Analysis of Potential Genes, Acute Phase Proteins and Hormonal Profiles Associated with Methicillin-Resistant Staphylococcus aureus (MRSA) Isolation from Pneumonic Sheep
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Study Design
2.2. Sampling
2.2.1. Blood Sampling
2.2.2. Nasal Swabs
2.3. Bacteriological Examination
2.3.1. Isolation and Identification of Staphylococcus aureus
2.3.2. Antimicrobial Susceptibility Testing
2.3.3. Antimicrobial Susceptibility Testing (AST) for MRSA Strains
2.4. Molecular Confirmation of MRSA Strains and Detection of Virulence and Resistance Genes
2.5. Genetic Polymorphisms Between Healthy and Pneumonic Ewes
2.5.1. Total RNA Extraction, Reverse Transcription and Quantitative Real Time PCR
2.5.2. DNA Sequencing and Polymorphism Detection
2.6. Biochemical Analysis
2.7. Statistical Analysis
3. Results
3.1. Clinical Findings
3.2. Staphylococcus Isolates
3.3. Molecular Identification
3.4. Antimicrobial Susceptibility of Staphylococcus aureus Isolated from Nasal Swabs of Sheep
3.5. Patterns for Transcript Levels of Immune Indicators
3.6. Genetic Polymorphisms of Immune Genes
3.7. Biochemical Profile
3.8. Correlation Between Gene Expression Pattern of Immunological Markers and Serum Profile of Acute Phase Proteins in Healthy and Pneumonic Ewes
4. Discussion
4.1. Clinical Examination
4.2. Prevalence of Staphylococcus aureus in Pneumonic Sheep
4.3. Emergence of MRSA in Sheep
4.4. Antimicrobial Resistance of S. aureus and MRSA
4.5. Nucleotide Sequence Variants of Investigated Immune Genes Linked to Pneumonia
4.6. Biochemical Markers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Deeb, W.M.; Elmoslemany, A.M. The diagnostic accuracy of acute phase proteins and proinflammatory cytokines in sheep with pneumonic pasteurellosis. PeerJ 2016, 4, e2161. [Google Scholar] [CrossRef]
- Arbaga, A.; Hassan, H.; Anis, A.; Othman, N.; Kamr, A. Clinicopathological and Electrophoretic Pattern of Serum Protein Alterations in Acute Pneumonic Sheep. Pak. Vet. J. 2023, 43, 303–308. [Google Scholar]
- Fernández, S.; Galapero, J.; Rey, J.; Pérez, C.; Ramos, A. Cytokines study in lungs infected with Pasteurellaceae and Mycoplasma spp. from fattening lambs. J. Med. Microbiol. Immunol. Res. 2018, 2, 1–7. [Google Scholar]
- Ramadan, M.A.; Abdel-Raoof, Y.M.; Zeineldin, M.M.; El Attar, H.E.M.; Abdelghany, A.H.; Ghanem, M.M. Assessment of pulmonary function test, acute phase proteins, cytokines and electrocardiographic changes in naturally occurring bovine respiratory disease of feedlot cattle calves. Benha Vet. Med. J. 2019, 37, 1–5. [Google Scholar] [CrossRef]
- Rizk, M.A.; El-Sayed, S.A.E.-S.; Salman, D.; Marghani, B.H.; Gadalla, H.E.; Sayed-Ahmed, M.Z. Immunomodulatory effect of vitamin C on proinflammatory cytokines production in Ossimi Lambs (Ovis aries) with Pneumonic Pasteurellosis. Animals 2021, 11, 3374. [Google Scholar] [CrossRef]
- Aly, M.M.; El-Sanousi, A.A.; Mahmoud, A.A. Environmental factors influencing respiratory infections in small ruminants in arid zones. Vet. World 2024, 17, 567–574. [Google Scholar]
- El-Sayed, A.; Ahmed, M.; Hassan, M.A. Etiological agents and risk factors of ovine respiratory diseases in Egypt: A comprehensive review. J. Vet. Med. Res. 2023, 30, 201–213. [Google Scholar]
- Cucarella, C.; Tormo, M.A.; Ubeda, C.; Trotonda, M.P.; Monzón, M.; Peris, C.; Amorena, B.; Lasa, Í.; Penadés, J.R. Role of biofilm-associated protein bap in the pathogenesis of bovine Staphylococcus aureus. Infect. Immun. 2004, 72, 2177–2185. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef]
- Zaher, H.A.; El Baz, S.; Alothaim, A.S.; Alsalamah, S.A.; Alghonaim, M.I.; Alawam, A.S.; Eraqi, M.M. Molecular basis of methicillin and vancomycin resistance in Staphylococcus aureus from cattle, sheep carcasses and slaughterhouse workers. Antibiotics 2023, 12, 205. [Google Scholar] [CrossRef]
- Gao, J.; Stewart, G.C. Regulatory elements of the Staphylococcus aureus protein A (Spa) promoter. J. Bacteriol. 2004, 186, 3738–3748. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Wichelhaus, T.; Schulze, J.; Hunfeld, K.; Schäfer, V.; Brade, V. Clonal heterogeneity, distribution, and pathogenicity of methicillin-resistant Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 1997, 16, 893–897. [Google Scholar] [CrossRef]
- Acar, J.; Rostel, B. Antimicrobial resistance: An overview. Rev. Sci. Tech.-Off. Int. Des Epizoot. 2001, 20, 797–810. [Google Scholar] [CrossRef]
- Moran, G.J.; Krishnadasan, A.; Gorwitz, R.J.; Fosheim, G.E.; McDougal, L.K.; Carey, R.B.; Talan, D.A. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 2006, 355, 666–674. [Google Scholar] [CrossRef]
- Conturba, B.; Lo Feudo, C.M.; Stucchi, L.; Stancari, G.; Olivieri, L.; Ferrucci, F. Recurrent equine capillary haemangioma treated with adjunctive laser photocoagulation therapy: A case report. Vet. Dermatol. 2021, 32, 290. [Google Scholar] [CrossRef]
- Lee, A.S.; De Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1334. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Wang, Y.; Yang, Y.; Liu, Y. Genomic adaptations contributing to linezolid and daptomycin resistance in clinical MRSA isolates. Front. Microbiol. 2022, 13, 853270. [Google Scholar]
- Shore, A.C.; Deasy, E.C.; Slickers, P.; Brennan, G.; O’Connell, B.; Monecke, S.; Ehricht, R.; Coleman, D.C. Detection of staphylococcal cassette chromosome mec type XI carrying highly divergent mecA, mecI, mecR1, blaZ, and ccr genes in human clinical isolates of clonal complex 130 methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 3765–3773. [Google Scholar] [CrossRef]
- Alghamdi, B.A.; Al-Johani, I.; Al-Shamrani, J.M.; Alshamrani, H.M.; Al-Otaibi, B.G.; Almazmomi, K.; Yusof, N.Y. Antimicrobial resistance in methicillin-resistant Staphylococcus aureus. Saudi J. Biol. Sci. 2023, 30, 103604. [Google Scholar] [CrossRef]
- David, M.Z.; Daum, R.S. Community-associated methicillin-resistant Staphylococcus aureus: Epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 2010, 23, 616–687. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Talaat, M.; Zayed, B.; Tolba, S.; Abdou, E.; Gomaa, M.; Itani, D.; Hutin, Y.; Hajjeh, R. Increasing antimicrobial resistance in world health organization eastern mediterranean region, 2017–2019. Emerg. Infect. Dis. 2022, 28, 717. [Google Scholar] [CrossRef]
- Jauro, S.; Hamman, M.M.; Malgwi, K.D.; Musa, J.A.; Ngoshe, Y.B.; Gulani, I.A.; Kwoji, I.D.; Iliya, I.; Abubakar, M.B.; Fasina, F.O. Antimicrobial resistance pattern of methicillin-resistant Staphylococcus aureus isolated from sheep and humans in Veterinary Hospital Maiduguri, Nigeria. Vet. World 2022, 15, 1141. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-resistant Staphylococcus aureus: Molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef]
- Saleha, A.; Zunita, Z. Methicillin resistant Staphylococcus aureus (MRSA): An emerging veterinary and zoonotic pathogen of public health concern and some studies in Malaysia. J. Anim. Vet. Adv. 2010, 9, 1094–1098. [Google Scholar] [CrossRef]
- Kralik, P.; Ricchi, M. A basic guide to real time PCR in microbial diagnostics: Definitions, parameters, and everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Yamamoto, Y. PCR in diagnosis of infection: Detection of bacteria in cerebrospinal fluids. Clin. Vaccine Immunol. 2002, 9, 508–514. [Google Scholar] [CrossRef]
- Kerremans, J.; Verboom, P.; Stijnen, T.; Hakkaart-van Roijen, L.; Goessens, W.; Verbrugh, H.; Vos, M. Rapid identification and antimicrobial susceptibility testing reduce antibiotic use and accelerate pathogen-directed antibiotic use. J. Antimicrob. Chemother. 2008, 61, 428–435. [Google Scholar] [CrossRef]
- Mostafa Abdalhamed, A.; Zeedan, G.S.G.; Ahmed Arafa, A.; Shafeek Ibrahim, E.; Sedky, D.; Abdel nabey Hafez, A. Detection of Methicillin-Resistant Staphylococcus aureus in Clinical and Subclinical Mastitis in Ruminants and Studying the Effect of Novel Green Synthetized Nanoparticles as One of the Alternative Treatments. Vet. Med. Int. 2022, 2022, 6309984. [Google Scholar] [CrossRef]
- Ceciliani, F.; Ceron, J.; Eckersall, P.; Sauerwein, H. Acute phase proteins in ruminants. J. Proteom. 2012, 75, 4207–4231. [Google Scholar] [CrossRef]
- Miglio, A.; Moscati, L.; Scoccia, E.; Maresca, C.; Antognoni, M.T.; Felici, A. Reference values for serum amyloid A, haptoglobin, lysozyme, zinc and iron in healthy lactating Lacaune sheep. Acta Vet. Scand. 2018, 60, 46. [Google Scholar] [CrossRef]
- Canelli, E.; Catella, A.; Borghetti, P.; Ferrari, L.; Ogno, G.; De Angelis, E.; Corradi, A.; Passeri, B.; Bertani, V.; Sandri, G. Phenotypic characterization of a highly pathogenic Italian porcine reproductive and respiratory syndrome virus (PRRSV) type 1 subtype 1 isolate in experimentally infected pigs. Vet. Microbiol. 2017, 210, 124–133. [Google Scholar] [CrossRef]
- Pierce, C.F.; Brown, V.R.; Olsen, S.C.; Boggiatto, P.; Pedersen, K.; Miller, R.S.; Speidel, S.E.; Smyser, T.J. Loci associated with antibody response in feral swine (Sus scrofa) infected with Brucella suis. Front. Vet. Sci. 2020, 7, 554674. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Lu, T.; Niu, L.; Wang, L.; Zhan, S.; Guo, J.; Cao, J.; Li, L.; Zhang, H. Nucleotide variants in TheTLR5 gene and promoter methylation with A susceptibility to brucellosis in Chinese goats. Folia Biol. (Kraków) 2022, 70, 55–66. [Google Scholar] [CrossRef]
- Sallam, A.M.; Abou-Souliman, I.; Reyer, H.; Wimmers, K.; Rabee, A.E. New insights into the genetic predisposition of brucellosis and its effect on the gut and vaginal microbiota in goats. Sci. Rep. 2023, 13, 20086. [Google Scholar] [CrossRef]
- Adams, L.; Schutta, C.J. Natural resistance against brucellosis: A review. Open Vet. Sci. J. 2010, 4, 61–71. [Google Scholar] [CrossRef]
- El-Shafaey, E.-S.; Ateya, A.; Ramadan, H.; Saleh, R.; Elseady, Y.; Abo El Fadl, E.; El-Khodery, S. Single nucleotide polymorphisms in IL8 and TLR4 genes as candidates for digital dermatitis resistance/susceptibility in Holstein cattle. Anim. Biotechnol. 2017, 28, 131–137. [Google Scholar] [CrossRef]
- Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Grünberg, W. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Uchida-Fujii, E.; Niwa, H.; Kinoshita, Y.; Nukada, T. Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) for identification of bacterial isolates from horses. J. Equine Vet. Sci. 2020, 95, 103276. [Google Scholar] [CrossRef]
- Rocha, L.A.; Aleixo, A.; Allen, G.; Almeda, F.; Baldwin, C.C.; Barclay, M.V.; Bates, J.M.; Bauer, A.; Benzoni, F.; Berns, C. Specimen collection: An essential tool. Science 2014, 344, 814–815. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Weinstein, M.P.; Lewis, J.S. The clinical and laboratory standards institute subcommittee on antimicrobial susceptibility testing: Background, organization, functions, and processes. J. Clin. Microbiol. 2020, 58, e01864-19. [Google Scholar] [CrossRef] [PubMed]
- McClure, J.-A.; Conly, J.M.; Lau, V.; Elsayed, S.; Louie, T.; Hutchins, W.; Zhang, K. Novel multiplex PCR assay for detection of the staphylococcal virulence marker Panton-Valentine leukocidin genes and simultaneous discrimination of methicillin-susceptible from-resistant staphylococci. J. Clin. Microbiol. 2006, 44, 1141–1144. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Boom, R.; Sol, C.; Salimans, M.; Jansen, C.; Wertheim-van Dillen, P.; Van der Noordaa, J. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990, 28, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Boesenberg-Smith, K.A.; Pessarakli, M.M.; Wolk, D.M. Assessment of DNA yield and purity: An overlooked detail of PCR troubleshooting. Clin. Microbiol. Newsl. 2012, 34, 1, 3–6. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Abdalla, O.; Haidy, G.; Yousseff, F.; Rehab, A. Clinicopathological alterations in blood and sera of sheep due to respiratory affections. J. Clin. Pathol. Forecast 2019, 2, 1006. [Google Scholar]
- Mandal, R.; Gupta, V.; Joshi, V.; Kumar, S.; Mondal, D. Study of Clinico Hematobiochemical Changes and Therapeutic Management of Naturally Infected Cases of Respiratory Disease in Non-Descript Goats of Bareilly Region. Int. J. Livest. Res. 2017, 7, 211–218. [Google Scholar] [CrossRef]
- Teng, T.; Yang, H.; Xu, T.; Sun, G.; Song, X.; Bai, G.; Shi, B. Activation of inflammatory networks in the lungs caused by chronic cold stress is moderately attenuated by glucose supplementation. Int. J. Mol. Sci. 2022, 23, 10697. [Google Scholar] [CrossRef]
- Bozukluhan, K.; Merhan, O.; Kiziltepe, S.; Egritag, H.E.; Akyuz, E.; Gokce, H.I. Determination of haptoglobin, some biochemical and oxidative stress parameters in calves with pneumonia. Fresenius Env. Bull 2021, 30, 9492–9496. [Google Scholar]
- Su, M.; She, Y.; Deng, M.; Guo, Y.; Li, Y.; Liu, G.; Sun, B.; Liu, D. Effect of capsaicin addition on antioxidant capacity, immune performance and upper respiratory microbiota in nursing calves. Microorganisms 2023, 11, 1903. [Google Scholar] [CrossRef]
- Saher, A.S.; Raza, A.; Qiu, F.; Mehmood, K.; Hussain, R.; Qayyum, A.; Idris, M.; Almutairi, M.H.; Li, K. Detection of haptoglobin and serum amyloid A as biomarkers in naturally infected Mycoplasma bovis calves. Acta Trop. 2024, 254, 107215. [Google Scholar] [CrossRef]
- AL-Dujaily, A.H.; Abeed, S.A.; Sahib, A.M. Hematological, Biochemical, Pathological, Serology and Molecular Detection of Mycoplasma Ovipneumoniae from Awassi Sheep in Al-Najaf Province, Iraq. Ann. For. Res 2023, 66, 1410–1422. [Google Scholar]
- Madhi, K.S.; Jasim, A.S.; Nasear, H.A.; Ibraheim, H.K.; Gharban, H.A. Phylogenetic analysis of Klebsiella pneumoniae isolates of respiratory tract infections in humans and sheep. Open Vet. J. 2024, 14, 2325. [Google Scholar] [CrossRef]
- Radostits, O.M.; Gay, C.C.; Hinchcliff, K.W.; Constable, P.D. Veterinary Medicine E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Risalde, M.; Molina, V.; Sánchez-Cordón, P.; Pedrera, M.; Panadero, R.; Romero-Palomo, F.; Gómez-Villamandos, J. Response of proinflammatory and anti-inflammatory cytokines in calves with subclinical bovine viral diarrhea challenged with bovine herpesvirus-1. Vet. Immunol. Immunopathol. 2011, 144, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Oraby, N.; Mohamed, A.; Mahmoud, H. The possibility of using zinc oxide nanoparticles in controlling some fungal and bacterial strains isolated from buffaloes. Egypt. J. Appl. Sci. 2014, 29, 58–83. [Google Scholar]
- Mohamed, D.; El-Gheit, A.A.; Eid, S.; Khadra, S.A.; Elsadik, G.; Mohamed, A.; Hamed, T. Prevalence of Staphylococcus aureus and Pasteurella multocida incriminated in calf pneumonia and their treatment. Egypt. J. Anim. Health 2023, 3, 21–39. [Google Scholar]
- Hassan, A.; Howayda, M.; Hanan, K.M. Antimicrobial Potential of Ozone on Fungal and Bacterial Contamination of Animal Feed That Caused Diseases in Some Buffalo Farms. In Proceedings of the 1st International Conference, Animal Health Research Institute, ARC, Giza, Egypt, 9–13 November 2017; pp. 9–13. [Google Scholar]
- Chu, C.; Yu, C.; Lee, Y.; Su, Y. Genetically divergent methicillin-resistant Staphylococcus aureus and sec-dependent mastitis of dairy goats in Taiwan. BMC Vet. Res. 2012, 8, 39. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Anderson, T.P.; Beynon, K.A.; Chua, A.; Fleming, A.M.; Laing, R.T.; Town, G.I.; Mills, G.D.; Chambers, S.T.; Jennings, L.C. Evaluation of a PCR assay for detection of Streptococcus pneumoniae in respiratory and nonrespiratory samples from adults with community-acquired pneumonia. J. Clin. Microbiol. 2003, 41, 63–66. [Google Scholar] [CrossRef]
- Sallam, M.G.A.E.; Khalifa, N.; Barakat, A.; Salem, L. Occurrence of Staphylococcus aureus in dairy farms and humans with reference to antimicrobial profile in Qalyobia Governorate. Benha Vet. Med. J. 2023, 45, 40–45. [Google Scholar] [CrossRef]
- Franco, M.F.; Gaeta, N.C.; Alemán, M.A.; Mellville, P.A.; Timenetsky, J.; Balaro, M.F.; Gregory, L. Bacteria isolated from the lower respiratory tract of sheep and their relationship to clinical signs of sheep respiratory disease. Pesqui. Vet. Bras. 2019, 39, 796–801. [Google Scholar] [CrossRef]
- Ali, A.; Abu-Zaid, K. Study on Klebsiella pneumoniae causing respiratory infection in small ruminants. Anim. Health Res. J 2019, 7, 57–67. [Google Scholar]
- El-Mashad, A.-B.I.; Moustafa, S.A.; Amin, A.; Samy, E.M. Pathological Studies on lung affections in sheep and goat at Kalubia Governorate. Benha Vet. Med. J. 2020, 38, 17–23. [Google Scholar] [CrossRef]
- IB, M.-S.; Okon, K.; Adamu, N.; Askira, U.; Isyaka, T.; Adamu, S.; Mohammed, A. Methicillin-resistant Staphylococcus aureus (MRSA) colonization rate among ruminant animals slaughtered for human consumption and contact persons in Maiduguri, Nigeria. Afr. J. Microbiol. Res. 2014, 8, 2643–2649. [Google Scholar]
- Graveland, H.; Duim, B.; Van Duijkeren, E.; Heederik, D.; Wagenaar, J.A. Livestock-associated methicillin-resistant Staphylococcus aureus in animals and humans. Int. J. Med. Microbiol. 2011, 301, 630–634. [Google Scholar] [CrossRef]
- Wirusanti, N.I.; Baldridge, M.T.; Harris, V.C. Microbiota regulation of viral infections through interferon signaling. Trends Microbiol. 2022, 30, 778–792. [Google Scholar] [CrossRef]
- Kluytmans, J. Methicillin-resistant Staphylococcus aureus in food products: Cause for concern or case for complacency? Clin. Microbiol. Infect. 2010, 16, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Calfee, D.P.; Salgado, C.D.; Milstone, A.M.; Harris, A.D.; Kuhar, D.T.; Moody, J.; Aureden, K.; Huang, S.S.; Maragakis, L.L.; Yokoe, D.S. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update. Infect. Control Hosp. Epidemiol. 2014, 35, S108–S132. [Google Scholar] [CrossRef] [PubMed]
- Al-Talib, H.; Yean, C.Y.; Al-Khateeb, A.; Hasan, H.; Ravichandran, M. Rapid detection of methicillin-resistant Staphylococcus aureus by a newly developed dry reagent-based polymerase chain reaction assay. J. Microbiol. Immunol. Infect. 2014, 47, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, S.; Bamidele, M.; Omonigbehin, E.; Akinsinde, K.; Smith, S.; Onile, B.; Olowe, A. Molecular epidemiology of methicillan-resistant Staphylococcus aureus in Ilorin, Nigeria. West Afr. J. Med. 2005, 24, 100–106. [Google Scholar] [CrossRef]
- Ikeh, E. Methicilin-resistant Staphylococcus aureus (MRSA) at Jos University Teaching Hospital. Afr. J. Clin. Exp. Microbiol. 2003, 4, 52–55. [Google Scholar] [CrossRef][Green Version]
- Tesfaye, K.; Gizaw, Z.; Haile, A.F. Prevalence of mastitis and phenotypic characterization of methicillin-resistant Staphylococcus aureus in lactating dairy cows of selected dairy farms in and around adama town, Central Ethiopia. Environ. Health Insights 2021, 15, 11786302211021297. [Google Scholar] [CrossRef]
- Rahimi, H.; Saei, H.D.; Ahmadi, M. Nasal carriage of Staphylococcus aureus: Frequency and antibiotic resistance in healthy ruminants. Jundishapur J. Microbiol. 2015, 8, e22413. [Google Scholar] [CrossRef]
- Ramadan, H.A.; El-Baz, A.M.; Goda, R.M.; El-Sokkary, M.M.; El-Morsi, R.M. Molecular characterization of enterotoxin genes in methicillin-resistant S. aureus isolated from food poisoning outbreaks in Egypt. J. Health Popul. Nutr. 2023, 42, 86. [Google Scholar] [CrossRef]
- Denton, M.; O’Connell, B.; Bernard, P.; Jarlier, V.; Williams, Z.; Henriksen, A.S. The EPISA study: Antimicrobial susceptibility of Staphylococcus aureus causing primary or secondary skin and soft tissue infections in the community in France, the UK and Ireland. J. Antimicrob. Chemother. 2008, 61, 586–588. [Google Scholar] [CrossRef]
- Aa, A.; Ibrahim, E.; Fouad, E.; Gaber, E. Antibiotic resistance of staphylococci concerning strains included in food industry in Egypt. Int. J. Pharm. Clin. Res. 2016, 8, 1583–1589. [Google Scholar]
- Naeim, D.E.; Eldesoukey, I.E.; Moawad, A.A.; Ahmed, A.M. Molecular detection of methicillin-resistant Staphylococcus aureus isolated from different foodstuffs in Egypt. Vet. Res. Forum 2023, 14, 243–248. [Google Scholar]
- Mehta, A.; Rodrigues, C.; Sheth, K.; Jani, S.; Hakiniyan, A.; Fazalbhoy, N. Control of methicillin resistant Staphylococcus aureus in a tertiary care Centre-A five year study. Indian J. Med. Microbiol. 1998, 16, 31–34. [Google Scholar]
- Pyatov, V.; Vrtková, I.; Knoll, A. Detection of selected antibiotic resistance genes using multiplex PCR assay in mastitis pathogens in the Czech Republic. Acta Vet. Brno 2017, 86, 167–174. [Google Scholar] [CrossRef]
- Kaliyeva, S.S.; Lavrinenko, A.V.; Tishkambayev, Y.; Zhussupova, G.; Issabekova, A.; Begesheva, D.; Simokhina, N. Microbial landscape and antibiotic susceptibility dynamics of skin and soft tissue infections in Kazakhstan 2018–2020. Antibiotics 2022, 11, 659. [Google Scholar] [CrossRef] [PubMed]
- Onwubiko, N.E.; Sadiq, N.M. Antibiotic sensitivity pattern of Staphylococcus aureus from clinical isolates in a tertiary health institution in Kano, Northwestern Nigeria. Pan Afr. Med. J. 2011, 8, 4. [Google Scholar] [CrossRef]
- Ndip, R.; Ebah, L.; Onile, B. Antibiogram of Staphylococcus aureus from clinical Syndromes in Ilorin, Nigeria. J. Med. Lab. Sci. 1997, 6, 24–26. [Google Scholar]
- Ikeagwu, I.; Amadi, E.; Iroha, I. Antibiotic sensitivity pattern of Staphylococcus aureus in Abakaliki. Pak. J. Med. Sci. 2008, 24, 231–235. [Google Scholar]
- Ashish, K.; Gurung, R.; Kinney, M.V.; Sunny, A.K.; Moinuddin, M.; Basnet, O.; Paudel, P.; Bhattarai, P.; Subedi, K.; Shrestha, M.P. Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: A prospective observational study. Lancet Glob. Health 2020, 8, e1273–e1281. [Google Scholar]
- Schulte, R.H.; Munson, E. Staphylococcus aureus resistance patterns in Wisconsin 2018 surveillance of Wisconsin organisms for trends in antimicrobial resistance and epidemiology (SWOTARE) Program Report. Clin. Med. Res. 2019, 17, 72–81. [Google Scholar] [CrossRef]
- Uwaezuoke, J.; Aririatu, L. A survey of antibiotic resistant Staphylococcus aureus strains from clinical sources in Owerri. J. Appl. Sci. Environ. Mgt. 2004, 8, 67–69. [Google Scholar] [CrossRef]
- Obiazi, H.; Nmorsi, O.; Ekundayo, A.; Ukwandu, N. Prevalence and antibiotic susceptibility pattern of Staphylococcus aureus from clinical isolates grown at 37 and 44oC from Irrua, Nigeria. Afr. J. Microbiol. Res. 2007, 1, 57–60. [Google Scholar]
- Naik, D.; Teclu, A. A study on antimicrobial susceptibility pattern in clinical isolates of Staphylococcus aureus in Eritrea. Pan Afr. Med. J. 2009, 3, 1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ateya, A.; Al-Sharif, M.; Abdo, M.; Fericean, L.; Essa, B. Individual genomic loci and mRNA levels of immune biomarkers associated with pneumonia susceptibility in baladi goats. Vet. Sci. 2023, 10, 185. [Google Scholar] [CrossRef]
- Abendaño, N.; Esparza-Baquer, A.; Bernales, I.; Reina, R.; de Andrés, D.; Jugo, B.M. Gene expression profiling reveals new pathways and genes associated with Visna/Maedi viral disease. Animals 2021, 11, 1785. [Google Scholar] [CrossRef]
- Sayed, A.E.; Hafez, A.; Ateya, A.; Darwish, A.; Tahoun, A. Single nucleotide polymorphisms, gene expression and evaluation of immunological, antioxidant, and pathological parameters associated with bacterial pneumonia in Barki sheep. Ir. Vet. J. 2025, 78, 11. [Google Scholar] [CrossRef]
- Wang, K.; Liu, X.; Li, Q.; Wan, K.; Gao, R.; Han, G.; Li, C.; Xu, M.; Jia, B.; Shen, X. MHC-DRB1 exon 2 polymorphism and its association with mycoplasma ovipneumonia resistance or susceptibility genotypes in sheep. J. Genet. 2020, 99, 22. [Google Scholar] [CrossRef]
- Leymaster, K.; Chitko-McKown, C.; Clawson, M.; Harhay, G.; Heaton, M. Effects of TMEM154 haplotypes 1 and 3 on susceptibility to ovine progressive pneumonia virus following natural exposure in sheep. J. Anim. Sci. 2013, 91, 5114–5121. [Google Scholar] [CrossRef] [PubMed]
- Barton, N.H. Mutation and the evolution of recombination. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1281–1294. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kala, D.; Dhiman, G.; Yadav, V.; Krokhotin, A.; Dokholyan, N.V. Predicting the functional consequences of non-synonymous single nucleotide polymorphisms in IL8 gene. Sci. Rep. 2017, 7, 6525. [Google Scholar] [CrossRef]
- Botos, I.; Segal, D.M.; Davies, D.R. The structural biology of Toll-like receptors. Structure 2011, 19, 447–459. [Google Scholar] [CrossRef]
- Fujita, M.; Into, T.; Yasuda, M.; Okusawa, T.; Hamahira, S.; Kuroki, Y.; Eto, A.; Nisizawa, T.; Morita, M.; Shibata, K.-I. Involvement of leucine residues at positions 107, 112, and 115 in a leucine-rich repeat motif of human Toll-like receptor 2 in the recognition of diacylated lipoproteins and lipopeptides and Staphylococcus aureus peptidoglycans. J. Immunol. 2003, 171, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Mura, M.; Andrade, C.F.; Okutani, D.; Lodyga, M.; dos Santos, C.C.; Keshavjee, S.; Matthay, M.; Liu, M. TNFα-induced long pentraxin PTX3 expression in human lung epithelial cells via JNK. J. Immunol. 2005, 175, 8303–8311. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, G.; Campo, S. Current understanding of PTX3 protective activity on Aspergillus fumigatus infection. Med. Mycol. 2012, 50, 225–233. [Google Scholar] [CrossRef][Green Version]
- Cremonesi, P.; Capoferri, R.; Pisoni, G.; Del Corvo, M.; Strozzi, F.; Rupp, R.; Caillat, H.; Modesto, P.; Moroni, P.; Williams, J.L. Response of the goat mammary gland to infection with Staphylococcus aureus revealed by gene expression profiling in milk somatic and white blood cells. BMC Genom. 2012, 13, 540. [Google Scholar] [CrossRef]
- Filipe, J.; Bronzo, V.; Curone, G.; Pollera, C.; Dall’Ara, P.; Luzzago, C.; Turin, L.; Vigo, D.; Casula, A.; Roccabianca, P. PTX3 is up-regulated in epithelial mammary cells during S. aureus intramammary infection in goat. Health Anim. Sci. Food Saf. 2015, 2, 8–16. [Google Scholar] [CrossRef]
- Essa, B.; Al-Sharif, M.; Abdo, M.; Fericean, L.; Ateya, A. New insights on nucleotide sequence variants and mRNA levels of candidate genes assessing resistance/susceptibility to mastitis in Holstein and montbéliarde dairy cows. Vet. Sci. 2023, 10, 35. [Google Scholar] [CrossRef]
- Arce, I.; Martínez-Muñoz, L.; Roda-Navarro, P.; Fernández-Ruiz, E. The human C-type lectin CLECSF8 is a novel monocyte/macrophage endocytic receptor. Eur. J. Immunol. 2004, 34, 210–220. [Google Scholar] [CrossRef]
- Brenaut, P.; Lefèvre, L.; Rau, A.; Laloë, D.; Pisoni, G.; Moroni, P.; Bevilacqua, C.; Martin, P. Contribution of mammary epithelial cells to the immune response during early stages of a bacterial infection to Staphylococcus aureus. Vet. Res. 2014, 45, 16. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, A.A.; Li, J.; Wu, Z.; Ni, P.; Adetula, A.A.; Wang, H.; Zhang, C.; Tang, X.; Bhuyan, A.A.; Zhao, S. Exploring the genetic resistance to gastrointestinal nematodes infection in goat using RNA-sequencing. Int. J. Mol. Sci. 2017, 18, 751. [Google Scholar] [CrossRef]
- Hedges, J.C.; Singer, C.A.; Gerthoffer, W.T. Mitogen-activated protein kinases regulate cytokine gene expression in human airway myocytes. Am. J. Respir. Cell Mol. Biol. 2000, 23, 86–94. [Google Scholar] [CrossRef]
- Russo, R.C.; Garcia, C.C.; Teixeira, M.M.; Amaral, F.A. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev. Clin. Immunol. 2014, 10, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Pozzoli, U.; Cagliani, R.; Comi, G.P.; Riva, S.; Clerici, M.; Bresolin, N.; Sironi, M. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J. Exp. Med. 2009, 206, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Ilie, D.E.; Kusza, S.; Sauer, M.; Gavojdian, D. Genetic characterization of indigenous goat breeds in Romania and Hungary with a special focus on genetic resistance to mastitis and gastrointestinal parasitism based on 40 SNPs. PLoS ONE 2018, 13, e0197051. [Google Scholar] [CrossRef] [PubMed]
- Krebs, D.L.; Hilton, D.J. SOCS proteins: Negative regulators of cytokine signaling. Stem Cells 2001, 19, 378–387. [Google Scholar] [CrossRef]
- Kukurba, K.R.; Montgomery, S.B. RNA sequencing and analysis. Cold Spring Harb. Protoc. 2015, 2015, pdb-top084970. [Google Scholar] [CrossRef]
- Burel, J.G.; Babor, M.; Pomaznoy, M.; Lindestam Arlehamn, C.S.; Khan, N.; Sette, A.; Peters, B. Host rranscriptomics as a tool to identify diagnostic and mechanistic immune signatures of tuberculosis. Front. Immunol 2019, 19, 221. [Google Scholar]
- Aslan, O.; Goksu, A.G.; Apaydin, Ν. The evaluation of oxidative stress in lambs with Pestivirus infection. J. Hell. Vet. Med. Soc. 2017, 68, 299–306. [Google Scholar] [CrossRef]
- Blattman, A.; Kinghorn, B.; Gray, G.; Hulme, D.; JBeh, K.; Woolaston, R. A search for associations between major histocompatibility complex restriction fragment length polymorphism bands and resistance to Haemonchus contortus infection in sheep. Anim. Genet. 1993, 24, 277–282. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; McManus, D.P. Concepts in immunology and diagnosis of hydatid disease. Clin. Microbiol. Rev. 2003, 16, 18–36. [Google Scholar] [CrossRef]
- El-Deeb, W.; Fayez, M.; Elsohaby, I.; Salem, M.; Alhaider, A.; Kandeel, M. Investigation of acute-phase proteins and cytokines response in goats with contagious caprine pleuropneumonia with special reference to their diagnostic accuracy. PeerJ 2020, 8, e10394. [Google Scholar] [CrossRef]
- Kaplan, Y.; Tekerli, M. Environmental Factors Affecting Economically Important Traits of Anatolian Buffalo in Yozgat. Kocatepe Vet. J. 2024, 17, 95–103. [Google Scholar] [CrossRef]
- Cruz-Topete, D.; Myers, P.; Foley, J.; Willis, M.; Cidlowski, J. Stress Hormones are Critical in Maintaining Cardiac Gene Expression and Function in Mice. FASEB J. 2015, 29, 1037.1. [Google Scholar] [CrossRef]
- Pérez-Aguilar, M.C.; Rondón-Mercado, R. Neuroimmunoendocrine system in health and disease. EC Microbiol 2018, 14, 2–6. [Google Scholar]
- Mehdi, S.F.; Qureshi, M.H.; Pervaiz, S.; Kumari, K.; Saji, E.; Shah, M.; Abdullah, A.; Zahoor, K.; Qadeer, H.A.; Katari, D.K. Endocrine and metabolic alterations in response to systemic inflammation and sepsis: A review article. Mol. Med. 2025, 31, 16. [Google Scholar] [CrossRef]
- Allam, T.S.; Saleh, N.S.; Abo-Elnaga, T.R.; Darwish, A.A. Cytokine Response and Immunological Studies in Camels (Camelus Dromedarius) with Respiratory Diseases at Matrouh Province. Alex. J. Vet. Sci. 2017, 53, 116–124. [Google Scholar] [CrossRef]
- Iliev, P.; Georgieva, T. Acute phase proteins in sheep and goats-function, reference ranges and assessment methods: An overview. Bulg. J. Vet. Med. 2018, 21, 1–16. [Google Scholar] [CrossRef]
- Reczyńska, D.; Zalewska, M.; Czopowicz, M.; Kaba, J.; Zwierzchowski, L.; Bagnicka, E. Acute phase protein levels as an auxiliary tool in diagnosing viral diseases in ruminants—A review. Viruses 2018, 10, 502. [Google Scholar] [CrossRef]
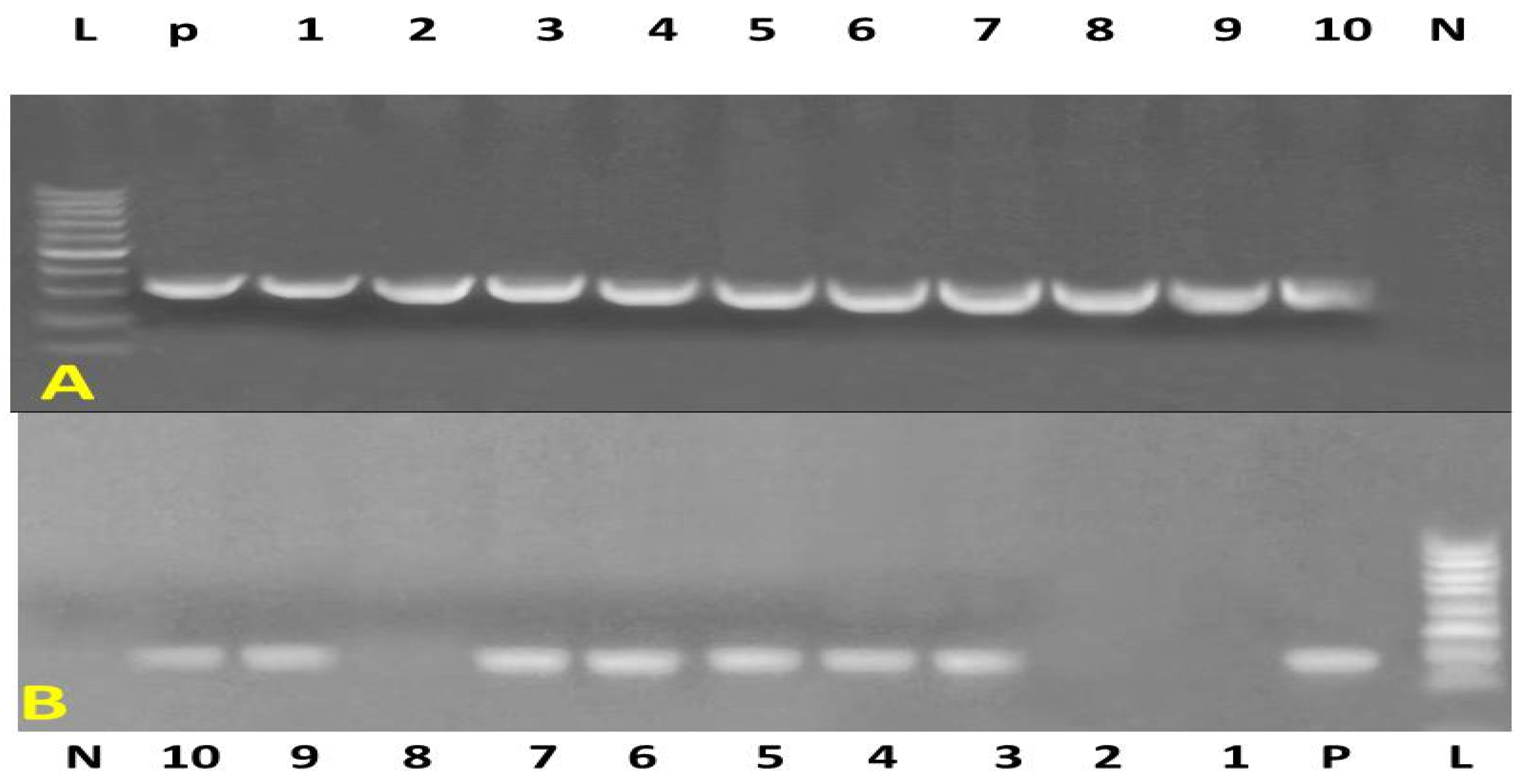
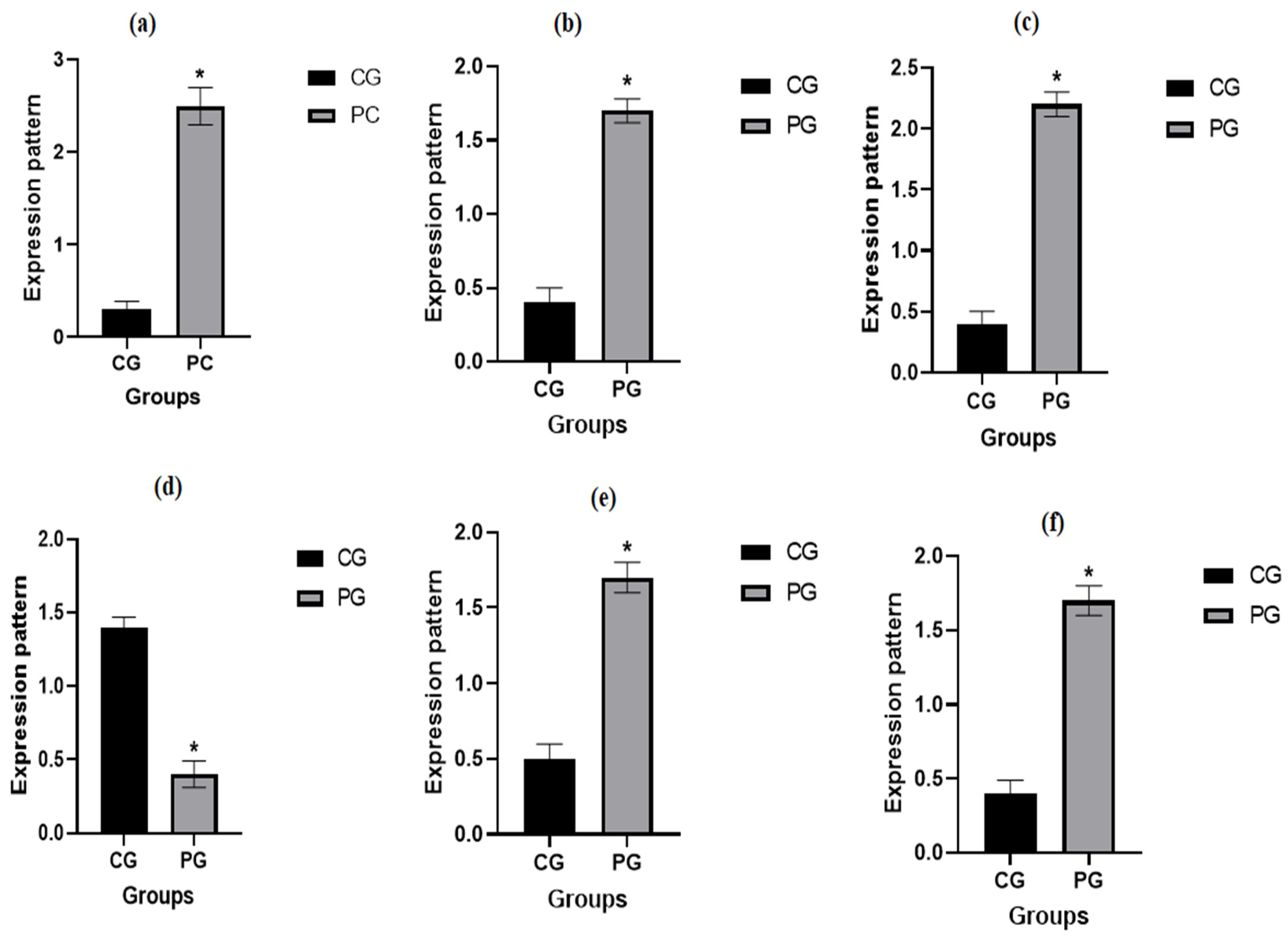
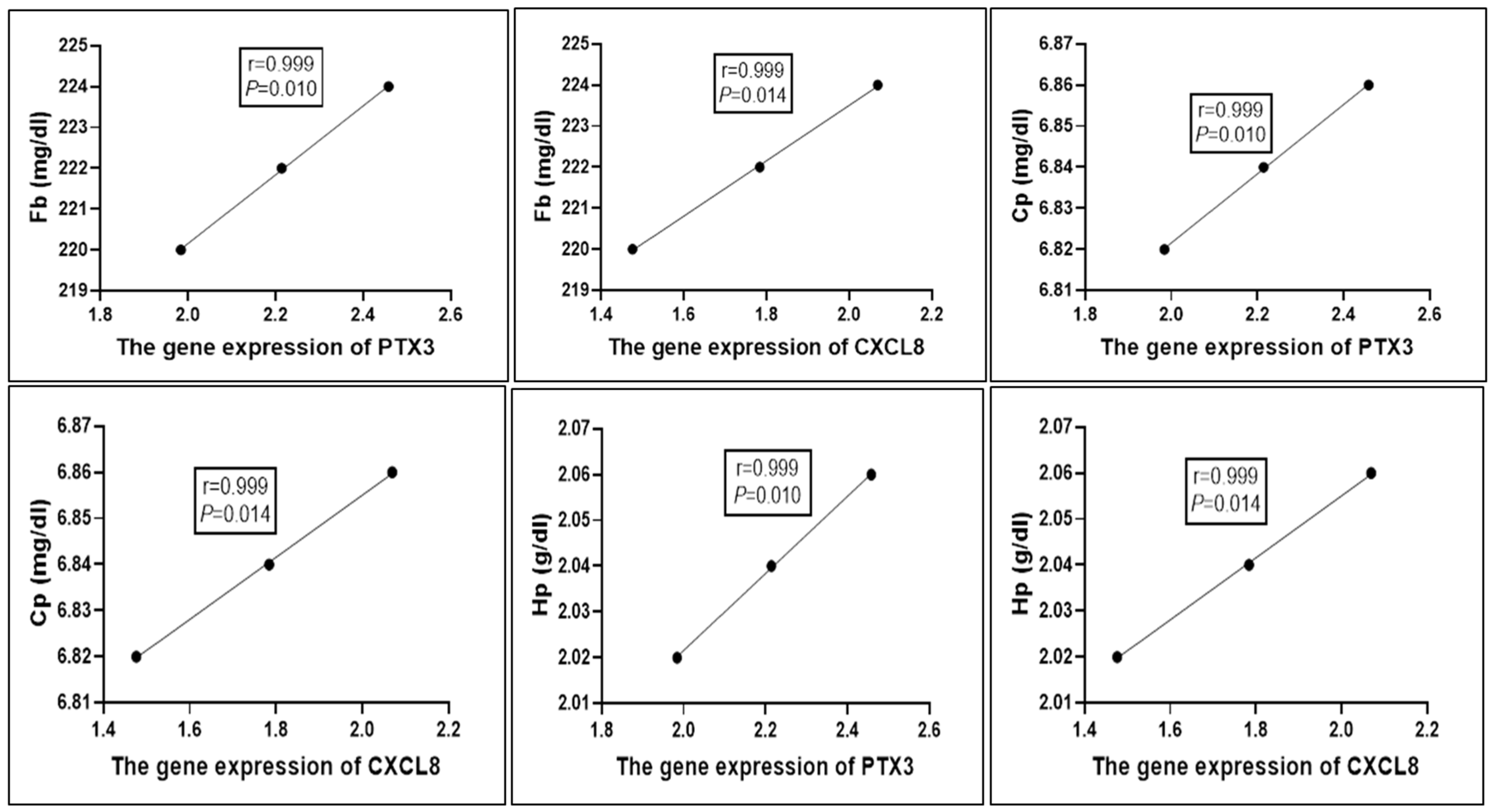
| Target Gene | Primers Sequences | Amplified Segment (bp) | Primary Denaturation | Amplification (35 Cycles) | Final Extension | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Secondary Denaturation | Annealing | Extension | ||||||
| Staph nuc | ATATGTATGGCAATCGTTTCAAT | 395 | 94 °C 5 min. | 94 °C 30 sec. | 55 °C 40 sec | 72 °C 40 sec. | 72 °C 10 min. | [12] |
| GTAAATGCACTTGCTTCAGGAC | ||||||||
| Staph mecA | GTA GAA ATG ACT GAA CGT CCG ATA A | 310 | 94 °C 5 min. | 94 °C 30 sec. | 50 °C 30 sec | 72 °C 30 sec | 72 °C 10 min | [46] |
| CCA ATT CCA CAT TGT TTC GGT CTA A | ||||||||
| Investigated Marker | Primer | Product Size (bp) | Annealing Temperature (°C) | GenBank Isolate | Origin |
|---|---|---|---|---|---|
| TLR2 | F5′-GCCTGGCTCCAGGCCAAGAGGA-3 R5′-TCCTCTTGGCCTGGAGCCAGGC-3′ | 354 | 58 | NM_001048231.1 | |
| CLEC4E | F5′-ATTCATCCACATCACCAGCATCA-3 R5′-GTGACCCTCGACCACCTGGTC-3′ | 443 | 55 | XM_042247586.1 | Present Research |
| PTX3 | F5′-CTGGAGGAGCTGCGGCGGACGC-3′ R5′-GGTGCTGCACAGATGGGTCCATG-3′ | 363 | 58 | XM_004003220.5 | |
| SOCS3 | F5′-ACCTTCCTCATCCGCGACAGCTC-3′ R5′-ATCGTACTGGTCCAGGAACTC-3′ | 480 | 58 | XM_027974200.3 | |
| CXCL8 | F5′-ATGACTTCCAAGCTGGCTGTTG-3′ R5′-ATCTTGCTTCTCAGCTCTCTTC-3′ | 300 | 55 | NM_001009401.2 | |
| IL15RA | F5′-CCGGCCACGCCGGGCATCACCTG-3′ R5′-CACCAGGCACACTGCAAAGAC-3′ | 378 | 58 | XM_042230436.2 | |
| GAPDH | F5′-GTGAAGGTCGGAGTGAACGG-3′ R5′-TTGACTGTGCCGTGGAACTT-3′ | 173 | 58 | HM043737.1 |
| Type of Antibiotic | No. Sensitive/27 | No. Resistant/27 | % Sensitive | % Resistant |
|---|---|---|---|---|
| Gentamicin | 21 | 6 | 77.7 | 22.2 |
| Amoxycillin | 8 | 19 | 29.6 | 70.4 |
| Amoxycillin/clavullanate | 17 | 10 | 63 | 37.0 |
| Ceftriaxone | 20 | 7 | 74 | 26 |
| Cloxacillin | 10 | 17 | 37 | 63 |
| Ciprofloxacin | 21 | 6 | 77.7 | 22.2 |
| Levofloxacin | 27 | 0 | 100 | 0 |
| Penicillin | 1 | 26 | 3.7 | 96.3 |
| Erythromycin | 14 | 13 | 51,8 | 48 |
| Chloramphenicol | 16 | 11 | 59.2 | 40.7 |
| Ofloxacin | 20 | 7 | 74 | 26 |
| Tetracycline | 8 | 19 | 29.6 | 70.3 |
| Cotrimoxazole | 4 | 23 | 14.8 | 85.2 |
| vancomycin | 17 | 0 | 100 | 0 |
| Type of Antibiotic | No. Sensitive/17 | No. Resistant/17 | % Sensitive | % Resistant |
|---|---|---|---|---|
| Gentamicin | 2 | 15 | 11.7 | 88.3 |
| Amoxycillin | 0 | 17 | 0 | 100 |
| Amoxycillin/clavullanate | 0 | 17 | 0 | 100 |
| Ceftriaxone | 7 | 10 | 41.2 | 58.8 |
| Cloxacillin | 0 | 17 | 0 | 100 |
| Ciprofloxacin | 9 | 8 | 53 | 47 |
| Levofloxacin | 16 | 1 | 94 | 5.8 |
| Penicillin | 0 | 17 | 0 | 100 |
| Erythromycin | 0 | 17 | 0 | 100 |
| Chloramphenicol | 0 | 17 | 0 | 100 |
| Ofloxacin | 12 | 5 | 70.6 | 29.4 |
| Tetracycline | 0 | 17 | 0 | 100 |
| Cotrimoxazole | 0 | 17 | 0 | 100 |
| vancomycin | 17 | 0 | 100 | 0 |
| Gene | SNPs | Healthy n = 100 | Pneumonia n = 100 | Total n = 200 | Chi Square X2 | p Value | Kind of Inherited Change | Amino Acid Order and Sort |
|---|---|---|---|---|---|---|---|---|
| TLR2 | C169T | 73/100 | -/100 | 73/200 | 115 | <0.005 | Non-synonymous | 57 L to F |
| CLEC4E | G43A | 59/100 | -/100 | 59/200 | 83.69 | <0.005 | Non-synonymous | 15 C to Y |
| PTX3 | G90C | -/100 | 84/100 | 84/200 | 144.8 | <0.005 | Synonymous | 30 A |
| C2383T | 39/100 | -/100 | 39/200 | 48.45 | <0.005 | Non-synonymous | 95 L to F | |
| SOCS3 | G438C | -/100 | 48/100 | 48/200 | 63.13 | <0.005 | Synonymous | 146 T |
| CXCL8 | C154T | -/100 | 74/100 | 74/200 | 117.5 | <0.005 | Synonymous | 52 L |
| IL15RA | A74G | 43/100 | -/100 | 43/200 | 54.8 | <0.005 | Non-synonymous | 25 S to N |
| Predicted Group Membership | Total | |||
|---|---|---|---|---|
| Healthy | Diseases | |||
| Count | Healthy | 100 | 0 | 100 |
| Diseased | 0 | 100 | 100 | |
| % | Healthy | 100.0 | 0.0 | 100.0 |
| Diseased | 0.0 | 100.0 | 100.0 | |
| Parameters | Control Group | Pneumonic Group |
|---|---|---|
| Fb (mg/dL) | 122.01 ± 8.49 | 225.01 ± 3.47 * |
| Cp (mg/dL) | 2.30 ± 1.15 | 6.24 ± 0.02 * |
| Hp (g/dL) | 0.15 ± 0.02 | 2.59 ± 0.49 * |
| SAA (mg/L) | 2.32 ± 0.15 | 6.96 ± 0.16 * |
| Cortisol (μg/dL) | 1.79 ± 0.16 | 6.44 ± 0.05 * |
| Insulin (μIU/mL) | 8.41 ± 0.15 | 7.08 ± 0.17 * |
| T3(ng/mL) | 1.74 ± 0.15 | 1.02 ± 0.02 * |
| T4 (μg/mL) | 0.85 ± 0.08 | 0.65 ± 0.02 * |
| TSH (μIU/mL) | 0.010 ± 0.002 | 0.022 ± 0.012 * |
| GH (ng/dL) | 12.39 ± 1.47 | 16.80 ± 0.05 * |
| SI (μg/dL) | 106.89 ± 2.46 | 89.13 ± 1.62 * |
| TIBC (μg/dL) | 327.39 ± 2.16 | 341.42 ± 3.11 * |
| UIBC (μg/dL) | 220.50 ± 2.24 | 252.29 ± 3.72 * |
| Transferrin(mg/dL) | 124.65 ± 2.74 | 86.47 ± 0.22 * |
| Tf sat. % | 32.65 ± 0.66 | 26.11 ± 0.56 * |
| Ferritin (ng/mL) | 13.60 ± 1.05 | 19.35 ± 0.53 * |
| Parameters | Cut-Off Point | Sensitivity | Specificity | LR | PPV | NPV | AR | % of Increase or Decrease |
|---|---|---|---|---|---|---|---|---|
| Fb (mg/dL) | 132.50 | 100% | 85% | 6.67 | 90.91% | 100% | 94% | 84.41% |
| Cp (mg/dL) | 3.60 | 100% | 80% | 5 | 88.24% | 100% | 92% | 171.30% |
| Hp (g/dL) | 0.185 | 100% | 90% | 10 | 93.75% | 100% | 96% | 1626.67% |
| SAA (mg/L) | 2.45 | 100% | 80% | 5 | 88.24% | 100% | 92% | 200% |
| Cortisol (μg/dL) | 1.99 | 100% | 85% | 6.67 | 90.91% | 100% | 94% | 259.77% |
| Insulin (μIU/mL) | 8. 26 | 100% | 80% | 5 | 88.24% | 100% | 92% | −15.81% |
| T3(ng/mL) | 1.57 | 100% | 95% | 20 | 96.77% | 100% | 98% | −41.37% |
| T4 (μg/mL) | 0.78 | 100% | 80% | 5 | 88.24% | 100% | 92% | −23.52% |
| TSH (μIU/mL) | 0.015 | 60% | 100% | - | 100% | 62.50% | 76% | 120% |
| GH (ng/dL) | 14.39 | 100% | 95% | 20 | 96.77% | 100% | 98% | 35.59% |
| SI (μg/dL) | 106 | 100% | 75% | 4 | 85.71% | 100% | 90% | −16.61% |
| TIBC (μg/dL) | 328 | 100% | 75% | 4 | 85.71% | 100% | 90% | 4.28% |
| UIBC (μg/dL) | 232.9 | 100% | 100% | - | 100% | 100% | 100% | 14.41% |
| Transferrin(mg/dL) | 121.50 | 100% | 85% | 6.67 | 90.91% | 100% | 94% | −30.62% |
| Tf sat. % | 32.51 | 100% | 75% | 4 | 85.71% | 100% | 90% | −20.03% |
| Ferritin (ng/mL) | 14.50 | 100% | 80% | 5 | 88.24% | 100% | 92% | 42.28% |
| LR = 0.5–5: low; LR = 5–10: moderate; LR > 10: high. | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, H.M.; Noaman, E.A.; El-Sayed, A.; Ragab, M.T.; Hafez, A.; Eissa, A.; Ateya, A.; Alwutayd, K.M.; Babaker, M.A.; Darwish, A. Analysis of Potential Genes, Acute Phase Proteins and Hormonal Profiles Associated with Methicillin-Resistant Staphylococcus aureus (MRSA) Isolation from Pneumonic Sheep. Vet. Sci. 2025, 12, 584. https://doi.org/10.3390/vetsci12060584
Alharbi HM, Noaman EA, El-Sayed A, Ragab MT, Hafez A, Eissa A, Ateya A, Alwutayd KM, Babaker MA, Darwish A. Analysis of Potential Genes, Acute Phase Proteins and Hormonal Profiles Associated with Methicillin-Resistant Staphylococcus aureus (MRSA) Isolation from Pneumonic Sheep. Veterinary Sciences. 2025; 12(6):584. https://doi.org/10.3390/vetsci12060584
Chicago/Turabian StyleAlharbi, Hanan M., Eman A. Noaman, Ahmed El-Sayed, Mohamed T. Ragab, Amani Hafez, Attia Eissa, Ahmed Ateya, Khairiah M. Alwutayd, Manal A. Babaker, and Asmaa Darwish. 2025. "Analysis of Potential Genes, Acute Phase Proteins and Hormonal Profiles Associated with Methicillin-Resistant Staphylococcus aureus (MRSA) Isolation from Pneumonic Sheep" Veterinary Sciences 12, no. 6: 584. https://doi.org/10.3390/vetsci12060584
APA StyleAlharbi, H. M., Noaman, E. A., El-Sayed, A., Ragab, M. T., Hafez, A., Eissa, A., Ateya, A., Alwutayd, K. M., Babaker, M. A., & Darwish, A. (2025). Analysis of Potential Genes, Acute Phase Proteins and Hormonal Profiles Associated with Methicillin-Resistant Staphylococcus aureus (MRSA) Isolation from Pneumonic Sheep. Veterinary Sciences, 12(6), 584. https://doi.org/10.3390/vetsci12060584





