The Multifaceted Zoonotic Risk of H9N2 Avian Influenza
Abstract
1. Introduction
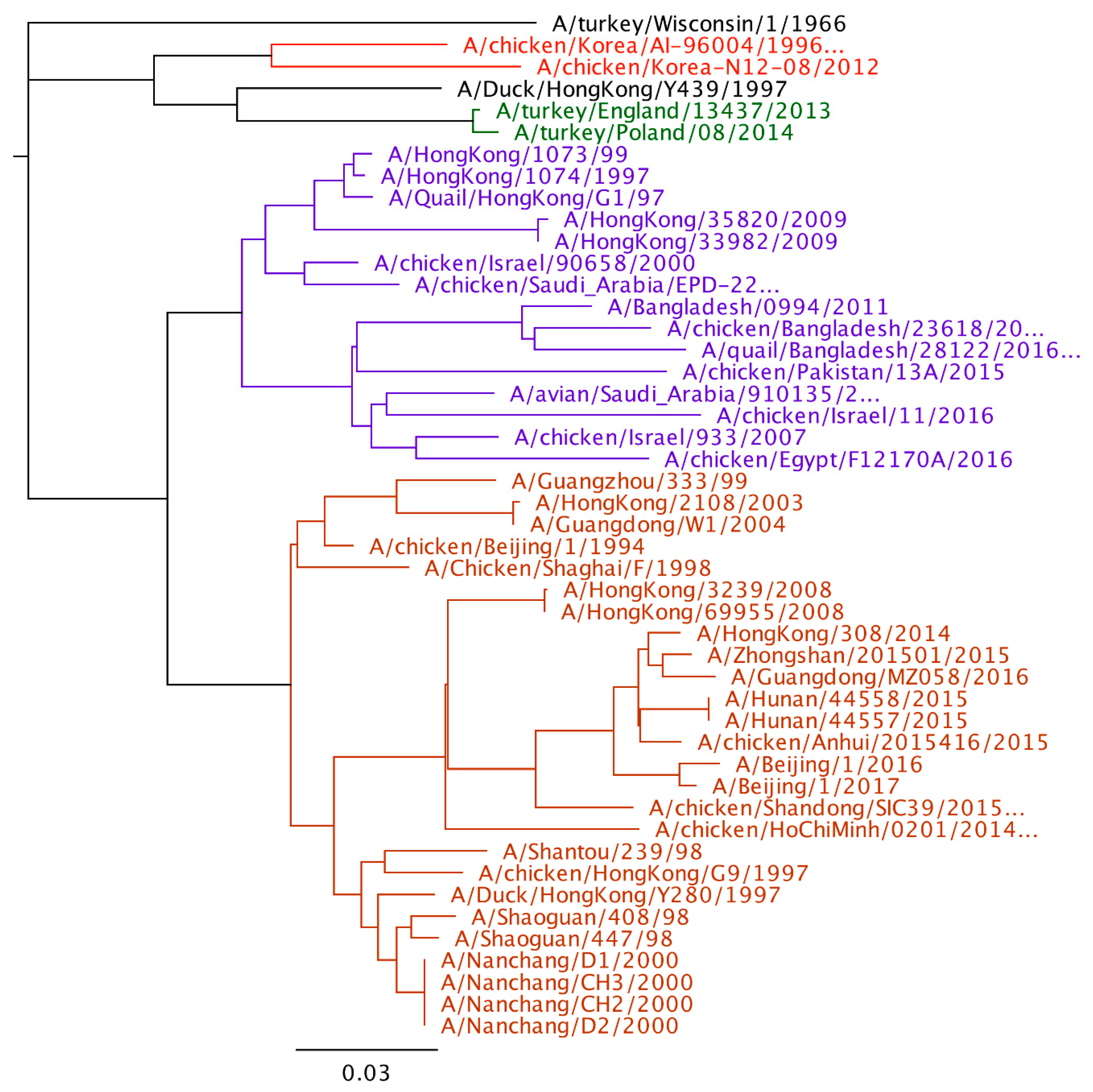
2. H9N2 Genetic Lineages
3. Evolution of H9N2
4. H9N2 in Mammals
5. H9N2 in Humans
5.1. Seroprevalence of H9N2 in Humans
5.2. Molecular Characterization of Human H9N2 Isolates
6. H9N2 Prevention
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iqbal, M.; Yaqub, T.; Reddy, K.; McCauley, J.W. Novel genotypes of H9N2 influenza A viruses isolated from poultry in Pakistan containing NS genes similar to highly pathogenic H7N3 and H5N1 Viruses. PLoS ONE 2009, 4, e5788. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Yaqub, T.; Mukhtar, N.; Shabbir, M.Z.; McCauley, J.W. Infectivity and transmissibility of H9N2 avian influenza virus in chickens and wild terrestrial birds. Vet. Res. 2013, 44, 100. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Shortridge, K.F.; Krauss, S.; Chin, P.S.; Dyrting, K.C.; Ellis, T.M.; Webster, R.G.; Peiris, M. H9N2 influenza Viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J. Virol. 2000, 74, 9372–9380. [Google Scholar] [CrossRef] [PubMed]
- Homme, P.J.; Easterday, B.C. Avian influenza virus infections. I. Characteristics of influenza A/Turkey/Wisconsin/1966 virus. Avian Dis. 1970, 14, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, J. H9N2 influenza virus in China: A cause of concern. Protein Cell 2014, 6, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Song, C.-S.; Lee, Y.-J.; Mo, I.-P.; Garcia, M.; Suarez, D.L.; Kim, S.-J. Sequence analysis of the hemagglutinin gene of H9N2 Korean avian influenza viruses and assessment of the pathogenic potential of isolate MS96. Avian Dis. 2000, 44, 527–535. [Google Scholar] [CrossRef] [PubMed]
- The SJCEIRS H9 Working Group. Assessing the fitness of distinct clades of influenza A (H9N2) viruses. Emerg. Microbes Infect. 2013, 2, e75. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M.; Siddique, S.; Idrees, M.; Tong, Y. Avian influenza A (H9N2): Computational molecular analysis and phylogenetic characterization of viral surface proteins isolated between 1997 and 2009 from the human population. Virol. J. 2010, 7, 319. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Anderson, B.D.; Heil, G.L.; Liang, S.; Gray, G.C. A systematic review and meta-analysis of the seroprevalence of influenza A (H9N2) infection among humans. J. Infect. Dis. 2015, 212, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Shortridge, K.F.; Krauss, S.; Webster, R.G. Molecular characterization of H9N2 influenza viruses: Were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc. Natl. Acad. Sci. USA 1999, 96, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.P.; Shaw, M.; Gregory, V.; Cameron, K.; Lim, W.; Klimov, A.; Subbarao, K.; Guan, Y.; Krauss, S.; Shortridge, K.; et al. Avian-to-human transmission of H9N2 subtype influenza A viruses: Relationship between H9N2 and H5N1 human isolates. Proc. Natl. Acad. Sci. USA 2000, 97, 9654–9658. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Perez, D.R. Amino acid 226 in the hemagglutinin of H9N2 influenza viruses determines cell tropism and teplication in human airway epithelial cells. J. Virol. 2007, 81, 5181–5191. [Google Scholar] [CrossRef] [PubMed]
- Avian influenza (infection with avian influenza viruses). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; World Organization for Animal Health (OIE): Paris, France, 2015.
- Gu, M.; Xu, L.; Wang, X.; Liu, X. Current situation of H9N2 subtype avian influenza in China. Vet. Res. 2017, 48, 49. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Pu, J.; Jiang, Z.; Guan, T.; Xia, Y.; Xu, Q.; Liu, L.; Ma, B.; Tian, F.; Brown, E.G.; et al. Genotypic evolution and antigenic drift of H9N2 influenza viruses in China from 1994 to 2008. Vet. Microbiol. 2010, 146, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, S.; Bing, G.; Carter, R.A.; Wang, Z.; Wang, J.; Wang, C.; Wang, L.; Wu, G.; Webster, R.G.; et al. Genetic evolution of influenza H9N2 viruses isolated from various hosts in China from 1994 to 2013. Emerg. Microbes Infect. 2017, 6, e106. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.M.; Banks, J.; Ceeraz, V.; Seekings, A.; Howard, W.A.; Puranik, A.; Collins, S.; Manvell, R.; Irvine, R.M.; Brown, I.H. The detection of a low pathogenicity avian influenza virus subtype H9 infection in a turkey breeder flock in the United Kingdom. Avian Dis. 2016, 60, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Śmietanka, K.; Minta, Z.; Świętoń, E.; Olszewska, M.; Jóźwiak, M.; Domańska-Blicharz, K.; Wyrostek, K.; Tomczyk, G.; Pikuła, A. Avian influenza H9N2 subtype in Poland–characterization of the isolates and evidence of concomitant infections. Avian Pathol. 2014, 43, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Kimble, B.; Ramirez Nieto, G.; Perez, D.R. Characterization of influenza virus sialic acid receptors in minor poultry species. Virol. J. 2010, 7, 365. [Google Scholar] [CrossRef] [PubMed]
- Lazniewski, M.; Dawson, W.K.; Szczepińska, T.; Plewczynski, D. The structural variability of the influenza A hemagglutinin receptor-binding site. Brief. Funct. Genom. 2017, 1–13. [Google Scholar] [CrossRef]
- Peiris, J.S.; Guan, Y.; Markwell, D.; Ghose, P.; Webster, R.G.; Shortridge, K.F. Cocirculation of avian H9N2 and contemporary “human” H3N2 influenza A viruses in pigs in southeastern China: Potential for genetic reassortment? J. Virol. 2001, 75, 9679–9686. [Google Scholar] [CrossRef] [PubMed]
- Li, K.S.; Xu, K.M.; Peiris, J.S.M.; Poon, L.L.M.; Yu, K.Z.; Yuen, K.Y.; Shortridge, K.F.; Webster, R.G.; Guan, Y. Characterization of H9 subtype influenza viruses from the ducks of southern China: A Candidate for the next influenza pandemic in humans? J. Virol. 2003, 77, 6988–6994. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Ozaki, H.; Webby, R.J.; Webster, R.G.; Peiris, J.S.; Poon, L.; Butt, C.; Leung, Y.H.C.; Guan, Y. Continuing evolution of H9N2 influenza viruses in doutheastern China. J. Virol. 2004, 78, 8609–8614. [Google Scholar] [CrossRef] [PubMed]
- Chrzastek, K.; Lee, D.; Gharaibeh, S.; Zsak, A.; Kapczynski, D.R. Characterization of H9N2 avian influenza viruses from the Middle East demonstrates heterogeneity at amino acid position 226 in the hemagglutinin and potential for transmission to mammals. Virology 2018, 518, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Swayne, D.E.; Sharma, P.; Rehmani, S.F.; Wajid, A.; Suarez, D.L.; Afonso, C. H9N2 low pathogenic avian influenza in Pakistan (2012–2015). Vet. Rec. Open 2016, 3, e000171. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-J.; Lin, X.-D.; Tian, J.-H.; Liao, Y.; Ying, X.-H.; Shao, J.-W.; Yu, B.; Guo, J.-J.; Wang, M.-R.; Peng, Y.; et al. Diversity, evolution and population dynamics of avian influenza viruses circulating in the live poultry markets in China. Virology 2017, 505, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Squires, R.B.; Noronha, J.; Hunt, V.; Garcia-Sastre, A.; Macken, C.; Baumgarth, N.; Suarez, D.L.; Pickett, B.E.; Zhang, Y.; Larsen, C.N.; et al. Influenza research database: An integrated bioinformatics resource for influenza virus research. Influenza Other Respir. Viruses 2012, 6, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Pantin-Jackwood, M.J.; Miller, P.J.; Spackman, E.; Swayne, D.E.; Susta, L.; Costa-Hurtado, M.; Suarez, D.L. Role of poultry in the spread of novel H7N9 influenza virus in China. J. Virol. 2014, 88, 5381–5390. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Fusaro, A.; Song, C.-S.; Suarez, D.L.; Swayne, D.E. Poultry vaccination directed evolution of H9N2 low pathogenicity avian influenza viruses in Korea. Virology 2016, 488, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Chen, H.; Li, Q.; Huang, J.; Zhao, M.; Gu, X.; Jiang, K.; Wang, X.; Peng, D.; Liu, X. Enzootic genotype S of H9N2 avian influenza viruses donates internal genes to emerging zoonotic influenza viruses in China. Vet. Microbiol. 2014, 174, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Wang, S.; Yin, Y.; Zhang, G.; Carter, R.A.; Wang, J.; Xu, G.; Sun, H.; Wang, M.; Wen, C.; et al. Evolution of the H9N2 influenza genotype that facilitated the genesis of the novel H7N9 virus. Proc. Natl. Acad. Sci. USA 2015, 112, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhou, X.; Shi, W.; Huang, L.; Xia, W.; Liu, D.; Li, H.; Chen, S.; Lei, F.; Cao, L.; et al. Genesis of the novel human-infecting influenza A (H10N8) virus and potential genetic diversity of the virus in poultry, China. Eurosurveillance 2014, 19, 1–13. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, W.; Xiaodan, L.; Bo, H.; Zhang, Y.; Zou, S.; Rongbao, G.; Dong, J.; Zhao, X.; Chen, W.; et al. Genesis and dissemination of highly pathogenic H5N6 avian influenza viruses. J. Virol. 2017, 91, e02199-16. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, J.; Guo, J.; Deng, G.; Zhang, Q.; Wang, J.; He, X.; Wang, K.; Chen, J.; Li, Y.; et al. Genetics, receptor binding property, and transmissibility in mammals of naturally isolated H9N2 avian influenza viruses. PLoS Pathog. 2014, 10, e1004508. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhou, Y.J.; Li, G.X.; Ma, J.H.; Yan, L.P.; Wang, B.; Yang, F.R.; Huang, M.; Tong, G.Z. Genetic diversity of H9N2 influenza viruses from pigs in China: A potential threat to human health? Vet. Microbiol. 2011, 149, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Krauss, S.; Senne, D.A.; Mo, I.P.; Lo, K.S.; Xiong, X.P.; Norwood, M.; Shortridge, K.F.; Webster, R.G.; Guan, Y. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology 2000, 267, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, M.; Hong, W.; Fan, X.; Chen, R.; Zheng, Z.; Zeng, Y.; Huang, R.; Zhang, Y.; Lam, T.T.-Y.; et al. Infectivity and transmissibility of avian H9N2 influenza viruses in pigs. J. Virol. 2016, 90, 3506–3514. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Z.; Chen, Z.; Zhang, Y.; Lv, Q.; An, X.; Tong, Y.; Carr, M.J.; Sun, S.; Shi, W. High genetic diversity and frequent genetic reassortment of avian influenza A (H9N2) viruses along the East Asian-Australian migratory flyway. Infect. Genet. Evol. 2016, 39, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, H.; Huang, J.; Chen, Y.; Gu, M.; Wang, X.; Hu, S.; Liu, X.; Liu, X. A nonpathogenic duck-origin H9N2 influenza A virus adapts to high pathogenicity in mice. Arch. Virol. 2014, 159, 2243–2252. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Punyadarsaniya, D.; Lambertz, R.L.O.; Lee, D.C.C.; Liang, C.H.; Höper, D.; Leist, S.R.; Hernández-Cáceres, A.; Stech, J.; Beer, M.; et al. Mutations during the adaptation of H9N2 avian influenza virus to the respiratory epithelium of pigs enhance sialic acid binding activity and virulence in mice. J. Virol. 2017, 91, e02125-16. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Chen, C.; Kai-yi, H.; Feng-xia, Z.; Yan-li, Z.; Zong-shuai, L.; Xing-xiao, Z.; Shi-jin, J.; Zhi-jing, X. Molecular characterization of H9N2 influenza virus isolated from mink and its pathogenesis in mink. Vet. Microbiol. 2015, 176, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Yong-feng, Z.; Fei-fei, D.; Jia-yu, Y.; Feng-xia, Z.; Chang-qing, J.; Jian-li, W.; Shou-yu, G.; Kai, C.; Chuan-yi, L.; Xue-hua, W.; et al. Intraspecies and interspecies transmission of mink H9N2 influenza virus. Sci. Rep. 2017, 7, 7429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xuan, Y.; Shan, H.; Yang, H.; Wang, J.; Wang, K.; Li, G.; Qiao, J. Avian influenza virus H9N2 infections in farmed minks. Virol. J. 2015, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, E.K.; London, W.; Murphy, B.R. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J. Virol. 1993, 67, 1761–1764. [Google Scholar] [PubMed]
- Massin, P.; van der Werf, S.; Naffakh, N. Residue 627 of PB2 is a determinant of cold sensitivity in RNA replication of avian influenza viruses. J. Virol. 2001, 75, 5398–5404. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Dewhurst, S.; Takimoto, T.; Kim, B. Biochemical impact of the host adaptation-associated PB2 E627K mutation on the temperature-dependent RNA synthesis kinetics of influenza A virus polymerase complex. J. Biol. Chem. 2011, 286, 34504–34513. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Sorrell, E.M.; Song, H.; Hossain, M.J.; Ramirez-Nieto, G.; Monne, I.; Stevens, J.; Cattoli, G.; Capua, I.; Chen, L.M.; et al. Replication and transmission of H9N2 influenza viruses in ferrets: Evaluation of pandemic potential. PLoS ONE 2008, 3, e2923. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Bai, T.; Li, X.; Xiong, Y.; Huang, Y.; Pan, M.; Zhang, Y.; Bo, H.; Zou, S.; Shu, Y. The comparison of pathology in ferrets infected by H9N2 avian influenza viruses with different genomic features. Virology 2016, 488, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Sang, X.; Wang, A.; Ding, J.; Kong, H.; Gao, X.; Li, L.; Chai, T.; Li, Y.; Zhang, K.; Wang, C.; et al. Adaptation of H9N2 AIV in guinea pigs enables efficient transmission by direct contact and inefficient transmission by respiratory droplets. Sci. Rep. 2015, 5, 15928. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.M. Avian influenza viruses in humans. Rev. Sci. Tech. 2009, 28, 161–173. [Google Scholar] [CrossRef]
- Yoo, S.J.; Kwon, T.; Lyoo, Y.S. Challenges of influenza A viruses in humans and animals and current animal vaccines as an effective control measure. Clin. Exp. Vaccine Res. 2018, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Matrosovich, M.; Stech, J.; Klenk, H.D. Influenza receptors, polymerase and host range. Rev. Sci. Tech. 2009, 28, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.; Govorkova, E. Continuing challenges in influenza. Ann. N. Y. Acad. Sci. 2014, 1323, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ju, L.; Liu, P.; Zhou, J.; Lv, X.; Li, L.; Shen, H.; Su, H.; Jiang, L.; Jiang, Q. Serological and virological surveillance of avian influenza A virus H9N2 subtype in humans and poultry in Shanghai, China, between 2008 and 2010. Zoonoses Public Health 2015, 62, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Peiris, M.; Yuen, K.; Leung, C.; Chan, K.; Ip, P.; Lai, R.; Orr, W.; Shortridge, K. Human infection with influenza H9N2. Lancet 1999, 354, 916–917. [Google Scholar] [CrossRef]
- Xu, J.; Li, S.; Yang, Y.; Liu, B.; Yang, H.; Li, T.; Zhang, L. Human infection with a further evolved avian H9N2 influenza A virus in Sichuan, China. Sci. China Life Sci. 2018, 61, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Butt, K.M.; Smith, G.J.D.; Chen, H.; Zhang, L.J.; Leung, H.C.; Xu, K.M.; Lim, W.; Webster, R.G.; Yuen, K.Y.; Peiris, J.S.M.; et al. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J. Clin. Microbiol. 2005, 43, 5760–5767. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Zhu, W.; You, R.; Chen, L.; Li, Y.; Liang, J.; Lin, Q.; Liao, M.; Xiao, C.; Ren, T. Human infections with avian influenza viruses in mainland China: A particular risk for southeastern China. J. Infect. 2017, 75, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Hoa, L.N.M.; Tuan, N.A.; My, P.H.; Huong, T.T.K.; Chi, N.T.Y.; Hau Thu, T.T.; Carrique-Mas, J.; Duong, M.T.; Tho, N.D.; Hoang, N.D.; et al. Assessing evidence for avian-to-human transmission of influenza A/H9N2 virus in rural farming communities in northern vietnam. J. Gen. Virol. 2017, 98, 2011–2016. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.J.; Putnam, S.D.; Krueger, W.S.; Chum, C.; Wierzba, T.F.; Heil, G.L.; Yasuda, C.Y.; Williams, M.; Kasper, M.R.; Friary, J.A.; et al. Evidence for avian H9N2 influenza virus infections among rural villagers in Cambodia. J. Infect. Public Health 2013, 6, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Okoye, J.; Eze, D.; Krueger, W.S.; Heil, G.L.; Friary, J.A.; Gray, G.C. Serologic evidence of avian influenza virus infections among Nigerian agricultural workers. J. Med. Virol. 2013, 85, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Heidari, A.; Mancin, M.; Nili, H.; Pourghanbari, G.H.; Lankarani, K.B.; Leardini, S.; Cattoli, G.; Monne, I.; Piccirillo, A. Serological evidence of H9N2 avian influenza virus exposure among poultry workers from Fars Province of Iran. Virol. J. 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ma, C.; Shi, W.; Cui, S.; Lu, G.; Peng, X.; Zhang, D.; Liu, Y.; Liang, H.; Zhang, Y.; et al. A serological survey of antibodies to H5, H7 and H9 avian influenza viruses amongst the duck-related workers in Beijing, China. PLoS ONE 2012, 7, e50770. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.C.; McCarthy, T.; Capuano, A.W.; Setterquist, S.F.; Alavanja, M.C.; Lynch, C.F. Evidence for avian influenza A infections among Iowa’s agricultural workers. Influenza Other Respir. Viruses 2008, 2, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Leibler, J.H.; Silbergeld, E.K.; Pekosz, A.; Gray, G.C. No evidence of infection with avian influenza among US poultry workers in the Delmarva Peninsula Maryland Virginia, USA. J. Agromedicine 2011, 16, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Samaha, H.; Ibrahim, M.S.; Ayoub, M.; Shaaban, S.I. Seroepidemiology of avian influenza viruses H5 and H9 in Beheira Governorate. Alexandria J. Vet. Sci. 2015, 44, 86–92. [Google Scholar] [CrossRef]
- Anvar, E.; Hosseini, S.M.; Kheiri, M.T.; Mazaheri, V.; Fazaei, K.; Shabani, M.; Alizadeh, E.; Tabatabaiean, M.; Torabi, A. serological survey of avian influenza (H9N2) among different occupational groups in Tehran and Qazvin Provinces in IR Iran. Jundishapur J. Microbiol. 2013, 6, 9–12. [Google Scholar] [CrossRef]
- Krueger, W.S.; Khuntirat, B.; Yoon, I.K.; Blair, P.J.; Chittagarnpitch, M.; Putnam, S.D.; Supawat, K.; Gibbons, R.V.; Bhuddari, D.; Pattamadilok, S.; et al. Prospective study of avian influenza virus infections among rural Thai Villagers. PLoS ONE 2013, 8, e72196. [Google Scholar] [CrossRef] [PubMed]
- Hadipour, M.M.; Pazira, S. Evaluation of antibody titers H9N2 influenza virus in hospital staff in Shiraz, Iran. J. Anim. Vet. Adv. 2011, 10, 832–834. [Google Scholar] [CrossRef]
- Pawar, S.D.; Tandale, B.V.; Raut, C.G.; Parkhi, S.S.; Barde, T.D.; Gurav, Y.K.; Kode, S.S.; Mishra, A.C. Avian influenza H9N2 seroprevalence among poultry workers in Pune, India, 2010. PLoS ONE 2012, 7, e36374. [Google Scholar] [CrossRef] [PubMed]
- Kayali, G.; Kandeil, A.; El-Shesheny, R.; Kayed, A.S.; Gomaa, M.M.; Maatouq, A.M.; Shehata, M.M.; Moatasim, Y.; Bagato, O.; Cai, Z.; et al. Active surveillance for avian influenza virus, Egypt, 2010–2012. Emerg. Infect. Dis. 2014, 20, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Uyeki, T.M.; Nguyen, D.C.; Rowe, T.; Lu, X.; Hu-Primmer, J.; Huynh, L.P.; Hang, N.L.K.; Katz, J.M. Seroprevalence of antibodies to avian influenza A (H5) and A (H9) viruses among market poultry workers, Hanoi, Vietnam, 2001. PLoS ONE 2012, 7, e43948. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, C.-X.; Zheng, B.-J. Antibodies against H5 and H9 avian influenza among poultry workers in China. N. Engl. J. Med. 2009, 360, 2583–2584. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhu, W.; Gu, H.; Fu, X.; Wang, L.; Zheng, Y.; He, S.; Ke, C.; Wang, H.; Yuan, Z.; et al. Avian influenza H9N2 seroprevalence among swine farm residents in China. J. Med. Virol. 2014, 86, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, Q.; Yang, K.; Zeng, F.; Lau, S.Y.; Wu, W.L.; Huang, S.; Zhang, J.; Chen, H.; Xia, N. Serological survey of antibodies to influenza A viruses in a group of people without a history of influenza vaccination. Clin. Microbiol. Infect. 2011, 17, 1347–1349. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, A.R.; Liu, Z.H.; Liang, W.; Li, X.X.; Tang, Y.J.; Miao, Z.M.; Chai, T.J. Seroprevalence of avian influenza H9N2 among poultry workers in Shandong Province, China. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, J.; White, S.K.; Cao, Z.; Zhen, Y.; He, S.; Zhu, W.; Ke, C.; Zhang, Y.; Su, S.; et al. Live poultry market workers are susceptible to both avian and swine influenza viruses, Guangdong Province, China. Vet. Microbiol. 2015, 181, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, Y.; Song, W.; Pang, Q.; Miao, Z. Avian influenza virus H9N2 seroprevalence and risk factors for infection in occupational poultry-exposed workers in Tai’an of China. J. Med. Virol. 2016, 88, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Hadipour, M.M. H9N2 avian influenza virus antibody titers in human population in Fars Province, Iran. Braz. J. Poult. Sci. 2010, 12, 160–164. [Google Scholar] [CrossRef]
- Jia, N.; de Vlas, S.J.; Liu, Y.-X.; Zhang, J.-S.; Zhan, L.; Dang, R.-L.; Ma, Y.-H.; Wang, X.-J.; Liu, T.; Yang, G.-P.; et al. Serological reports of human infections of H7 and H9 avian influenza viruses in northern China. J. Clin. Virol. 2009, 44, 225–229. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.W.; Hung, I.F.N.; Lui, Y.-M.M.; Mok, F.K.Y.; Chan, A.S.F.; Li, P.T.W.; Wong, T.-L.L.; Ho, D.T.Y.; Chan, J.F.W.; Chan, K.-H.; et al. Ongoing transmission of avian influenza A viruses in Hong Kong despite very comprehensive poultry control measures: A prospective seroepidemiology study. J. Infect. 2016, 72, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kayali, G.; Ortiz, E.J.; Chorazy, M.L.; Gray, G.C. Evidence of previous avian influenza infection among US Turkey workers. Zoonoses Public Health 2010, 57, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Rabbani, M.; Yaqub, T.; Younus, M.; Mahmood, A.; Shabbir, M.Z.; Fatima, Z.; Khalid, R.K.; Rasheed, M. Serosurveillance to H9 and H7 avian influenza virus among poultry workers in Punjab Province, Pakistan. Pak. Vet. J. 2013, 33, 107–112. [Google Scholar] [CrossRef]
- Ahad, A.; Thornton, R.N.; Rabbani, M.; Yaqub, T.; Younus, M.; Muhammad, K.; Mahmood, A.; Shabbir, M.Z.; Kashem, M.A.; Islam, M.Z.; et al. Risk factors for H7 and H9 infection in commercial poultry farm workers in provinces within Pakistan. Prev. Vet. Med. 2014, 117, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, B.; Jianfang, Z.; Yongkun, C.; Xiaodan, L.; Wenfei, Z.; Yan, L.; Jing, T.; Junfeng, G.; Tao, C.; et al. A comprehensive retrospective study of the seroprevalence of H9N2 avian influenza viruses in occupationally exposed populations in China. PLoS ONE 2017, 12, e0178328. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, E.; Hosseini, S.M.; Kheiri, M.T.; Bashar, R.; Tabatabaeian, M. Avian influenza (H9N2) among poultry workers in Iran. Iran. J. Microbiol. 2009, 1, 3–6. [Google Scholar]
- Uyeki, T.M.; Chong, Y.-H.; Katz, J.M.; Lim, W.; Ho, Y.-Y.; Wang, S.S.; Tsang, T.H.F.; Au, W.W.; Chan, S.; Rowe, T.; et al. Lack of evidence for human-to-human transmission of avian influenza A (H9N2) viruses in Hong Kong, China, 1999. Emerg. Infect. Dis. 2002, 8, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Khuntirat, B.P.; Yoon, I.K.; Blair, P.J.; Krueger, W.S.; Chittaganpitch, M.; Putnam, S.D.; Supawat, K.; Gibbons, R.V.; Pattamadilok, S.; Sawanpanyalert, P.; et al. Evidence for subclinical avian influenza virus infections among rural thai villagers. Clin. Infect. Dis. 2011, 53, e107–e116. [Google Scholar] [CrossRef] [PubMed]
- Coman, A.; Maftei, D.N.; Krueger, W.S.; Heil, G.L.; Friary, J.A.; Chereches, R.M.; Sirlincan, E.; Bria, P.; Dragnea, C.; Kasler, I.; et al. Serological evidence for avian H9N2 influenza virus infections among Romanian agriculture workers. J. Infect. Public Health 2013, 6, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Kayali, G.; Setterquist, S.F.; Capuano, A.W.; Myers, K.P.; Gill, J.S.; Gray, G.C. Testing human sera for antibodies against avian influenza viruses: Horse RBC hemagglutination inhibition vs. microneutralization assays. J. Clin. Virol. 2008, 43, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Ma, W.; Sun, N.; Huang, L.; Li, Y.; Zeng, Z.; Wen, Y.; Zhang, Z.; Li, H.; Li, Q.; et al. PB2-588 V promotes the mammalian adaptation of H10N8, H7N9 and H9N2 avian influenza viruses. Sci. Rep. 2016, 6, 19474. [Google Scholar] [CrossRef] [PubMed]
- Swayne, D.E. Avian influenza vaccines and therapies for poultry. Comp. Immunol. Microbiol. Infect. Dis. 2009, 32, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Lee, J.; Mena, I.; Henningson, J.; Li, Y.; Ma, J.; Duff, M.; Li, Y.; Lang, Y.; Yang, J.; et al. Recombinant Newcastle disease virus expressing H9 HA protects chickens against heterologous avian influenza H9N2 virus challenge. Vaccine 2016, 34, 2537–2545. [Google Scholar] [CrossRef] [PubMed]
- Tretyakova, I.; Pearce, M.B.; Florese, R.; Tumpey, T.M.; Pushko, P. Intranasal vaccination with H5, H7 and H9 hemagglutinins co-localized in a virus-like particle protects ferrets from multiple avian influenza viruses. Virology 2013, 442, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Pushko, P.; Pearce, M.B.; Ahmad, A.; Tretyakova, I.; Smith, G.; Belser, J.A.; Tumpey, T.M. Influenza virus-like particle can accommodate multiple subtypes of hemagglutinin and protect from multiple influenza types and subtypes. Vaccine 2011, 29, 5911–5918. [Google Scholar] [CrossRef] [PubMed]
- Bertran, K.; Balzli, C.; Kwon, Y.-K.; Tumpey, T.M.; Clark, A.; Swayne, D.E. Airborne transmission of highly pathogenic influenza virus during processing of infected poultry. Emerg. Infect. Dis. 2017, 23, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Wu, J.; Lau, E.H.Y.; Cheng, K.L.; Zhong, Z.; Song, Y.; Ji, X.; Zhou, L.; Ke, C.; Peiris, J.S.M.; et al. Monitoring avian influenza viruses from chicken carcasses sold at markets, China, 2016. Emerg. Infect. Dis. 2017, 23, 1714–1717. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Antigenic and genetic characteristics of zoonotic influenza viruses and development of candidate vaccine viruses for pandemic preparedness. Wkly. Epidemiol. Rec. 2018, 93, 133–142. [Google Scholar] [CrossRef]
| Origin | Glutamine | Leucine | Total Isolates |
|---|---|---|---|
| North America 1966–2017 a | 100% b | 0% | 68 |
| South Korea 1996–2012 | 100% | 0% | 128 |
| China 1980–1997 | 59.50% | 40.50% | 42 |
| China 1998–2000 | 39.80% | 60.10% | 153 |
| China 2001–2003 | 25.70% | 74.30% | 268 |
| China 2004–2006 | 26.70% | 73.30% | 236 |
| China 2007–2009 | 14.20% | 85.80% | 513 |
| China 2010–2012 | 6.90% | 93.10% | 1182 |
| China 2013–2015 | 2.80% | 97.20% | 688 |
| China 2016-2018 | 3.00% | 97.00% | 66 |
| rest of world 1980–2000 | 61.10% | 38.90% | 90 |
| rest of world 2001–2003 | 32% | 68% | 147 |
| rest of world 2004–2006 | 32% | 68% | 128 |
| rest of world 2007–2009 | 30.70% | 69.30% | 150 |
| rest of world 2010–2012 | 9.50% | 90.50% | 190 |
| rest of world 2013–2015 | 4.20% | 95.80% | 237 |
| rest of world 2016–2018 | 19.10% | 80.90% | 157 |
| swine 1998–2015 | 54.2% | 45.8% | 48 |
| human 1998–2016 | 8.7% | 91.3% | 23 |
| H9N2 Sequenced Human Viruses | Poultry Lineage |
|---|---|
| A/Shaoguan/447/1998 | Y280 |
| A/Shaoguan/408/1998 | Y280 |
| A/Shantou/239/1998 | Y280 |
| A/Hong Kong/1073/1999 | G1 |
| A/Hong Kong/1074/1999 | G1 |
| A/Guangzhou/333/1999 | Y280 |
| A/Nanchang/CH3/2000 | Y280 |
| A/Nanchang/D1/2000 | Y280 |
| A/Nanchang/D2/2000 | Y280 |
| A/Nanchang/CH2/2000 | Y280 |
| A/HK/2108/2003 | Y280 |
| A/Guangdong/W1/2004 | Y280 |
| A/Hong Kong/3239/2008 | Y280 |
| A/Hong Kong/69955/2008 | Y280 |
| A/Hong Kong/35820/2009 | G1 |
| A/Hong Kong/33982/2009 | G1 |
| A/Bangladesh/0994/2011 | G1 |
| A/Hong Kong/308/2014 | Y280 |
| A/Hunan/44557/2015 | Y280 |
| A/Zhongshan/201501/2015 | Y280 |
| A/Hunan/44558/2015 | Y280 |
| A/Beijing/1/2016 | Y280 |
| A/Guangdong/MZ058/2016 | Y280 |
| A/Beijing/1/2017 | Y280 |
| Country | Dates Sampled | Occupational Exposure | Percent Positiv (%) | General Exposure | Percent Positive (%) | HI Cutoff | Occupational Exposure | Percent Positive (%) | General Exposure | Percent Positive (%) | MN Cutoff | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| China 1 | 1999 | 0/54 | 0 | 1/110 | 0.91 | 1600 | [87] | |||||
| Vietnam 1 | 2001 | 1/200 | 0.5 | 0/200 | 0 | 1/40 | [72] | |||||
| UnitedStates 2 | 2004 | 1/385 | 0.26 | 1/484 | 0.21 | 1/40 | [64] | |||||
| China 1 | Apr. 2006–Feb. 2008 | 12/1060 | 1.1 | 0/407 | 0 | 1/160 | [80] | |||||
| Iran 1 | Nov. 2006 | 48/127 | 37.7 | 0/25 | 0 | 1/20 | [86] | |||||
| UnitedStates 2 | Mar. 2007–Apr. 2008 | 4/93 | 4.3 | 1/78 | 1.28 | 1/10 | [82] | |||||
| China 1 | Mar. 2007–Jul. 2008 | 95/1890 | 5.03 | 4/301 | 1.33 | 1/20 | [73] | |||||
| China 1 | Jan. 2008–Dec. 2010 | 103/840 | 12.47 | 47/1663 | 2.83 | 1/40 | 1/20 | [54] | ||||
| Cambodia 3 | Apr. 2008–Oct. 2008 | 21/777 | 2.7 | 1/10 | [60] | |||||||
| Thailand 3 | Apr. 2008–Oct. 2010 Enrollment | 38/800 | 4.7 | 1/10 | [88] | |||||||
| 12 Month | 21/768 | 2.7 | 1/10 | [68] | ||||||||
| 24 Month | 40/784 | 5.1 | 1/10 | |||||||||
| Nigeria 2 | Dec. 2008–Jun. 2009 | 4/316 | 1.27 | 0/54 | 0 | 1/10 | [61] | |||||
| United States 2 | 2009–2010 | 1/157 | 0.63 | 0/78 | 0 | [65] | ||||||
| China 1 | 2009–2011 | 1912/14,896 | 12.8 | 1/40 | 453/14,896 | 3.04 | 1/40 | [85] | ||||
| 489/13,453 | 3.6 | 159/13,453 | 1.18 | |||||||||
| Romania 2 | Feb. 2009–Jan. 2010 | 31/312 | 0.7 | 2/51 | 3.92 | 1/40 | 29/312 | 10.14 | 4/51 | 5.19 | 1/10 | [89] |
| China 1 | Mar. 2009–Dec. 2012 | 37/2006 | 1.8 | 0/83 | 0 | 1/160 | 24/37 | 64.86 | 0 | [74] | ||
| China 1 | May 2010 | 18/1039 | 1.73 | 1/40 | [75] | |||||||
| India 1 | Jul. 2010–Dec. 2010 | 21/338 | 6.2 | 0/249 | 0 | 1/40 | 19/338 | 5.6 | 1/40 | [70] | ||
| Iran 1 | Dec. 2010–Jul. 2011 | 3/182 | 1.6 | 0 | 0 | 1/20 | [67] | |||||
| Pakistan 1 | 2010–2011 | 165/384 | 43.0 | 1/160 | [84] | |||||||
| China 1 | Jan. 2011–Dec. 2013 | 51/600 | 8.5 | 11/600 | 1.8 | 1/40 | 51/600 | 8.5 | 11/600 | 1.8 | 1/40 | [78] |
| China 1 | Mar. 2011 | 12/1741 | 0.7 | 1/40 | [63] | |||||||
| China 1 | Dec. 2011–Feb. 2012 | 9/382 | 2.3 | 0/100 | 0 | 1/40 | 7/382 | 1.80 | 0/100 | 0 | 1/40 | [76] |
| Iran 1 | Sep. 2012–Jan. 2013 | 12/100 | 12.0 | 2/100 | 2 | 1/40 | 17/100 | 17 | 3/100 | 3 | 1/40 | [62] |
| China 1 | Oct. 2013–Jul. 2014 | 56/171 | 32.7 | 1/40 | [81] | |||||||
| China 1 | Dec. 2013–Jan. 2014 | 50/546 | 9.2 | 5/264 | 1.89 | 1/40 | [77] | |||||
| Vietnam 1 | 2013–2015 | 28/784 | 3.6 | 0 | 0 | 1/40 | [59] | |||||
| Iran 1 | not reported | 98/300 | 32.6 | 8/300 | 2.5 | 1/40 | [69] | |||||
| Iran 1 | not reported | 163/240 | 67.9 | 14/60 | 23.3 | 1/20 | [79] | |||||
| Pakistan 1 | not reported | 209/465 | 45.0 | 0/25 | 0 | 1/160 | [83] | |||||
| Egypt 1 | not reported | 0/60 | 0.0 | 1/40 | [66] |
| Gene Segment | Avian > Mammalian | Avian Isolates | Human Isolates | Activity |
|---|---|---|---|---|
| PB2 | K526R | 3.20% | 6.30% | increase polymerase activity, enhance virus replication |
| PB2 | A588V | 11.90% | 25% | higher polymerase activity, increased virulence mice |
| PB2 | E627K | 0.80% | 6.30% | associated with mammalian adaptation |
| PB2 | D701N | 0.05% | 6.30% | aerosol transmission guinea pigs |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pusch, E.A.; Suarez, D.L. The Multifaceted Zoonotic Risk of H9N2 Avian Influenza. Vet. Sci. 2018, 5, 82. https://doi.org/10.3390/vetsci5040082
Pusch EA, Suarez DL. The Multifaceted Zoonotic Risk of H9N2 Avian Influenza. Veterinary Sciences. 2018; 5(4):82. https://doi.org/10.3390/vetsci5040082
Chicago/Turabian StylePusch, Elizabeth A., and David L. Suarez. 2018. "The Multifaceted Zoonotic Risk of H9N2 Avian Influenza" Veterinary Sciences 5, no. 4: 82. https://doi.org/10.3390/vetsci5040082
APA StylePusch, E. A., & Suarez, D. L. (2018). The Multifaceted Zoonotic Risk of H9N2 Avian Influenza. Veterinary Sciences, 5(4), 82. https://doi.org/10.3390/vetsci5040082





