The Presence and Size of the Corpus Luteum Influence the In Vitro Production of Sheep Embryos
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. COC Recovery
2.3. Maturation, Fertilization, and In Vitro Embryo Development
2.4. Experimental Design
2.5. Statistical Analysis
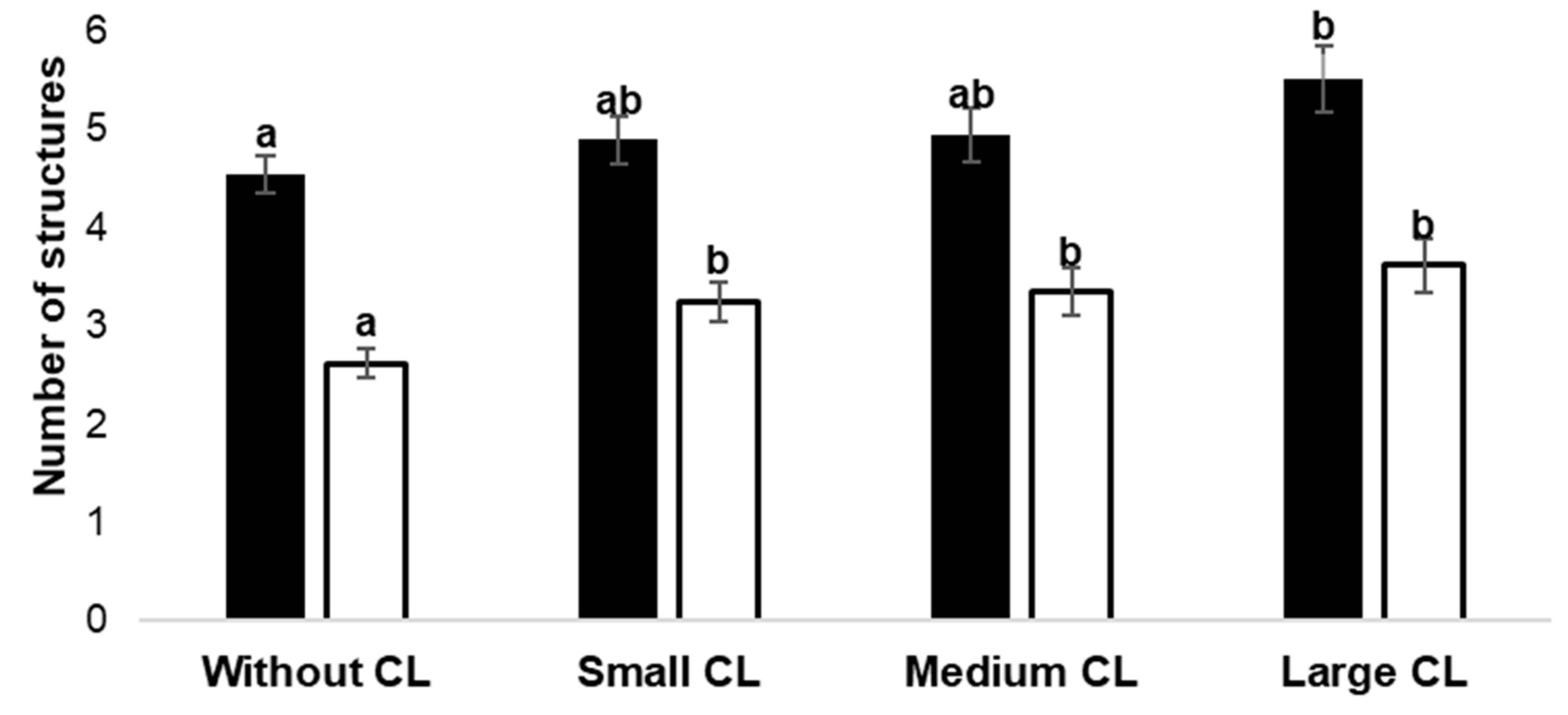
3. Results
Number of Follicles and COC Collection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krisher, R.L. The effect of oocyte quality on development. J. Anim. Sci. 2004, 82 (Suppl. 13), E14–E23. [Google Scholar] [PubMed]
- Bols, P.; Ysebaert, M.; Van Soom, A.; de Kruif, A. Effects of needle tip bevel and aspiration procedure on the morphology and developmental capacity of bovine compact cumulus oocyte complexes. Theriogenology 1997, 47, 1221–1236. [Google Scholar] [CrossRef] [PubMed]
- Penitente-Filho, J.M.; Carrascal, E.; Oliveira, F.A.; Zolini, A.M.; Oliveira, C.T.; Soares, Í.A.C.; Torres, C.A.A. Influence of dominant follicle and corpus luteum on recovery of good quality oocytes for in vitro embryo production in cattle. Br. Biotechnol. J. 2014, 4, 1305–1312. [Google Scholar] [CrossRef]
- Penitente-Filho, J.M.; Jimenez, C.R.; Zolini, A.M.; Carrascal, E.; Azevedo, J.L.; Silveira, C.O.; Oliveira, F.A.; Torres, C.A.A. Influence of corpus luteum and ovarian volume on the number and quality of bovine oocytes. Anim. Sci. J. 2015, 86, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; Sarwar, Z.; Saleem, M.; Arshad, U.; Shahzad, M.; Mushtaq, M.H.; Husnain, A.; Riaz, A.; Ahmad, N. Effect of plasma progesterone on oocyte recovery, oocyte quality, and early in-vitro developmental competence of embryos in Bos indicus dairy cows. Anim. Reprod. Sci. 2019, 202, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Sangha, G.; Sharma, R.; Guraya, S. Biology of corpus luteum in small ruminants. Small Rumin. Res. 2002, 43, 53–64. [Google Scholar] [CrossRef]
- Boediono, A.; Rajamahendran, R.; Saha, S.; Sumantri, C.; Suzuki, T. Effect of the presence of a CL in the ovary on oocyte number, cleavage rate and blastocyst production in vitro in cattle. Theriogenology 1995, 43, 169. [Google Scholar] [CrossRef]
- Bisinotto, R.S.; Chebel, R.C.; Santos, J.E. Follicular wave of the ovulatory follicle and not cyclic status influences fertility of dairy cows. J. Dairy Sci. 2010, 93, 3578–3587. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, P. Influence of progesterone on oocyte quality and embryo development in cows. Theriogenology 2011, 76, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Pursley, J.R.; Martins, J.P.N. Impact of circulating concentrations of progesterone and antral age of the ovulatory follicle on fertility of high-producing lactating dairy cows. Reprod. Fertil. Dev. 2011, 24, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Jerez, E.R.M.; García, A.A.; Caccia, M.; Rodríguez, A.C.; Gonzales, S.J.R.; Waltero, E.M.M.; Marín, D.F.D. Effect of the presence and location of corpus luteum on competence of bovine cumulus-oocyte complexes. Anim. Reprod. 2022, 19, e20210074. [Google Scholar] [CrossRef] [PubMed]
- Azari-Dolatabad, N.; Benedetti, C.; Velez, D.A.; Montoro, A.F.; Sadeghi, H.; Residiwati, G.; Leroy, J.L.; Van Soom, A.; Pascottini, O.B. Oocyte developmental capacity is influenced by intrinsic ovarian factors in a bovine model for individual embryo production. Anim. Reprod. Sci. 2023, 249, 107185. [Google Scholar] [CrossRef] [PubMed]
- Argudo, D.E.; Tenemaza, M.A.; Merchán, S.L.; Balvoa, J.A.; Méndez, M.S.; Soria, M.E.; Galarza, L.R.; Ayala, L.E.; Hernández-Fonseca, H.J.; Perea, M.S.; et al. Intraovarian influence of bovine corpus luteum on oocyte morphometry and developmental competence, embryo production and cryotolerance. Theriogenology 2020, 155, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Rosa, P.M.d.S.; Bridi, A.; Ferronato, G.d.Á.; Nociti, R.P.; dos Santos, A.C.; Cataldi, T.R.; dos Santos, G.; Chiaratti, M.R.; Silva, L.A.; Pugliesi, G.; et al. Corpus luteum proximity alters molecular signature of the small extracellular vesicles and cumulus cells in the bovine ovarian follicle environment. Mol. Cell. Endocrinol. 2024, 592, 112347. [Google Scholar] [CrossRef] [PubMed]
- Quirk, S.M.; Cowan, R.G.; Harman, R.M. Progesterone Receptor and the Cell Cycle Modulate Apoptosis in Granulosa Cells. Endocrinology 2004, 145, 5033–5043. [Google Scholar] [CrossRef] [PubMed]
- Abdelnaby, E.A.; El-Maaty, A.M.A.; Ragab, R.S.; Seida, A.A. Dynamics of uterine and ovarian arteries flow velocity waveforms and their relation to follicular and luteal growth and blood flow vascularization during the estrous cycle in Friesian cows. Theriogenology 2018, 121, 112–121. [Google Scholar] [CrossRef] [PubMed]
- de Bulnes, A.G.; Moreno, J.S.; Brunet, A.G.; Sebastian, A.L. Relationship between ultrasonographic assessment of the corpus luteum and plasma progesterone concentration during the oestrous cycle in monovular ewes. Reprod. Domest. Anim. 2000, 35, 65–68. [Google Scholar] [CrossRef]
- Contreras-Solis, I.; Diaz, T.; Lopez, G.; Caigua, A.; Lopez-Sebastian, A.; Gonzalez-Bulnes, A. Systemic and intraovarian effects of corpus luteum on follicular dynamics during estrous cycle in hair breed sheep. Anim. Reprod. Sci. 2008, 104, 47–55. [Google Scholar] [CrossRef] [PubMed]
- NOM-033-SAG/ZOO-2014; Métodos para dar Muerte a los Animales Domésticos y Silvestres. Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (Actualmente SADER): Mexico City, Mexico, 2015.
- Crocomo, L.; Ariu, F.; Bogliolo, L.; Bebbere, D.; Ledda, S.; Bicudo, S. In vitro Developmental Competence of Adult Sheep Oocytes Treated with Roscovitine. Reprod. Domest. Anim. 2016, 51, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Davachi, N.D.; Kohram, H.; Zainoaldini, S. Cumulus cell layers as a critical factor in meiotic competence and cumulus expansion of ovine oocytes. Small Rumin. Res. 2012, 102, 37–42. [Google Scholar] [CrossRef]
- Paramio, M.-T.; Izquierdo, D. Recent advances in in vitro embryo production in small ruminants. Theriogenology 2016, 86, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Romão, R.; Marques, C.; Baptista, M.; Vasques, M.; Barbas, J.; Horta, A.; Carolino, N.; Bettencourt, E.; Plancha, C.; Rodrigues, P.; et al. Evaluation of two methods of in vitro production of ovine embryos using fresh or cryopreserved semen. Small Rumin. Res. 2013, 110, 36–41. [Google Scholar] [CrossRef]
- Younglai, E.; Holt, D.; Brown, P.; Jurisicova, A.; Casper, R. Sperm swim-up techniques and DNA fragmentation. Hum. Reprod. 2001, 16, 1950–1953. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, H.; Akiyama, K.; Tajima, T. Classification of Morphological Changes Based on the Number of Cleavage Divisions in Bovine Embryos. J. Reprod. Dev. 2009, 55, 83–87. [Google Scholar] [CrossRef] [PubMed]
- SAS. SAS User’s Guide: Statistics, Version 9.1.3; SAS Inst. Inc.: Cary, NC, USA, 2012.
- Gbur, E.E.; Stroup, W.W.; McCarter, K.S.; Durham, S.; Young, L.J.; Christman, M.; West, M.; Kramer, M. Analysis of Generalized Linear Mixed Models in the Agricultural and Natural Resources Sciences; ASA, CSSA, and SSSA Series; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Aguila, L.; Treulen, F.; Therrien, J.; Felmer, R.; Valdivia, M.; Smith, L.C. Oocyte selection for in vitro embryo production in bovine species: Noninvasive approaches for new challenges of oocyte competence. Animals 2020, 10, 2196. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bulnes, A.; Berlinguer, F.; Cocero, M.; Garcia-Garcia, R.; Leoni, G.; Naitana, S.; Rosati, I.; Succu, S.; Veiga-Lopez, A. Induction of the presence of corpus luteum during superovulatory treatments enhances in vivo and in vitro blastocysts output in sheep. Theriogenology 2005, 64, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Kor, N.M. The effect of corpus luteum on hormonal composition of follicular fluid from different sized follicles and their relationship to serum concentrations in dairy cows. Asian Pac. J. Trop. Med. 2014, 7, S282–S288. [Google Scholar] [CrossRef] [PubMed]
- Rosa, P.M.d.S.; Bridi, A.; Ferronato, G.d.Á.; Prado, C.M.; Bastos, N.M.; Sangalli, J.R.; Meirelles, F.V.; Perecin, F.; da Silveira, J.C. Corpus luteum presence in the bovine ovary increase intrafollicular progesterone concentration: Consequences in follicular cells gene expression and follicular fluid small extracellular vesicles miRNA contents. J. Ovarian Res. 2024, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, I.; Garcia-Herreros, M.; O’SHea, L.; Hensey, C.; Lonergan, P.; Fair, T. Expression, regulation, and function of progesterone receptors in bovine cumulus oocyte complexes during in vitro maturation. Biol. Reprod. 2011, 84, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Borman, S.M.; Chaffin, C.L.; Schwinof, K.M.; Stouffer, R.L.; Zelinski-Wooten, M.B. Progesterone promotes oocyte maturation, but not ovulation, in nonhuman primate follicles without a gonadotropin surge. Biol. Reprod. 2004, 71, 366–373. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Group | Diameter of COCs, µm (LSM ± SE) * | COCs Area, µm2 (LSM ± SE) * |
|---|---|---|
| Without CL | 248.5 ± 8.83 a | 49,175 ± 4136.40 a |
| Small CL (≤3 mm) | 285.8 ± 10.56 b | 66,560 ± 4943.94 b |
| Medium CL (4–8 mm) | 254.0 ± 9.58 ab | 51,325 ± 4486.55 ab |
| Large CL (>8 mm) | 272.2 ± 8.83 ab | 59,259 ± 4136.40 ab |
| Group | Number of COCs | Number of Morulae | Morulae (LSM ± SE, %) * |
|---|---|---|---|
| Without CL | 339 | 264 | 78.59 ± 3.13 a |
| Small CL (≤3 mm) | 274 | 224 | 81.74 ± 2.58 ab |
| Medium CL (4–8 mm) | 197 | 168 | 85.30 ± 3.53 ab |
| Large CL (>8 mm) | 185 | 162 | 88.01 ± 3.70 b |
| Group | Number of Oocytes | Number of Blastocysts | Blastocysts (LSM ± SE, %) * | Hatching Capacity (LSM ± SE, %) * |
|---|---|---|---|---|
| Without CL | 339 | 132 | 39.48 ± 3.64 a | 22.41 ± 5.41 a |
| Small CL (≤3 mm) | 274 | 123 | 44.89 ± 3.29 a | 20.22 ± 4.23 a |
| Medium CL (4–8 mm) | 197 | 102 | 52.63 ± 5.48 b | 19.61 ± 5.52 a |
| Large CL (>8 mm) | 185 | 94 | 51.19 ± 5.63 ab | 24.39 ± 6.76 a |
| Group | Blastocysts, n | Blastocyst Diameter (LSM ± SE, µm) |
|---|---|---|
| Without CL | 69 | 211.7 ± 6.40 |
| Small CL (≤3 mm) | 59 | 212.3 ± 4.48 |
| Medium CL (4–8 mm) | 60 | 207.6 ± 6.29 |
| Large CL (>8 mm) | 51 | 202.0 ± 7.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo-Torres, A.; Rangel-Santos, R.; Bautista-Pérez, Y.V.; González-Maldonado, J. The Presence and Size of the Corpus Luteum Influence the In Vitro Production of Sheep Embryos. Vet. Sci. 2025, 12, 690. https://doi.org/10.3390/vetsci12080690
Lorenzo-Torres A, Rangel-Santos R, Bautista-Pérez YV, González-Maldonado J. The Presence and Size of the Corpus Luteum Influence the In Vitro Production of Sheep Embryos. Veterinary Sciences. 2025; 12(8):690. https://doi.org/10.3390/vetsci12080690
Chicago/Turabian StyleLorenzo-Torres, Alfredo, Raymundo Rangel-Santos, Yuri Viridiana Bautista-Pérez, and Juan González-Maldonado. 2025. "The Presence and Size of the Corpus Luteum Influence the In Vitro Production of Sheep Embryos" Veterinary Sciences 12, no. 8: 690. https://doi.org/10.3390/vetsci12080690
APA StyleLorenzo-Torres, A., Rangel-Santos, R., Bautista-Pérez, Y. V., & González-Maldonado, J. (2025). The Presence and Size of the Corpus Luteum Influence the In Vitro Production of Sheep Embryos. Veterinary Sciences, 12(8), 690. https://doi.org/10.3390/vetsci12080690




