Red Cotton Stamen Extracts Mitigate Ferrous Sulfate-Induced Oxidative Stress and Enhance Quality in Bull Frozen Semen
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection and Extraction
2.2. Total Phenolic and Total Tannin Content Determination
2.3. Total Anthocyanins Determination
2.4. Total Flavonoid Determination
2.5. Lycopene Content Determination
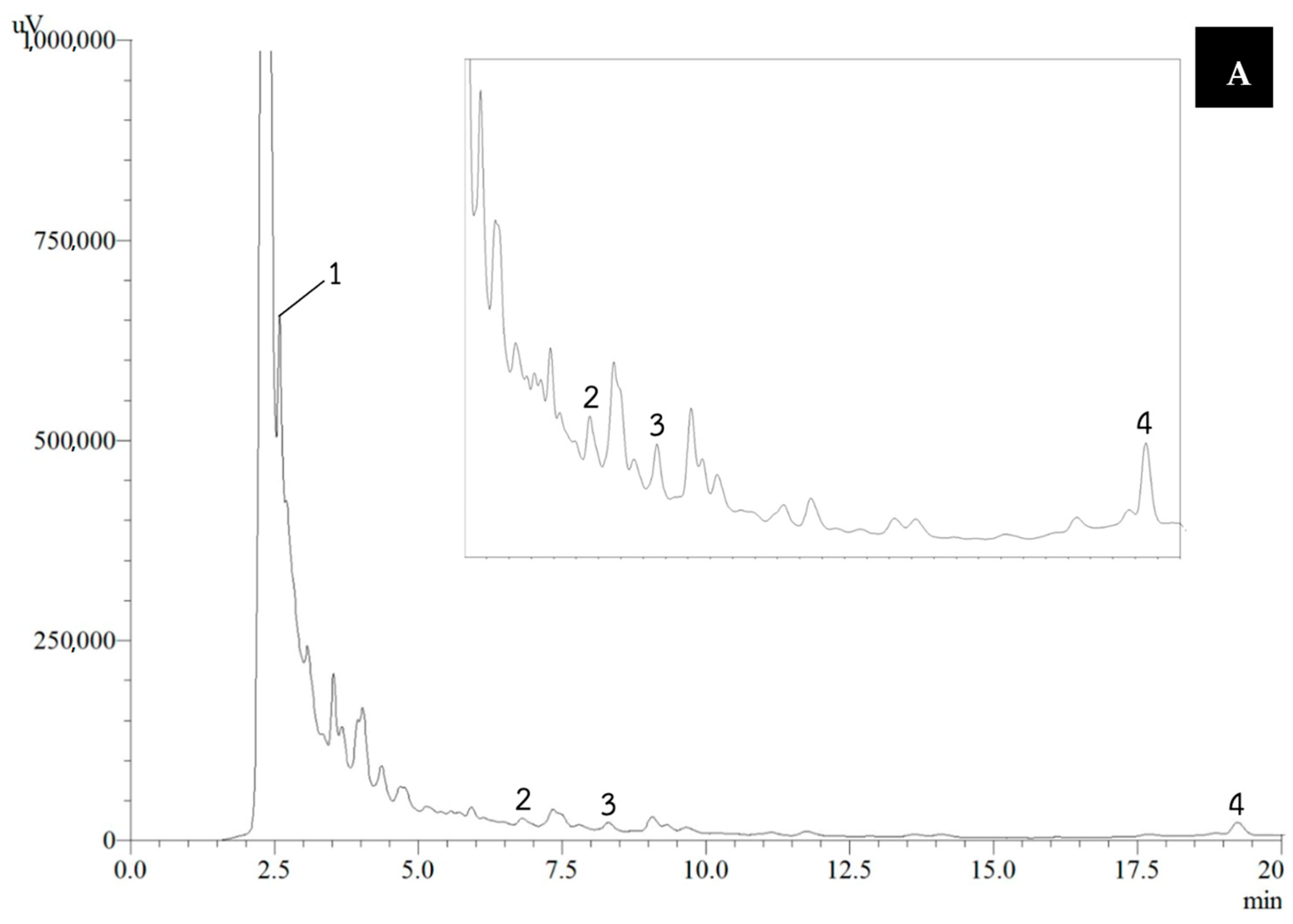
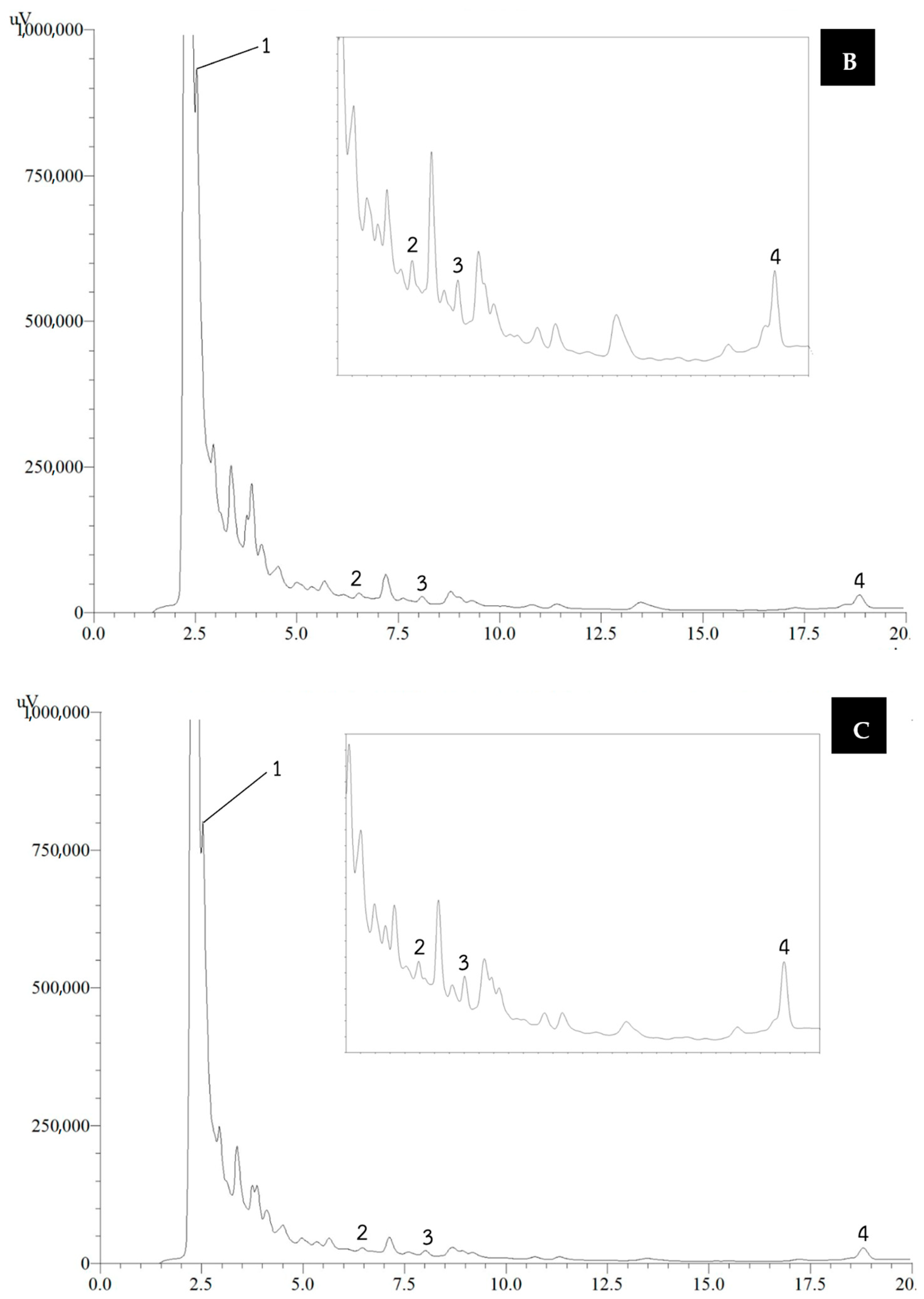
2.6. High-Performance Liquid Chromatography (HPLC) Analysis of Phenolic Compounds
2.7. Antioxidant Capacity Determination
2.7.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
2.7.2. 2,2′-Azino-di-[3-Ethylbenzthiazoline Sulfonate] (ABTS) Radical Scavenging Assay
2.8. Inhibition of Protein Denaturation Assay
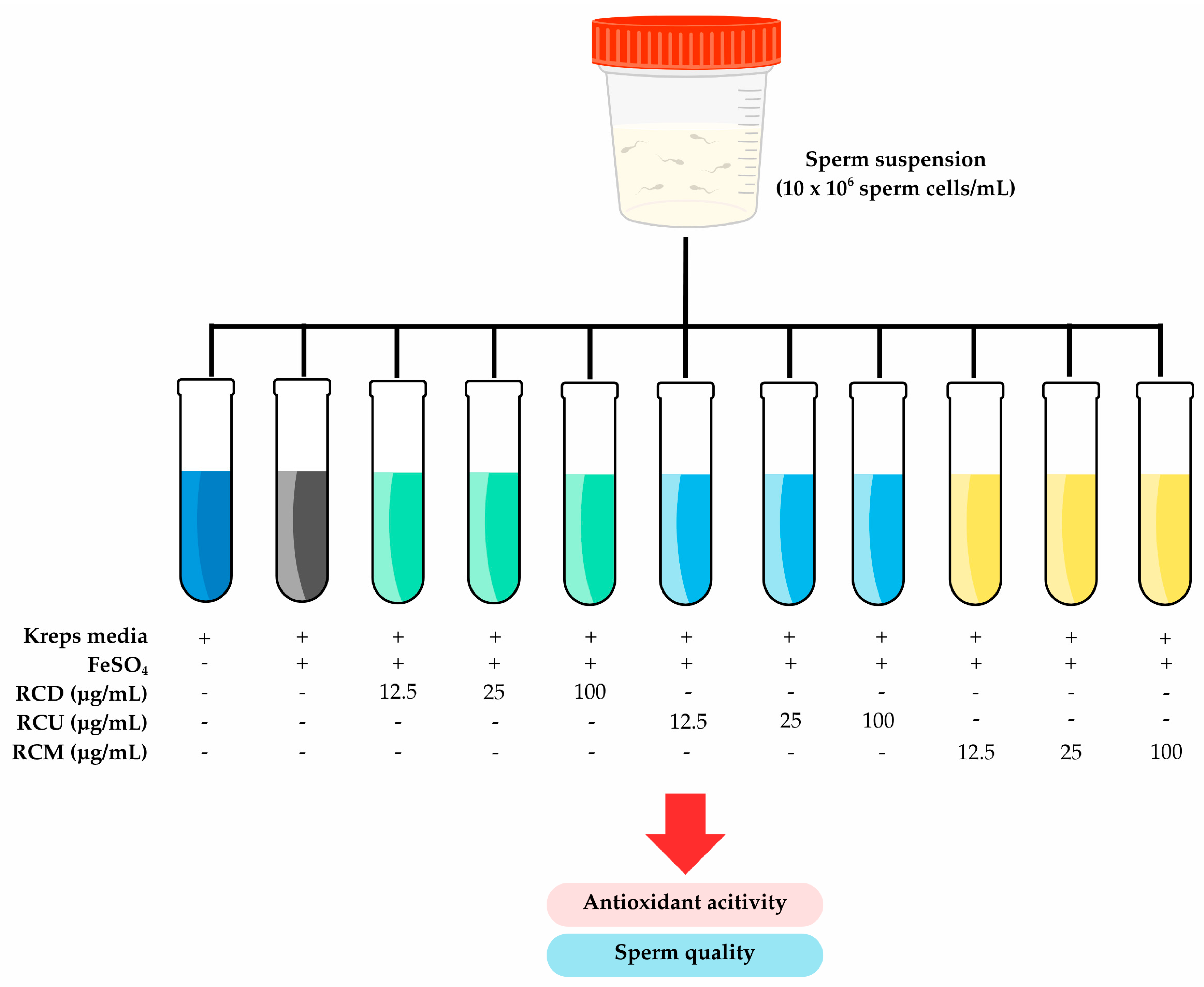
2.9. Experimental Design
2.10. Determination of Sperm Reactive Oxygen Species (ROS) Production
2.11. Determination of Inhibition of Advanced Glycation End Products (AGEs) Formation
2.12. Sperm Qualities Evaluation
2.12.1. Determination of Sperm Motility
2.12.2. Determination of Sperm Viability
2.12.3. Classification of Sperm Morphology
2.13. Statistical Analysis
3. Results
3.1. Identification and Quantification of Phytochemical Compounds
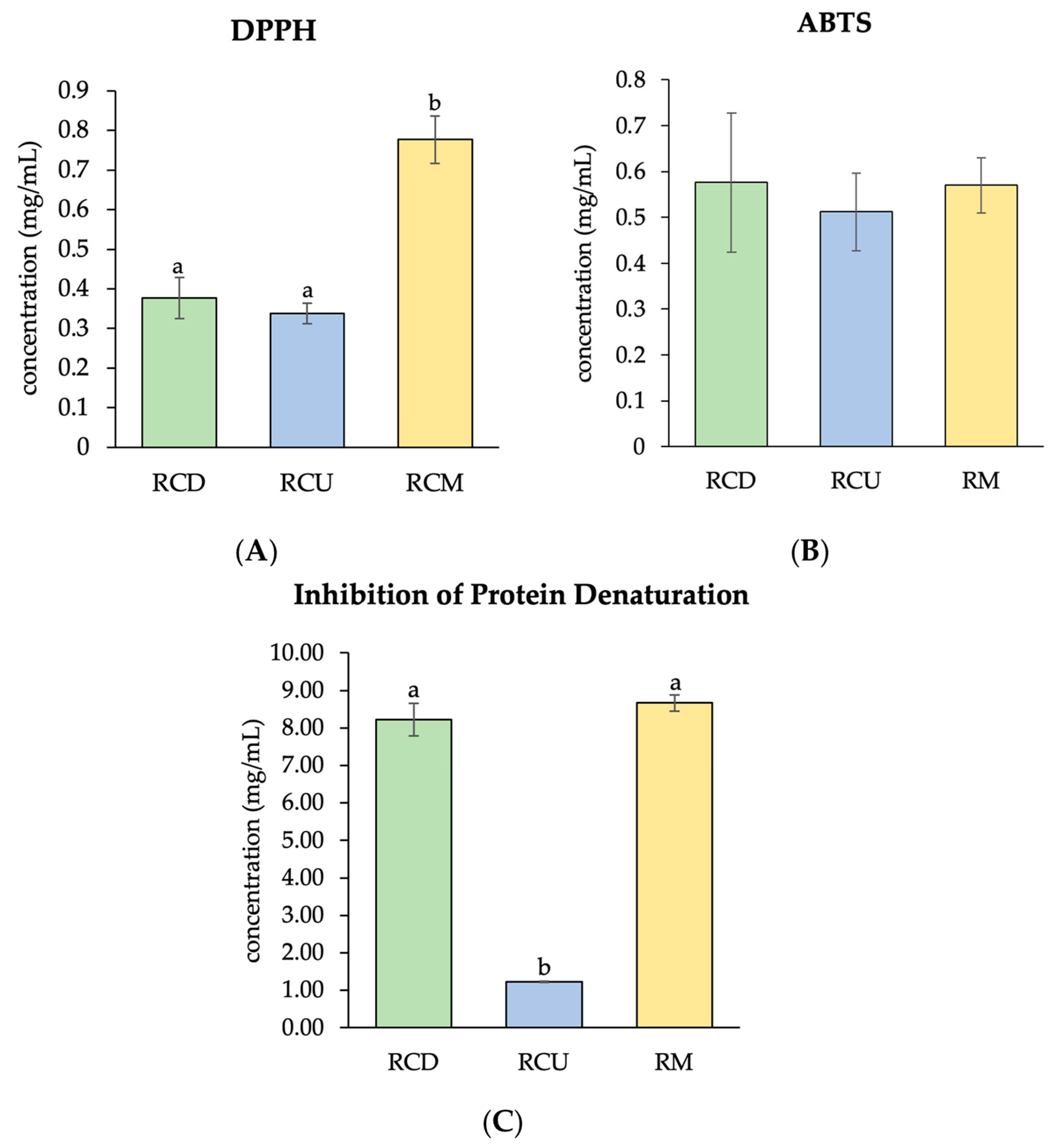
3.2. Antioxidant Capacities and Inhibition of Protein Denaturation in Cell-Free System
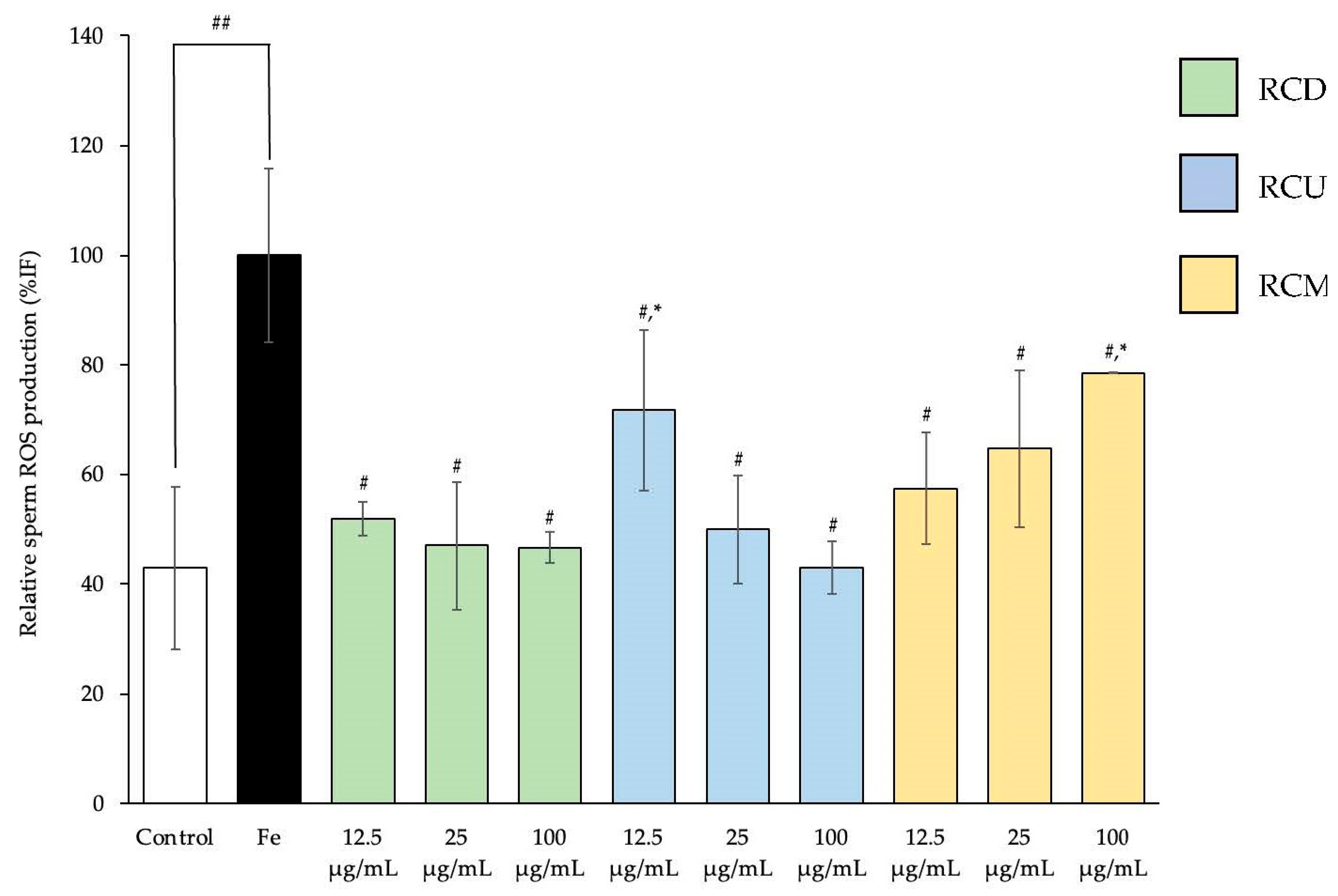
3.3. Sperm Reactive Oxygen Species (ROS)
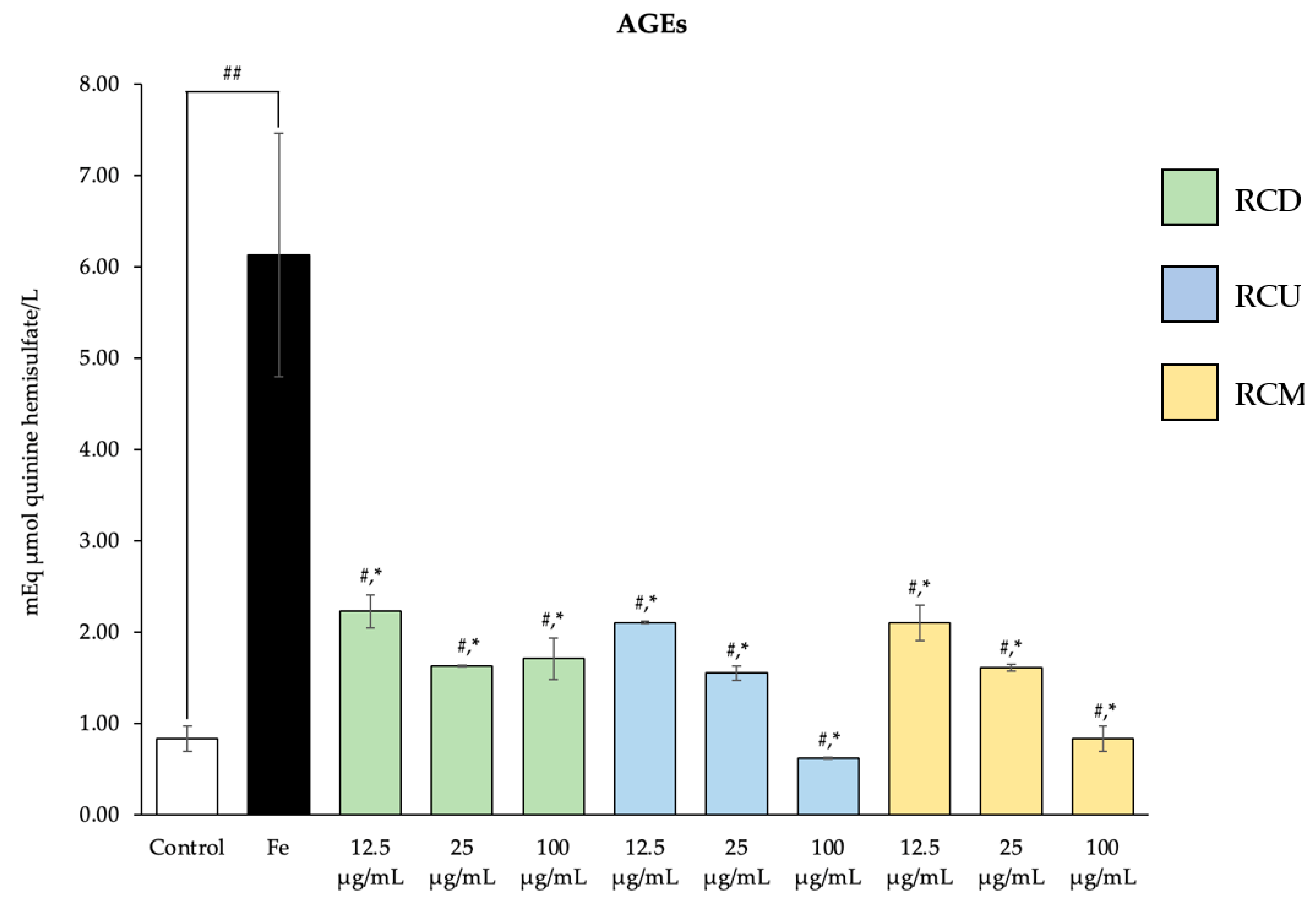
3.4. Inhibition of Advanced Glycation End Products (AGEs)
3.5. Sperm Quality
3.5.1. Sperm Motility
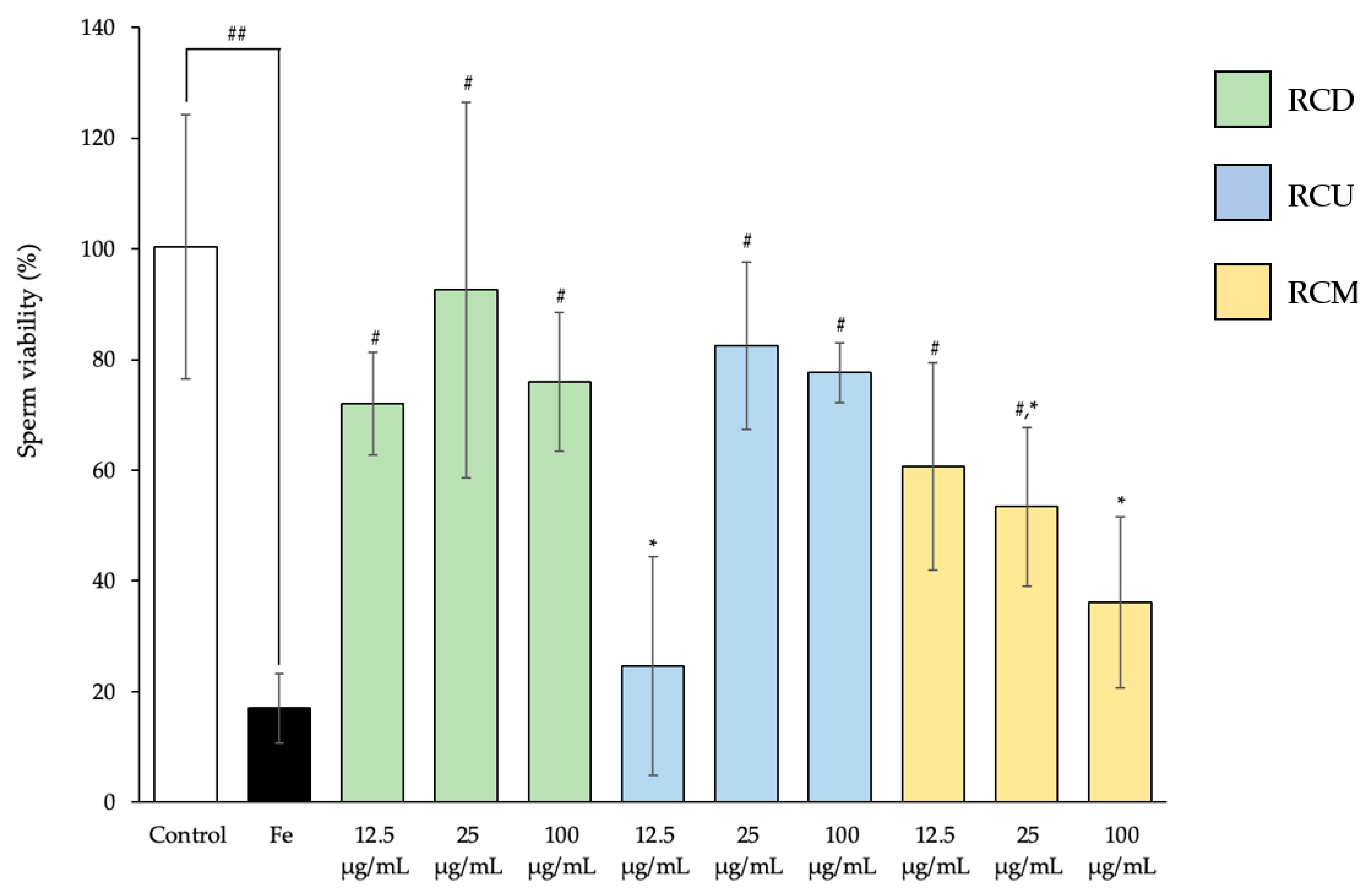
3.5.2. Sperm Viability
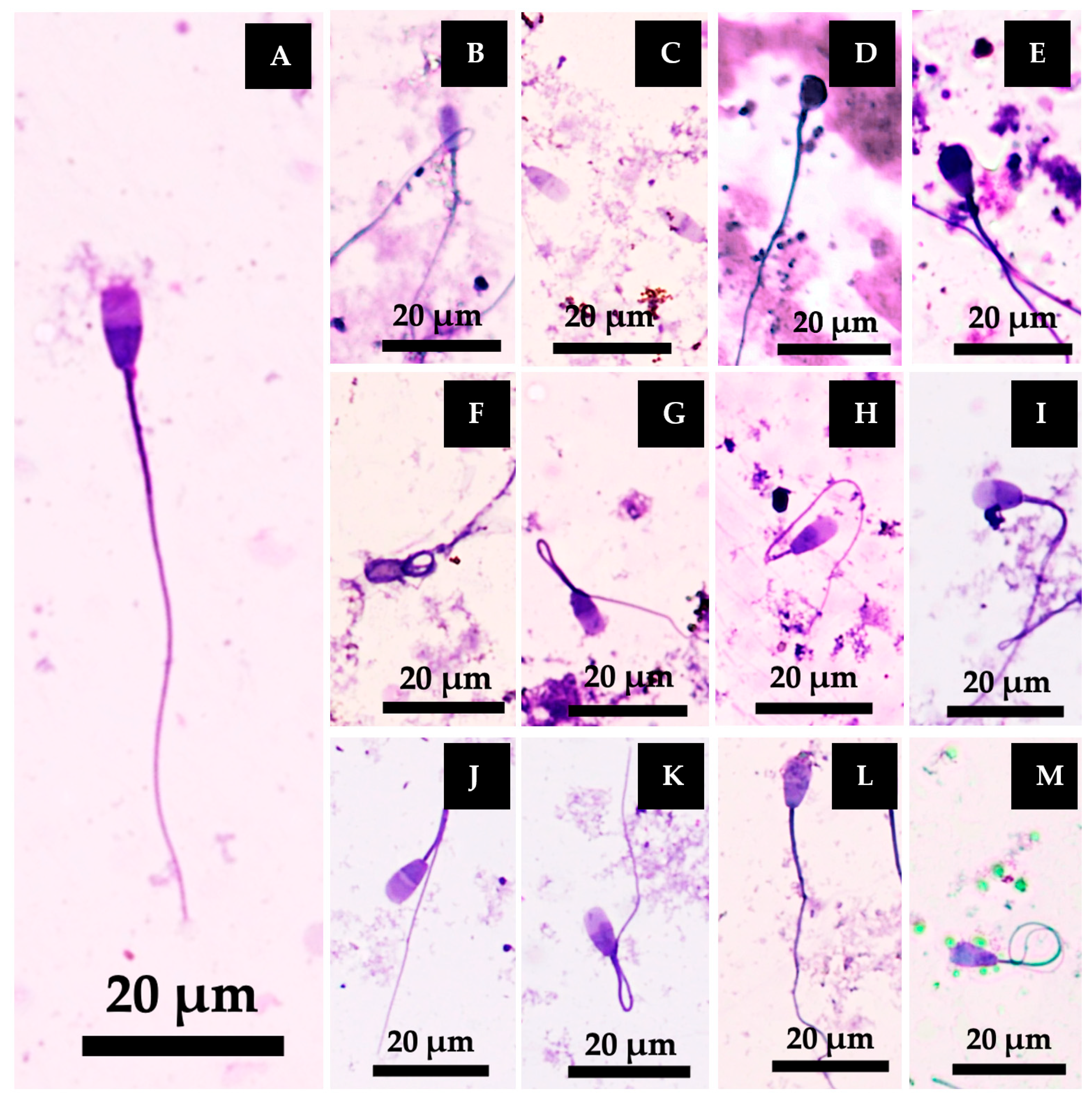
3.5.3. Sperm Morphology Classification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boivin, J.; Bunting, L.; Collins, J.A.; Nygren, K.G. International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility medical care. Hum. Reprod. 2007, 22, 1506–1512. [Google Scholar] [CrossRef]
- De Jonge, C.; Barratt, C.L. The present crisis in male reproductive health: An urgent need for a political, social, and research roadmap. Andrology 2019, 7, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Virk, G.; Ong, C.; Du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Mens Health 2014, 32, 1. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Saleh, R.A. Role of oxidants in male infertility: Rationale, significance, and treatment. Urol. Clin. 2002, 29, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Sedha, S.; Kumar, S.; Shukla, S. Role of oxidative stress in male reproductive dysfunctions with reference to phthalate compounds. Urol. J. 2015, 12, 2304–2316. [Google Scholar] [CrossRef]
- Bansal, A.; Bilaspuri, G. Effect of ferrous sulphate and ascorbic acid on motility, viability and lipid peroxidation of crossbred cattle bull spermatozoa. Animal 2008, 2, 100–104. [Google Scholar] [CrossRef]
- Makker, K.; Agarwal, A.; Sharma, R. Oxidative stress & male infertility. Indian J. Med. Res. 2009, 129, 357–367. [Google Scholar]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Sapanidou, V.; Taitzoglou, I.; Tsakmakidis, I.; Kourtzelis, I.; Fletouris, D.; Theodoridis, A.; Lavrentiadou, S.; Tsantarliotou, M. Protective effect of crocetin on bovine spermatozoa against oxidative stress during in vitro fertilization. Andrology 2016, 4, 1138–1149. [Google Scholar] [CrossRef]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 2018, 16, 87. [Google Scholar] [CrossRef]
- Cocuzza, M.; Sikka, S.C.; Athayde, K.S.; Agarwal, A. Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: An evidence based analysis. Int. Braz. J. Urol. 2007, 33, 603–621. [Google Scholar] [CrossRef]
- Bozkurt, Y.; Yavaş, İ.; Bucak, M.N.; Kıran, T.R.; Gül, A. Cryoprotective effect of vitamin E supplementation of different extenders on quality and fertilizing ability of frozen-thawed brown trout sperm. Biopreserv. Biobank. 2021, 19, 171–177. [Google Scholar] [CrossRef]
- Kefer, J.C.; Agarwal, A.; Sabanegh, E. Role of antioxidants in the treatment of male infertility. Int. J. Urol. 2009, 16, 449–457. [Google Scholar] [CrossRef]
- Mbah, C.J.; Orabueze, I.; Okorie, N.H. Antioxidants properties of natural and synthetic chemical compounds: Therapeutic effects on biological system. Acta Sci. Pharm. Sci 2019, 3, 28–42. [Google Scholar] [CrossRef]
- Sharples, F.M.; Van Haselen, R.; Fisher, P. NHS patients’ perspective on complementary medicine: A survey. Complement. Ther. Med. 2003, 11, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Shahat, A.A.; Hassan, R.A.; Nazif, N.M.; Van Miert, S.; Pieters, L.; Hammuda, F.M.; Vlietinck, A.J. Isolation of mangiferin from Bombax malabaricum and structure revision of shamimin. Planta Medica 2003, 69, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Thongmee, O.; Rodhetbhai, C.; Siltragool, W. Lanna food: The cultural management strategy for the creative economy development. Humanit. Arts Soc. Sci. Stud. 2015, 15, 105–120. [Google Scholar]
- Davis, T. Stamen number and pollen size in levo-and dextro-rotary flowers of Bombacaceae. Rev. Palaeobot. Palynol. 1967, 3, 133–139. [Google Scholar] [CrossRef]
- Kriintong, N.; Katisart, T. In vitro antioxidant and antidiabetic activities of leaf and flower extracts from Bombax ceiba. Pharmacogn. Res. 2020, 12, 194–198. [Google Scholar] [CrossRef]
- Chaudhary, P.H.; Tawar, M.G. Pharmacognostic and phytopharmacological overview on Bombax ceiba. Syst. Rev. Pharm. 2019, 10, 20–25. [Google Scholar] [CrossRef]
- Wang, G.K.; Lin, B.B.; Rao, R.; Zhu, K.; Qin, X.Y.; Xie, G.Y.; Qin, M.J. A new lignan with anti-HBV activity from the roots of Bombax ceiba. Nat. Prod. Res. 2013, 27, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Laoung-On, J.; Ounjaijean, S.; Sudwan, P.; Boonyapranai, K. Phytochemical screening, antioxidant effect and sperm quality of the Bomba ceiba stamen extracts on Charolais cattle sperm induced by ferrous sulfate. Plants 2024, 13, 960. [Google Scholar] [CrossRef] [PubMed]
- Kopjar, M.; Orsolic, M.; Pilizota, V. Anthocyanins, phenols, and antioxidant activity of sour cherry puree extracts and their stability during storage. Int. J. Food Prop. 2014, 17, 1393–1405. [Google Scholar] [CrossRef]
- Suwanaruang, T. Analyzing lycopene content in fruits. Agric. Agric. Sci. Proc. 2016, 11, 46–48. [Google Scholar] [CrossRef]
- Laoung-On, J.; Jaikang, C.; Saenphet, K.; Sudwan, P. Phytochemical screening, antioxidant and sperm viability of Nelumbo nucifera petal extracts. Plants 2021, 10, 1375. [Google Scholar] [CrossRef]
- Dharmadeva, S.; Galgamuwa, L.S.; Prasadinie, C.; Kumarasinghe, N. In vitro anti-inflammatory activity of Ficus racemosa L. bark using albumin denaturation method. AYU 2018, 39, 239–242. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Calogero, A.E.; Singh, R.; Cannarella, R.; Sengupta, P.; Dutta, S. Coenzyme Q10, oxidative stress, and male infertility: A review. Clin. Exp. Reprod. Med. 2021, 48, 97. [Google Scholar] [CrossRef]
- Benedetti, S.; Catalani, S.; De Stefani, S.; Primiterra, M.; Fraternale, A.; Palma, F.; Palini, S. A microplate-based DCFH-DA assay for the evaluation of oxidative stress in whole semen. Heliyon 2022, 8, e10642. [Google Scholar] [CrossRef]
- Laoung-On, J.; Saenphet, K.; Jaikang, C.; Sudwan, P. Effect of Moringa oleifera Lam. leaf tea on sexual behavior and reproductive function in male rats. Plants 2021, 10, 2019. [Google Scholar] [CrossRef]
- Komori, K.; Tsujimura, A.; Ishijima, S.; Tanjapatkul, P.; Fujita, K.; Matsuoka, Y.; Takao, T.; Miyagawa, Y.; Takada, S.; Okuyama, A. Comparative study of sperm motility analysis system and conventional microscopic semen analysis. Reprod. Med. Biol. 2006, 5, 195–200. [Google Scholar] [CrossRef]
- Laoung-On, J.; Nuchniyom, P.; Intui, K.; Jaikang, C.; Saenphet, K.; Boonyapranai, K.; Konguthaithip, G.; Outaitaveep, N.; Phankhieo, S.; Sudwan, P. The potential effect of Bualuang (white Nelumbo nucifera Gaertn.) extract on sperm quality and metabolomic profiles in mancozeb-induced oxidative stress in male rats. Life 2024, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Laoung-On, J.; Jaikang, C.; Saenphet, K.; Sudwan, P. Effect of Nelumbo nucifera petals extract on antioxidant activity and sperm quality in Charolais cattle sperm induced by mancozeb. Plants 2022, 11, 637. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xiong, X.; Huang, G. Ultrasound-assisted extraction and characteristics of maize polysaccharides from different sites. Ultrason. Sonochem. 2023, 95, 106416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, J.; Dai, X.; Li, X. Extraction and analysis of chemical compositions of natural products and plants. Separations 2023, 10, 598. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of temperatures on polyphenols during extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Akowuah, G.; Mariam, A.; Chin, J. The effect of extraction temperature on total phenols and antioxidant activity of Gynura procumbens leaf. Pharmacogn. Mag. 2009, 5, 81–85. [Google Scholar]
- Vo, T.P.; Pham, N.D.; Pham, T.V.; Nguyen, H.Y.; Vo, L.T.V.; Tran, T.N.H.; Tran, T.N.; Nguyen, D.Q. Green extraction of total phenolic and flavonoid contents from mangosteen (Garcinia mangostana L.) rind using natural deep eutectic solvents. Heliyon 2023, 9, e14884. [Google Scholar] [CrossRef]
- Aprodu, I.; Milea, Ș.A.; Enachi, E.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Thermal degradation kinetics of anthocyanins extracted from purple maize flour extract and the effect of heating on selected biological functionality. Foods 2020, 9, 1593. [Google Scholar] [CrossRef]
- Oancea, S.; Stoia, M.; Coman, D. Effects of extraction conditions on bioactive anthocyanin content of Vaccinium corymbosum in the perspective of food applications. Proc. Eng. 2012, 42, 489–495. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Melis, M.R.A. A dopamine and sexual behavior. Neurosci. Biobehav. Rev. 1995, 19, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Shayan-Nasr, M.; Ghaniei, A.; Eslami, M.; Zadeh-Hashem, E. Ameliorative role of trans-ferulic acid on induced oxidative toxicity of rooster semen by β-cyfluthrin during low temperature liquid storage. Poult. Sci. 2021, 100, 101308. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Yang, L.; Wu, L.; He, H.; Geng, G.; Xu, D.; Chen, H.; Li, Q. Combined effect of apigenin and ferulic acid on frozen-thawed boar sperm quality. Anim. Sci. J. 2018, 89, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Eslami, M.; Farrokhi-Ardabili, F. Influence of trans-ferulic acid on the quality of ram semen upon cold preservation. Vet. Med. Sci. 2023, 9, 1369–1378. [Google Scholar] [CrossRef]
- Zhang, F.; Han, S.; Zhang, N.; Chai, J.; Xiong, Q. Effect of ferulic acid on semen quality of goat bucks during liquid storage at 17 °C. Animals 2023, 13, 2469. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Factors influencing the antioxidant activity determined by the ABTS•+ radical cation assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, X.; Li, H. Mechanisms of oxidative stress-induced sperm dysfunction. Front. Endocrinol. 2025, 16, 1520835. [Google Scholar] [CrossRef]
- Meng, F.; Tong, H.; Feng, C.; Huang, Z.; Wu, P.; Zhou, J.; Hua, J.; Wu, F.; Liu, C. Structural Fe(II)-induced generation of reactive oxygen species on magnetite surface for aqueous As(III) oxidation during oxygen activation. Water Res. 2024, 252, 121232. [Google Scholar] [CrossRef]
| Sample | Yield (%) | Total Phenolic (µg GAE/g Dried Weight) | Total Tannin (µg TAE/g Dried Weight) | Total Monomeric Anthocyanins (µg Cyanidin-3-Glucoside E/g Dried Weight) | Total Flavonoid (µg QE/g Dried Weight) | Lycopene Content (×102 µg/g Dried Weight) |
|---|---|---|---|---|---|---|
| RCD | 21.81 ± 6.27 | 5.55 ± 0.27 b,c | 4.77 ± 0.23 b,c | 6.85 ± 1.04 a,b | 0.11 ± 0.06 | 41.71 ± 8.89 |
| RCU | 22.21 ± 4.90 | 5.84 ± 0.21 c | 4.98 ± 0.19 c | 7.99 ± 0.26 c | 0.13 ± 0.03 | 56.91 ± 28.90 |
| RCM | 21.19 ± 6.60 | 5.19 ± 0.21 a,b | 4.45 ± 0.17 a,b | 6.46 ± 0.26 a | 0.15 ± 0.01 | 40.30 ± 13.96 |
| Sample | µg/Mg Plant Extract | |||
|---|---|---|---|---|
| Gallic Acid | Chlorogenic Acid | Caffeic Acid | Ferulic Acid | |
| RCD | 0.61 ± 0.05 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 a,b |
| RCU | 0.63 ± 0.06 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 a |
| RCM | 0.65 ± 0.05 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 b |
| Group | Concentration (µg/mL) | Percentage of Sperm Motility | Percentage of Sperm Non-Motility | ||
|---|---|---|---|---|---|
| Progressive | Circle | Non-Progressive | |||
| Control | 1.67 ± 0.27 | 1.67 ± 1.04 | 15.83 ± 0.29 | 80.83 ± 0.76 | |
| Ferrous sulfate (Fe) | 20 µg/mL | 0.00 ± 0.00 ## | 0.00 ± 0.00 ## | 0.17 ± 0.29 ## | 99.83 ± 0.29 ## |
| RCD | 12.5 µg/mL | 2.66 ± 1.60 # | 0.00 ± 0.00 * | 21.50 ± 1.00 *,# | 75.84 ± 1.04 *,# |
| 25 µg/mL | 2.34 ± 1.04 # | 0.00 ± 0.00 * | 22.33 ± 8.81 *,# | 75.33 ± 9.83 # | |
| 100 µg/mL | 0.84 ± 0.29 *,# | 0.00 ± 0.00 * | 13.33 ± 2.84 # | 81.83 ± 3.06 # | |
| RCU | 12.5 µg/mL | 2.17 ± 0.29 # | 0.00 ± 0.00 * | 13.00 ± 3.28 # | 84.84 ± 3.55 # |
| 25 µg/mL | 5.00 ± 1.50 *,# | 0.00 ± 0.00 * | 44.17 ± 16.65 *,# | 50.83 ± 18.11 *,# | |
| 100 µg/mL | 3.66 ± 1.26 *,# | 0.00 ± 0.00 * | 44.34 ± 10.50 *,# | 52.00 ± 11.76 *,# | |
| RCM | 12.5 µg/mL | 0.16 ± 0.29 * | 0.00 ± 0.00 * | 12.84 ± 0.58 *,# | 87.00 ± 0.50 *,# |
| 25 µg/mL | 0.00 ± 0.00 * | 0.00 ± 0.00 * | 9.17 ± 0.77 *,# | 90.83 ± 0.77 *,# | |
| 100 µg/mL | 0.50 ± 0.00 *,# | 0.00 ± 0.00 * | 12.67 ± 2.26 *,# | 86.83 ± 2.25 *,# | |
| Group | Concentration (µg/mL) | Number of Normal Sperm | Number of Abnormal Sperm | ||
|---|---|---|---|---|---|
| Head Only | Head and Tail | Tail Only | |||
| Control | 45.33 ± 5.51 | 2.67 ± 0.58 | 6.33 ± 1.53 | 45.67 ± 3.51 | |
| Ferrous sulfate (Fe) | 20 µg/mL | 36.00 ± 6.00 | 6.00 ± 4.00 | 17.67 ± 6.51 ## | 40.33 ± 4.51 |
| RCD | 12.5 µg/mL | 69.33 ± 0.58 *,# | 0.67 ± 0.58 *,# | 0.00 ± 0.00 *,# | 30.00 ± 0.01 *,# |
| 25 µg/mL | 51.67 ± 13.50 | 0.67 ± 0.58 *,# | 3.33 ± 0.58 *,# | 44.33 ± 12.50 | |
| 100 µg/mL | 51.67 ± 13.50 | 0.67 ± 0.58 *,# | 1.67 ± 2.08 *,# | 46.00 ± 11.00 | |
| RCU | 12.5 µg/mL | 58.33 ± 6.51 *,# | 1.33 ± 1.15 | 1.33 ± 0.58 *,# | 39.00 ± 8.00 |
| 25 µg/mL | 64.67 ± 0.58 *,# | 0.67 ± 0.58 *,# | 0.33 ± 0.58 *,# | 34.33 ± 0.58 *,# | |
| 100 µg/mL | 61.67 ± 3.51 *,# | 0.00 ± 0.00 *,# | 1.33 ± 1.53 *,# | 37.00 ± 2.00 * | |
| RCM | 12.5 µg/mL | 51.67 ± 1.53 # | 0.67 ± 0.58 *,# | 1.67 ± 1.15 *,# | 46.00 ± 1.00 |
| 25 µg/mL | 57.67 ± 19.50 | 1.67 ± 1.53 | 1.67 ± 1.53 *,# | 39.00 ± 16.52 | |
| 100 µg/mL | 52.00 ± 7.00 # | 0.00 ± 0.00 *,# | 1.00 ± 1.00 *,# | 47.00 ± 8.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laoung-on, J.; Jitjumnong, J.; Sudwan, P.; Outaitaveep, N.; Ounjaijean, S.; Boonyapranai, K. Red Cotton Stamen Extracts Mitigate Ferrous Sulfate-Induced Oxidative Stress and Enhance Quality in Bull Frozen Semen. Vet. Sci. 2025, 12, 674. https://doi.org/10.3390/vetsci12070674
Laoung-on J, Jitjumnong J, Sudwan P, Outaitaveep N, Ounjaijean S, Boonyapranai K. Red Cotton Stamen Extracts Mitigate Ferrous Sulfate-Induced Oxidative Stress and Enhance Quality in Bull Frozen Semen. Veterinary Sciences. 2025; 12(7):674. https://doi.org/10.3390/vetsci12070674
Chicago/Turabian StyleLaoung-on, Jiraporn, Jakree Jitjumnong, Paiwan Sudwan, Nopparuj Outaitaveep, Sakaewan Ounjaijean, and Kongsak Boonyapranai. 2025. "Red Cotton Stamen Extracts Mitigate Ferrous Sulfate-Induced Oxidative Stress and Enhance Quality in Bull Frozen Semen" Veterinary Sciences 12, no. 7: 674. https://doi.org/10.3390/vetsci12070674
APA StyleLaoung-on, J., Jitjumnong, J., Sudwan, P., Outaitaveep, N., Ounjaijean, S., & Boonyapranai, K. (2025). Red Cotton Stamen Extracts Mitigate Ferrous Sulfate-Induced Oxidative Stress and Enhance Quality in Bull Frozen Semen. Veterinary Sciences, 12(7), 674. https://doi.org/10.3390/vetsci12070674





