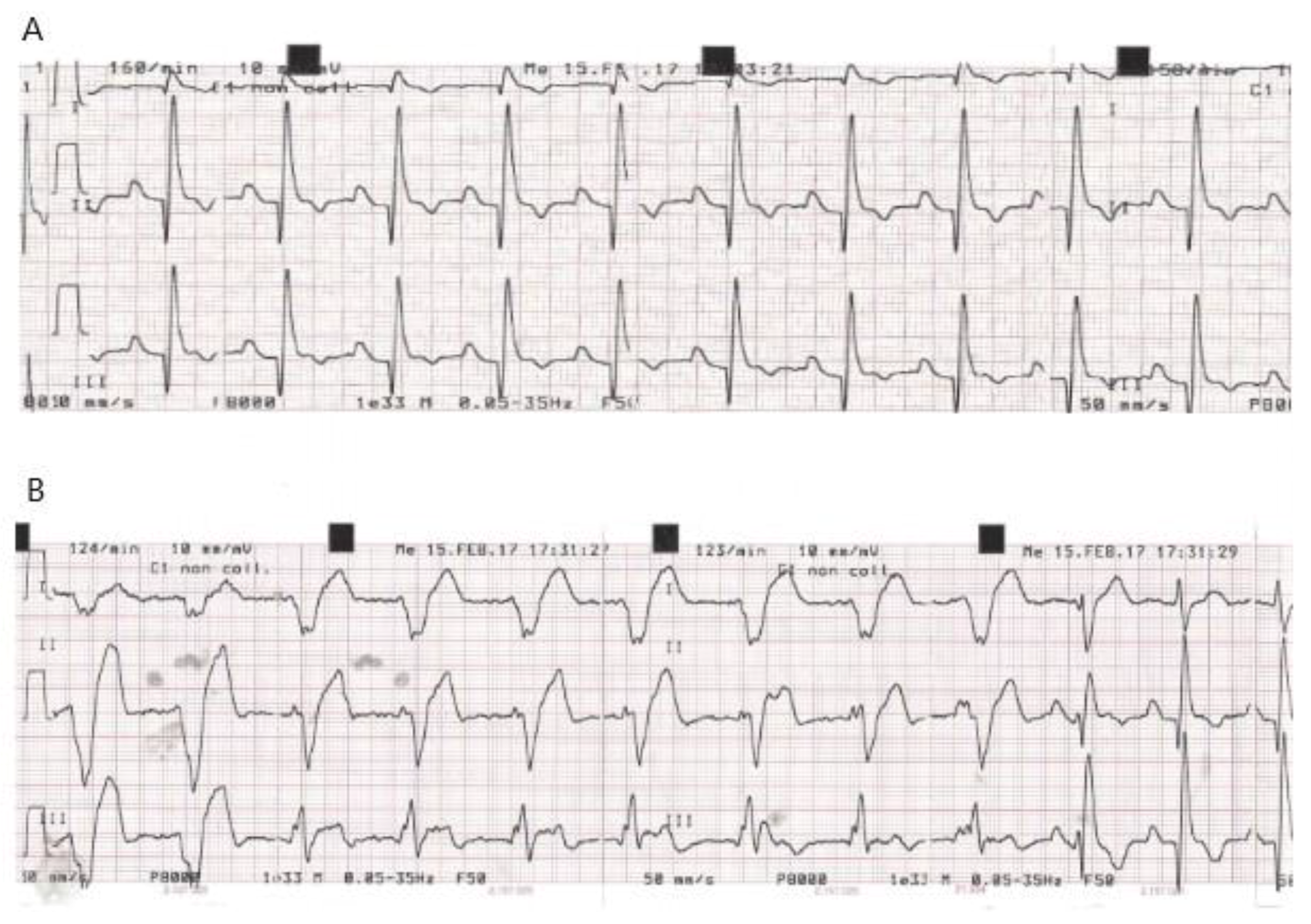
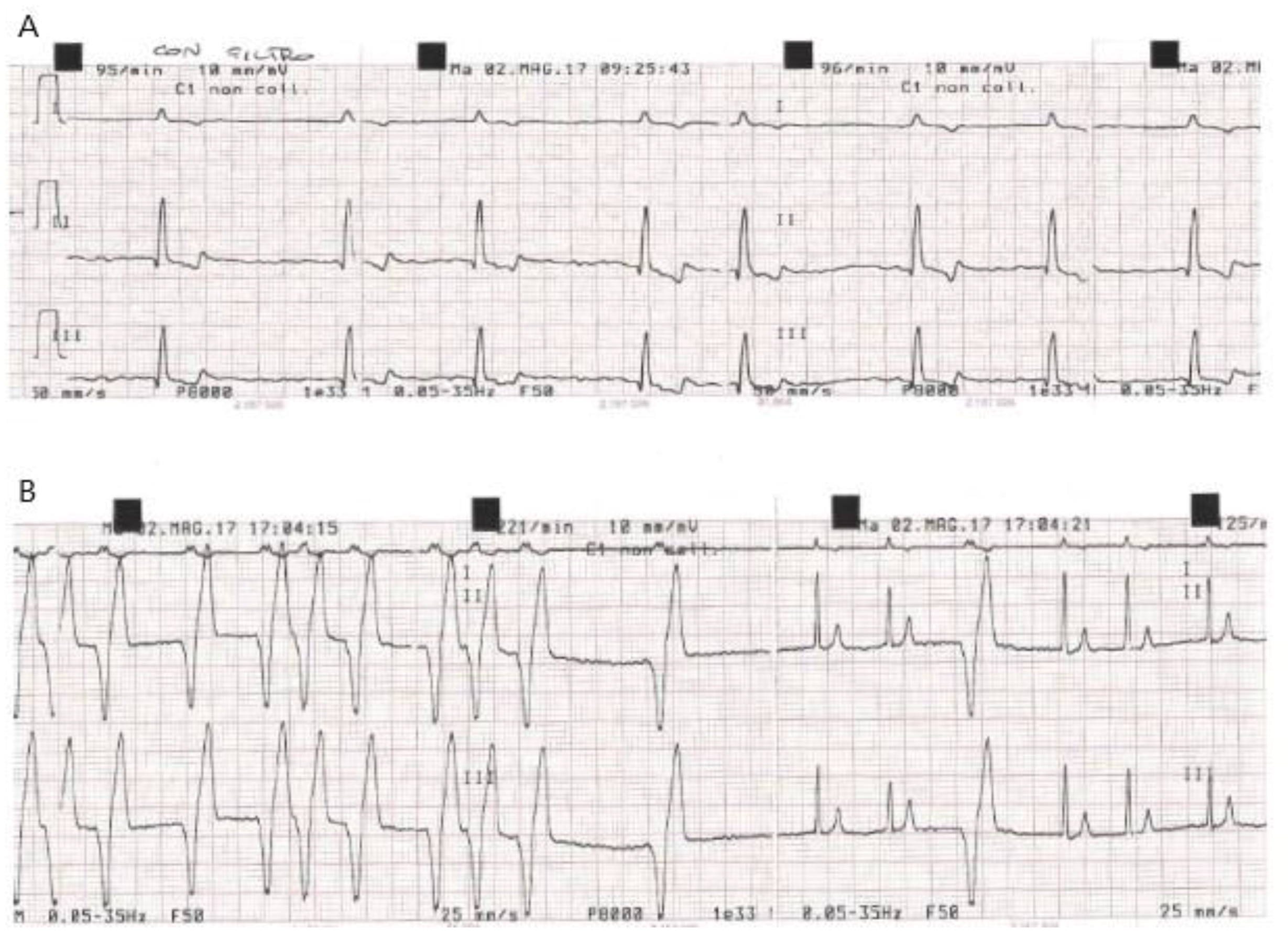
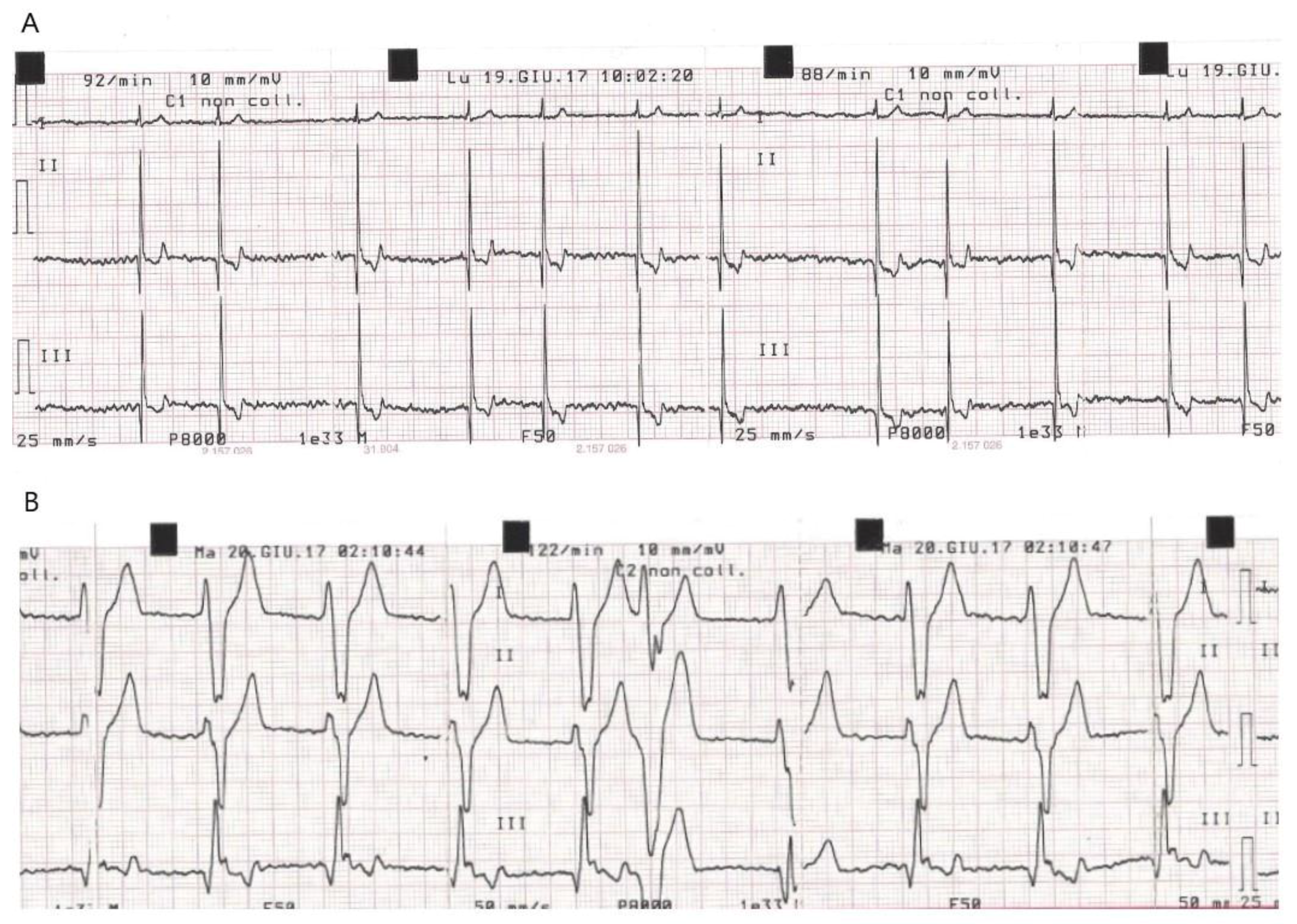
| Dog | ECG at Baseline (T0) | New Conduction Abnormalities Appeared in the PPM (First Few Hours during Post-Anaesthesia Recovery) | Time of Discharge | ECG at D7 Compared to D0: | K+ Serum Concentration (mEq/L) at Weak Up |
|---|---|---|---|---|---|
| C 1 | Afib, HR = 134/min | No changes | 12 h | No changes | n.d. |
| C 2 | Afib, HR = 121/min | No changes | 12 h | No changes | n.d. |
| C 3 | Afib, HR = 147/min | No changes | 12 h | No changes | K+ = 4.1 |
| C 4 | Synus rhythm HR = 160/min | VR (5h) | 48 h | No changes | K+ = 3 |
| C 5 | Afib, HR = 95/min | Sporadic VPC (4h) exiting in VR (6h) | 48 h Sporadic monomorphic VPCs | Residual sporadic monomorphic VPCs | K+ = 4.4 |
| C 6 | Synus rhythm, WP, HR = 51/min, sporadic PVC | No changes | 12 h | No changes | K+ = 4 |
| C 7 | Afib, HR = 88/min. | No changes | 12 h | No changes | K+ =3.6 |
| C 8 | Afib, HR = 150/min | No changes | 12 h | No changes | K+ = 4.2 |
| C 9 | Afib, HR = 150/min | No changes | 12 h | No changes | K+ = 4.3 |
| C 10 | Afib, HR = 85/min | Afib + VPC (2h) exiting in VR (4h). | 48 h | No changes | K+ = 4.2 |
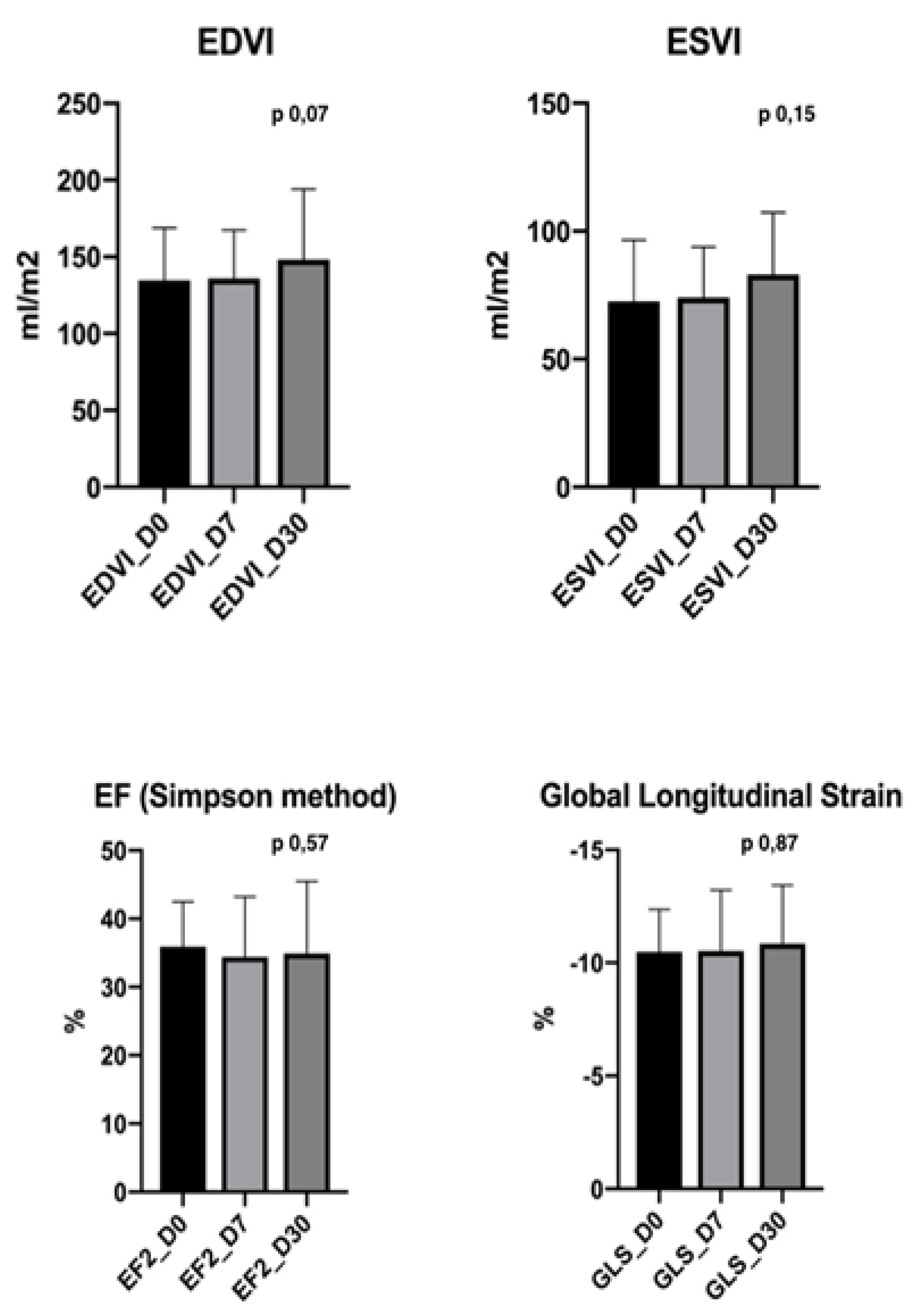
| Dog | EF (%) D0 | EF (%) D30 | EDVI (mL/m2) D0 | EDVI (mL/m2) D30 | ESVI (mL/m2) D0 | ESVI (mL/m2) D30 | GLS (%) D0 | GLS (%) D30 |
|---|---|---|---|---|---|---|---|---|
| C 1 | 21 | 19 | 79 | 93 | 51 | 61 | 7,7 | 12,1 |
| C 2 | 38 | 40 | 98 | 100 | 53 | 52 | 9,6 | 9,7 |
| C 3 | 36 | 35 | 186 | 213 | 82 | 122 | 10,7 | 9,9 |
| C 4 | 19 | 22 | 163 | 181 | 115 | 130 | 9,4 | 9,8 |
| C 5 | 46 | 46 | 133 | 150 | 76 | 78 | 9,7 | 10,1 |
| C 6 | 28 | 29 | 155 | 153 | 105 | 110 | 10,6 | 12,1 |
| C 7 | 38 | 36 | 106 | 105 | 52 | 64 | 11,4 | 11,6 |
| C 8 | 44 | 44 | 189 | 195 | 88 | 100 | 11,6 | 10,9 |
| C 9 | 36 | 39 | 193 | 182 | 90 | 110 | 10,2 | 12,2 |
| C 10 | 38 | 39 | 137 | 137 | 79 | 69 | 10,1 | 8,27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paradies, P.; Carlucci, L.; Woitek, F.; Staffieri, F.; Lacitignola, L.; Ceci, L.; Romano, D.; Sasanelli, M.; Zentilin, L.; Giacca, M.; et al. Intracoronary Gene Delivery of the Cytoprotective Factor Vascular Endothelial Growth Factor-B167 in Canine Patients with Dilated Cardiomyopathy: A Short-Term Feasibility Study. Vet. Sci. 2019, 6, 23. https://doi.org/10.3390/vetsci6010023
Paradies P, Carlucci L, Woitek F, Staffieri F, Lacitignola L, Ceci L, Romano D, Sasanelli M, Zentilin L, Giacca M, et al. Intracoronary Gene Delivery of the Cytoprotective Factor Vascular Endothelial Growth Factor-B167 in Canine Patients with Dilated Cardiomyopathy: A Short-Term Feasibility Study. Veterinary Sciences. 2019; 6(1):23. https://doi.org/10.3390/vetsci6010023
Chicago/Turabian StyleParadies, Paola, Lucia Carlucci, Felix Woitek, Francesco Staffieri, Luca Lacitignola, Luigi Ceci, Daniela Romano, Mariateresa Sasanelli, Lorena Zentilin, Mauro Giacca, and et al. 2019. "Intracoronary Gene Delivery of the Cytoprotective Factor Vascular Endothelial Growth Factor-B167 in Canine Patients with Dilated Cardiomyopathy: A Short-Term Feasibility Study" Veterinary Sciences 6, no. 1: 23. https://doi.org/10.3390/vetsci6010023
APA StyleParadies, P., Carlucci, L., Woitek, F., Staffieri, F., Lacitignola, L., Ceci, L., Romano, D., Sasanelli, M., Zentilin, L., Giacca, M., Salvadori, S., Crovace, A., & Recchia, F. A. (2019). Intracoronary Gene Delivery of the Cytoprotective Factor Vascular Endothelial Growth Factor-B167 in Canine Patients with Dilated Cardiomyopathy: A Short-Term Feasibility Study. Veterinary Sciences, 6(1), 23. https://doi.org/10.3390/vetsci6010023






