A Review of Immunotherapeutic Strategies in Canine Malignant Melanoma
Abstract
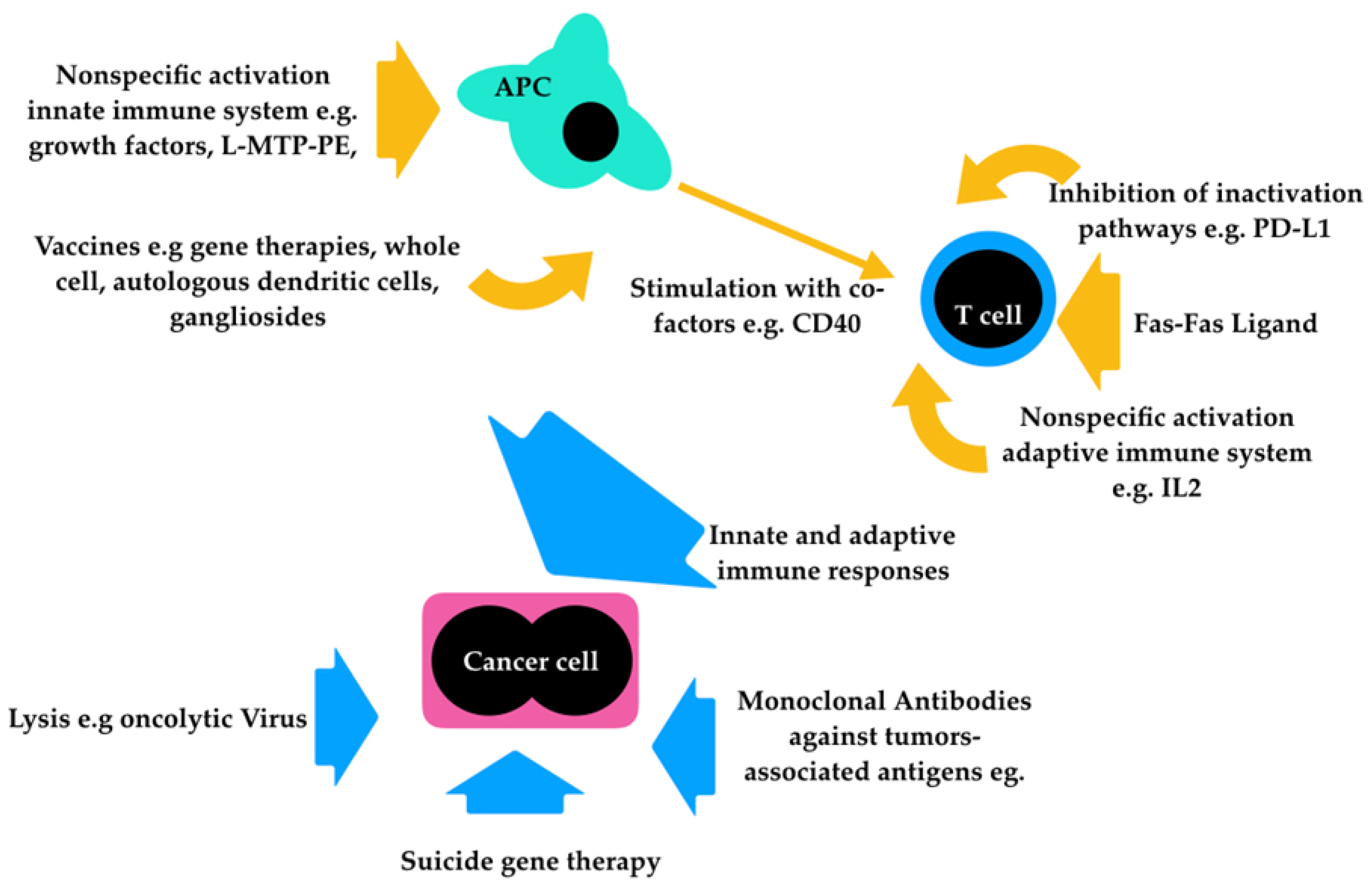
1. Introduction
2. Monoclonal Antibodies
2.1. Checkpoint Inhibitors
2.2. Antiganglioside Monoclonal Antibodies
3. Nonspecific Immunotherapy Activated by Bacteria
4. Oncolytic Virotherapy
5. Vaccines
6. Gene Therapy
7. Lymphokine-activated Killer (LAK) Cell Therapy
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Von Euler, H.; Sadeghi, A.; Carlsson, B.; Rivera, P.; Loskog, A.; Segall, T.; Korsgren, O.; Tötterman, T.H. Efficient adenovector CD40 ligand immunotherapy of canine malignant melanoma. J. Immunother. 2008, 31, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.J.; Kent, M.S.; Farese, J.P. Melanoma. In Withrow and MacEwen’s Small Animal Clinical Oncology; Withrow, S., Vail, D., Page, R., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2013; pp. 321–334. ISBN 978-1-4377-2362-5. [Google Scholar]
- Clifford, C.A.; de Lorimier, L.P.; Fan, T.; Garrett, L.D. Neoplastic and non-neoplastic tumors. In Muller and Kirk’s Small Animal Dermatology; Miller, W.H., Griffin, C.E., Campbell, K.L., Eds.; Elsevier Mosby: St. Louis, MO, USA, 2013; pp. 774–843. ISBN 978-1-4160-0028-0. [Google Scholar]
- La-Beck, N.M.; Jean, G.W.; Huynh, C.; Alzghari, S.K.; Lowe, D.B. Immune Checkpoint Inhibitors: New In-sights and Current Place in Cancer Therapy. Pharmacotherapy 2015, 35, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 7378, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Pavlin, D.; Cemazar, M.; Sersa, G.; Tozon, N. IL-12 based gene therapy in veterinary medicine. J. Transl. Med. 2012, 10, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Bujak, J.K.; Pingwara, R.; Nelson, M.H.; Majchrzak, K. Adoptive cell transfer: New perspective treatment in veterinary oncology. Acta Vet. Scand. 2018, 60, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Takagi, S.; Kagawa, Y.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Deguchi, T.; Nakajima, C.; et al. A canine chimeric monoclonal antibody targeting PD-L1 and its clinical efficacy in canine oral malignant melanoma or undifferentiated sarcoma. Sci. Rep. 2017, 7, 8951–8963. [Google Scholar] [CrossRef] [PubMed]
- Kwok, G.; Yau, T.C.; Chiu, J.W.; Tse, E.; Kwong, Y.L. Pembrolizumab (Keytruda). Hum. Vaccin. Immunother. 2016, 12, 2777–2789. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Shah, M.; Suarez-Almazor, M.E. Adverse events associated with immune checkpoint blockade in patients with cancer: A systematic review of case reports. PLoS ONE 2016, 11, e0160221. [Google Scholar] [CrossRef]
- Nguyen, S.M.; Thamm, D.H.; Vail, D.M.; London, C.A. Response evaluation criteria for solid tumors in dogs (v1.0): A Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 2015, 13, 176–183. [Google Scholar] [CrossRef]
- Owen, L.N. TNM Classification of Tumors in Domestic Animals; World Health Organization: Geneve, Switzerland, 1980. [Google Scholar]
- Helfand, S.C.; Soergel, S.A.; Donner, R.L.; Gan, J.; Hank, J.A.; Lindstrom, M.J.; Sondel, P.M. Potential to in-volve multiple effector cells with human recombinant interleukin-2 and antiganglioside monoclonal anti-bodies in a canine malignant melanoma immunotherapy model. J. Immunother. Emphasis Tumor Immunol. 1994, 16, 188–197. [Google Scholar] [CrossRef]
- Soergel, S.A.; MacEwen, E.G.; Vail, D.M.; Potter, D.M.; Sondel, P.M.; Helfand, S.C. The immunotherapeutic potential of activated canine alveolar macrophages and antitumor monoclonal antibodies in metastatic ca-nine melanoma. J. Immunother. 1999, 22, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflamma-tory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.B.; Imai, A. The immunopathogenesis of staphylococcal skin infections—A review. Comp. Immunol. Microbiol. Infect. Dis. 2016, 49, 8–28. [Google Scholar] [CrossRef] [PubMed]
- MacEwen, E.G.; Patnaik, A.K.; Harvey, H.J.; Hayes, A.A.; Matus, R. Canine oral melanoma: Comparison of surgery versus surgery plus Corynebacterium parvum. Cancer Investig. 1986, 4, 397–402. [Google Scholar] [CrossRef]
- MacEwen, E.G.; Kurzman, I.D.; Vail, D.M.; Dubielzig, R.R.; Everlith, K.; Madewell, B.R.; Rodriguez, C.O., Jr.; Phillips, B.; Zwahlen, C.H.; Obradovich, J.; et al. Adjuvant therapy for melanoma in dogs: Results of randomized clinical trials using surgery, liposome-encapsulated muramyl triptide, and granulocyte macro-phage colony-stimulang factor. Clin. Cancer Res. 1999, 5, 4249–4258. [Google Scholar] [PubMed]
- Patil, S.S.; Gentschev, I.; Nolte, I.; Ogilvie, G.; Szalay, A.A. Oncolytic virotherapy in veterinary medicine: Current status and future prospects for canine patients. J. Transl. Med. 2012, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, D.; Cesarman-Maus, G.; Amador-Molina, A.; Lizano, M. Oncolytic Viruses for Canine Cancer Treatment. Cancers 2018, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Igase, M.; Hwang, C.C.; Coffey, M.; Okuda, M.; Noguchi, S.; Mizuno, T. The oncolytic effects of reovirus in canine solid tumor cell lines. J. Vet. Med. Sci. 2015, 77, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Arendt, M.; Nasir, L.; Morgan, I.M. Oncolytic gene therapy for canine cancers: Teaching old dog viruses new tricks. Vet. Comp. Oncol. 2009, 7, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Gentschev, I.; Patil, S.S.; Adelfinger, M.; Weibel, S.; Geissinger, U.; Frentzen, A.; Chen, N.G.; Yu, Y.A.; Zhang, Q.; Ogilvie, G.; et al. Characterization and evaluation of a new oncolytic vaccinia virus strain LIVP6.1.1 for canine cancer therapy. Bioengineered 2013, 4, 84–89. [Google Scholar] [CrossRef]
- Cejalvo, T.; Perisé-Barrios, A.J.; Del Portillo, I.; Laborda, E.; Rodriguez-Milla, M.A.; Cubillo, I.; Vázquez, F.; Sardón, D.; Ramirez, M.; Alemany, R.; et al. Remission of Spontaneous Canine Tumors after Systemic Cellular Viroimmunotherapy. Cancer Res. 2018, 78, 4891–4901. [Google Scholar] [CrossRef] [PubMed]
- Kimpel, J.; Urbiola, C.; Koske, I.; Tober, R.; Banki, Z.; Wollmann, G.; von Laer, D. The Oncolytic Virus VSV-GP Is Effective against Malignant Melanoma. Viruses 2018, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Constantino, J.; Gomes, C.; Falcão, A.; Neves, B.M.; Cruz, M.T. Dendritic cell-based immunotherapy: A basic review and recent advances. Immunol. Res. 2017, 65, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, F.; Aurisicchio, L.; Mancini, R.; Ciliberto, G. Xenogene vaccination in the therapy of cancer. Expert Opin. Biol. Ther. 2014, 14, 1427–1442. [Google Scholar] [CrossRef] [PubMed]
- Atherton, M.J.; Morris, J.S.; McDermott, M.R.; Lichty, B.D. Cancer immunology and canine malignant melanoma: A comparative review. Vet. Immunol. Immunopathol. 2016, 169, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Gyorffy, S.; Rodriguez-Lecompte, J.C.; Woods, J.P.; Foley, R.; Kruth, S.; Liaw, P.C.; Gauldie, J. Bone marrow-derived dendritic cell vaccination of dogs with naturally occurring melanoma by using human gp100 antigen. J. Vet. Intern. Med. 2005, 19, 56–63. [Google Scholar] [PubMed]
- Tamura, K.; Yamada, M.; Isotani, M.; Arai, H.; Yagihara, H.; Ono, K.; Washizu, T.; Bonkobara, M. Induction of dendritic cell-mediated immune responses against canine malignant melanoma cells. Vet. J. 2008, 175, 126–129. [Google Scholar] [CrossRef]
- Regan, D.; Guth, A.; Coy, J.; Dow, S. Cancer immunotherapy in veterinary medicine: Current options and new developments. Vet. J. 2016, 207, 20–28. [Google Scholar] [CrossRef]
- Alexander, A.; Huelsmeyer, M.; Mitzey, A.; Dubielzig, R.; Kurzman, I.; MacEwen, E.; Vail, D. Development of an allogenic whole-cell tumor vaccine expressing xenogenic gp100 and implementation in phase II clinical trial in canine patients with malignant melanoma. Cancer Immunol. Immunother. 2006, 55, 433–442. [Google Scholar] [CrossRef]
- Witlox, M.A.; Lamfers, M.L.; Wuisman, P.I.; Curiel, D.T.; Siegal, G.P. Evolving gene therapy approaches for osteosarcoma using viral vectors: Review. Bone 2007, 40, 797–812. [Google Scholar] [CrossRef]
- Glikin, G.C.; Finocchiaro, L.M. Clinical trials of immunogene therapy for spontaneous tumors in companion animals. Sci. World J. 2014, 2014, 718520. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Yang, J.C.; Topalian, S.L.; Schwartzentruber, D.J.; Weber, J.S.; Parkinson, D.R.; Seipp, C.A.; Einhorn, J.H.; White, D.E. Treatment of 283 consecutive patients with metastatic mela-noma or renal cell cancer using high-dose bolus interleukin 2. JAMA 1994, 271, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Quintin-Colonna, F.; Devauchelle, P.; Fradelizi, D.; Mourot, B.; Faure, T.; Kourilsky, P.; Roth, C.; Mehtali, M. Gene therapy of spontaneous canine melanoma and feline fibrosarcoma by intratumoral administration of histoincompatible cells expressing human interleu-kin-2. Gene Ther. 1996, 3, 1104–1112. [Google Scholar] [PubMed]
- Cutrera, J.; King, G.; Jones, P.; Kicenuik, K.; Gumpel, E.; Xia, X.; Li, S. Safety and efficacy of tumor-targeted interleukin 12 gene therapy in treated and non-treated, metastatic lesions. Curr. Gene Ther. 2015, 15, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Cicchelero, L.; Denies, S.; Vanderperren, K.; Stock, E.; Van Brantegem, L.; de Rooster, H.; Sanders, N.N. Im-munological, anti-angiogenic and clinical effects of intratumoral interleukin 12 electrogene therapy com-bined with metronomic cyclophosphamide in dogs with spontaneous cancer: A pilot study. Cancer Lett. 2016, 15, 44–54. [Google Scholar]
- Reed, S.D.; Fulmer, A.; Buckholz, J.; Cutrera, J.; Shiomitsu, K.; Li, S.; Zhang, B. Bleomycin/interleukin-12 electrochemogene therapy for treating naturally occurring spontaneous neoplasms in dogs. Cancer Gene Ther. 2010, 17, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, L.M.E.; Glikin, G.C. Recent clinical trials of cancer immunogene therapy in companion animals. World J. Exp. Med. 2017, 7, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Thamm, D.H.; Kurzman, I.D.; MacEwen, E.G.; Feinmehl, R.; Towell, T.L.; Longhofer, S.L.; Johnson, C.M.; Geoly, F.J.; Stinchcomb, D.T. Intralesional lipid-complexed cytokine/superantigen immunogene therapy for spontaneous canine tumors. Cancer Immunol. Immunother. 2003, 52, 473–480. [Google Scholar] [CrossRef]
- Dow, S.W.; Walsh, P.M.; Kummer, D.; Roche, L.; Gorman, C.; Potter, T.A. In vivo tumor transfection with superantigen plus cytokine genes induces tumor regression and prolongs survival in dogs with malignant melanoma. J. Clin. Investig. 1998, 101, 2406–2414. [Google Scholar] [CrossRef]
- Bianco, S.R.; Sun, J.; Fosmire, S.P.; Hance, K.; Padilla, M.L.; Ritt, M.G.; Getzy, D.M.; Duke, R.C.; Withrow, S.J.; Lana, S.; et al. Enhancing antimelanoma immune responses through apoptosis. Cancer Gene Ther. 2003, 10, 726–736. [Google Scholar] [CrossRef]
- Finocchiaro, L.M.E.; Fiszman, G.L.; Karara, A.L.; Glikin, G.C. Suicide gene and cytokines combined nonviral gene therapy for spontaneous canine melanoma. Cancer Gene Ther. 2008, 15, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, L.M.E.; Glikin, G.C. Cytokine-enhanced vaccine and suicide gene therapy as surgery adjuvant treatments for spontaneous canine melanoma. Gene Ther. 2008, 15, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, L.M.; Fondello, C.; Gil-Cardeza, M.L.; Rossi, U.A.; Villaverde, M.S.; Riveros, M.D.; Glikin, G.C. Cytokine-Enhanced Vaccine and Interferon-β plus Suicide Gene Therapy as Surgery Adjuvant Treatments for Spontaneous Canine Melanoma. Hum. Gene Ther. 2015, 26, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, L.M.; Glikin, G.C. Cytokine-enhanced vaccine and suicide gene therapy as surgery adjuvant treatments for spontaneous canine melanoma: 9 years of follow-up. Cancer Gene Ther. 2012, 19, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Riccardo, F.; Iussich, S.; Maniscalco, L.; Mayayo, S.L.; La Rosa, G.; Arigoni, M.; De Maria, R.; Gattino, F.; Lanzardo, S.; Lardone, E.; et al. CSPG4-specific immunity and survival prolongation in dogs with oral malignant melanoma immunized with human CSPG4 DNA. Clin. Cancer Res. 2014, 20, 3753–3762. [Google Scholar] [CrossRef] [PubMed]
- Piras, L.A.; Riccardo, F.; Iussich, S.; Gattino, F.; Morello, E.; Mayayo, S.L.; Rolih, V.; Garavaglia, F.; Lardone, E.; Collivignarelli, F.; et al. Prolongation of survival of dogs with oral malignant melanoma treated by en bloc surgical resection and adjuvant CSPG4-antigen electrovaccination. Vet. Comp. Oncol. 2016, 15, 996–1013. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Oncept Melanoma: Withdrawal of the marketing authorisation application. Available online: https://www.ema.europa.eu/en/medicines/veterinary/withdrawn-applications/oncept-melanoma (accessed on 11 December 2018).
- Zuleger, C.L.; Kang, C.; Ranheim, E.A.; Kurzman, I.D.; Macklin, M.D.; Newton, M.A.; Wolchok, J.D.; Vail, D.M.; Eriksson, E.; Albertini, M.R.; et al. Pilot study of safety and feasibility of DNA microseeding for treatment of spontaneous canine melanoma. Vet. Med. Sci. 2017, 3, 134–145. [Google Scholar] [CrossRef]
- Grosenbaugh, D.A.; Leard, A.T.; Bergman, P.J.; Klein, M.K.; Meleo, K.; Susaneck, S.; Hess, P.R.; Jankowski, M.K.; Jones, P.D.; Leibman, N.F.; et al. Safety and efficacy of a xenogeneic DNA vaccine encod-ing for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am. J. Vet. Res. 2011, 72, 1631–1638. [Google Scholar] [CrossRef]
- Ottnod, J.M.; Smedley, R.C.; Walshaw, R.; Hauptman, J.G.; Kiupel, M.; Obradovich, J.E. A retrospective analysis of the efficacy of Oncept vaccine for the adjunct treatment of canine oral malignant melanoma. Vet. Comp. Oncol. 2013, 11, 219–229. [Google Scholar] [CrossRef]
- Boston, S.E.; Lu, X.; Culp, W.T.; Montinaro, V.; Romanelli, G.; Dudley, R.M.; Liptak, J.M.; Mestrinho, L.A.; Buracco, P. Efficacy of systemic adjuvant therapies administered to dogs after exci-sion of oral malignant melanomas: 151 cases (2001-2012). J. Am. Vet. Med. Assoc. 2014, 245, 401–407. [Google Scholar] [CrossRef]
- McLean, J.L.; Lobetti, R.G. Use of the melanoma vaccine in 38 dogs: The South African experience. J. S. Afr. Vet. Assoc. 2015, 86, 1246. [Google Scholar] [CrossRef] [PubMed]
- Treggiari, E.; Grant, J.P.; North, S.M. A retrospective review of outcome and survival following surgery and adjuvant xenogeneic DNA vaccination in 32 dogs with oral malignant melanoma. J. Vet. Med. Sci. 2016, 78, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Verganti, S.; Berlato, D.; Blackwood, L.; Amores-Fuster, I.; Polton, G.A.; Elders, R.; Doyle, R.; Taylor, A.; Murphy, S. Use of Oncept melanoma vaccine in 69 canine oral malignant melanomas in the UK. J. Small Anim. Pract. 2017, 58, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Vail, D.M. Levels of evidence in canine oncology trials—A case in point. Vet. Comp. Oncol. 2013, 11, 167–168. [Google Scholar] [CrossRef] [PubMed]
- Manley, C.A.; Leibman, N.F.; Wolchok, J.D.; Rivière, I.; Bartido, S.; Craft, D.; Bergman, P. Xenogeneic murine tyrosinase DNA vaccine for malignant melanoma of the digit of dogs. J. Vet. Intern. Med. 2011, 25, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Westberg, S.; Sadeghi, A.; Svensson, E.; Segall, T.; Dimopoulou, M.; Korsgren, O.; Hemminki, A.; Loskog, A.; Tötterman, T.H.; Von Euler, H.; et al. Treatment efficacy and immune stimulation by AdCD40L gene therapy of spontaneous canine malignant melanoma. J. Immunother. 2013, 36, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Mie, K.; Shimada, T.; Akiyoshi, H.; Hayashi, A.; Ohashi, F. Change in peripheral blood lymphocyte count in dogs following adoptive immunotherapy using lymphokine-activated T killer cells combined with pallia-tive tumor resection. Vet. Immunol. Immunopathol. 2016, 177, 58–63. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.M.; Sheppard, S.; Hartline, C.A.; Huls, H.; Palla, S.L.; Maiti, S.; Ma, W.; Craig, S.; Lee, D.A.; Wilson, H.; et al. Adoptive T-cell therapy improves treatment of canine non-Hodgkin lymphoma post chemotherapy. Sci. Rep. 2012, 2, 249. [Google Scholar] [CrossRef]
- Hoshino, Y.; Takagi, S.; Osaki, T.; Okumura, M.; Fujinaga, T. Phenotypic analysis and effects of sequential administration of activated canine lymphocytes on healthy beagles. J. Vet. Med. Sci. 2008, 70, 581–588. [Google Scholar] [CrossRef]
| Stage I | Stage II | Stage III | Stage IV |
|---|---|---|---|
| ≤2 cm diameter | 2–4 cm diameter | >4 cm diameter | Any size |
| No involvement of lymph nodes | No involvement of lymph nodes | +/− metastatic lymph nodes | Distant metastasis |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almela, R.M.; Ansón, A. A Review of Immunotherapeutic Strategies in Canine Malignant Melanoma. Vet. Sci. 2019, 6, 15. https://doi.org/10.3390/vetsci6010015
Almela RM, Ansón A. A Review of Immunotherapeutic Strategies in Canine Malignant Melanoma. Veterinary Sciences. 2019; 6(1):15. https://doi.org/10.3390/vetsci6010015
Chicago/Turabian StyleAlmela, Ramón M., and Agustina Ansón. 2019. "A Review of Immunotherapeutic Strategies in Canine Malignant Melanoma" Veterinary Sciences 6, no. 1: 15. https://doi.org/10.3390/vetsci6010015
APA StyleAlmela, R. M., & Ansón, A. (2019). A Review of Immunotherapeutic Strategies in Canine Malignant Melanoma. Veterinary Sciences, 6(1), 15. https://doi.org/10.3390/vetsci6010015





