Faecal Microbiota Transplantation as an Adjuvant Treatment for Extraintestinal Disorders: Translating Insights from Human Medicine to Veterinary Practice
Simple Summary
Abstract
1. Introduction
2. Methods
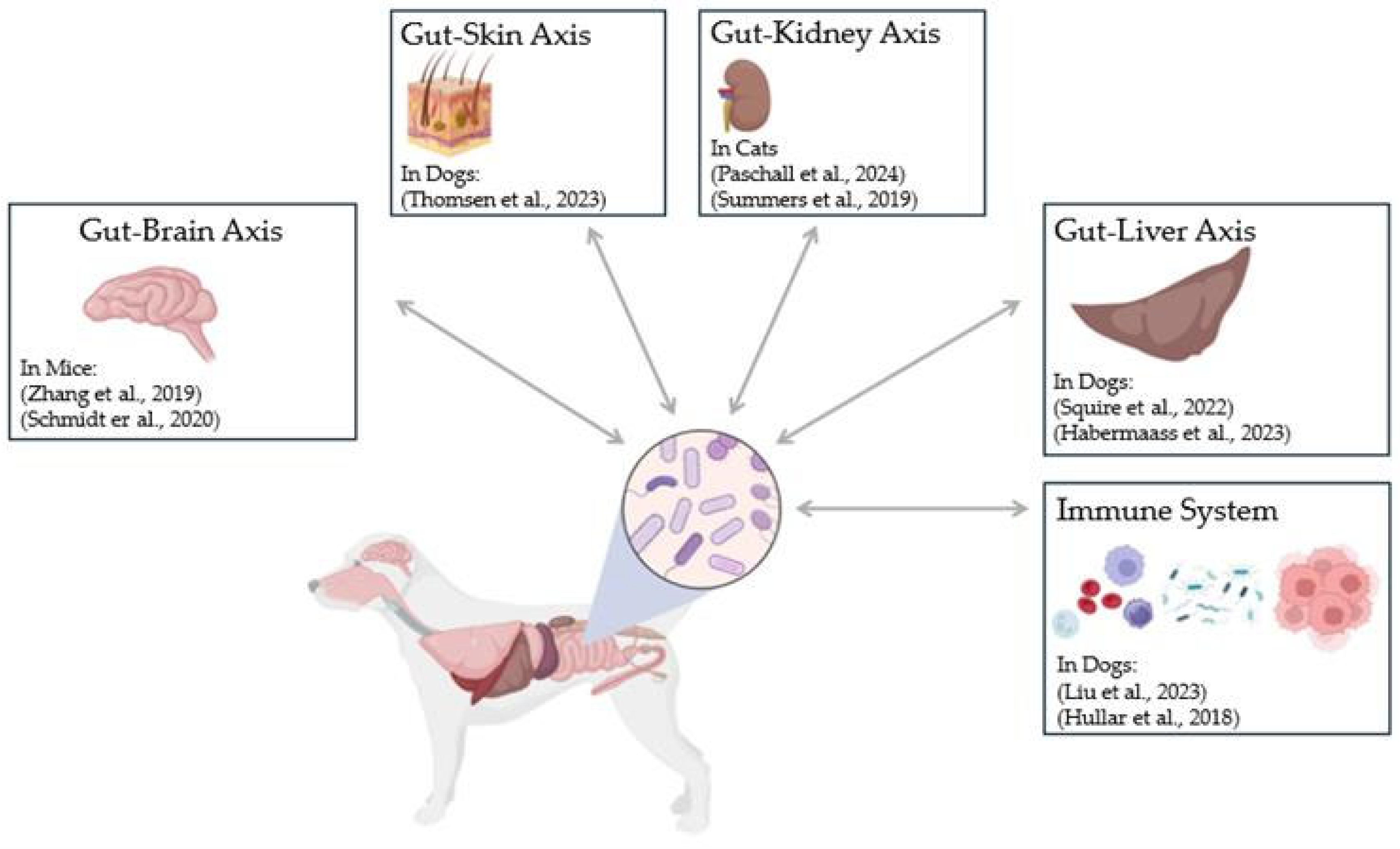
3. Discussion
3.1. Current Applications of FMT in Domestic Dogs
3.2. Dermatological Disorders
3.3. Infection, and Susceptibility to Multidrug-Resistant Organisms
3.4. Hepatic Disorders
3.5. Neurological Disorders
3.6. Metabolic Disorders
3.7. Oncological Applications
3.8. Immune Mediated Disorders
3.9. Kidney Disorders
4. Conclusions
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Y.C.; Yang, Y.S. Fecal microbiota transplantation: Current status and challenges in China. JGH Open 2018, 2, 114–116. [Google Scholar] [CrossRef] [PubMed]
- DePeters, E.J.; George, L.W. Rumen transfaunation. Immunol. Lett. 2014, 162, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Joshi, V.; Gautam, D.; GE, C.; Lekshman, A. Rumen transfaunation an effective method for treating simple indigestion in ruminants. North-East Vet. 2017, 17, 31. [Google Scholar]
- de Groot, P.F.; Frissen, M.N.; de Clercq, N.C.; Nieuwdorp, M. Fecal microbiota transplantation in metabolic syndrome: History, present and future. Gut Microbes 2017, 8, 253–267. [Google Scholar] [CrossRef]
- Baunwall, S.M.D.; Lee, M.M.; Eriksen, M.K.; Mullish, B.H.; Marchesi, J.R.; Dahlerup, J.F.; Hvas, C.L. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. EClinicalMedicine 2020, 29–30, 100642. [Google Scholar] [CrossRef]
- Aguirre, A.M.; Adegbite, A.O.; Sorg, J.A. Clostridioides difficile bile salt hydrolase activity has substrate specificity and affects biofilm formation. NPJ Biofilms Microbiomes 2022, 8, 94. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Faecal microbiota transplant for recurrent Clostridioides difficile infection (NICE Guideline No. MTG71). 2022. Available online: https://www.nice.org.uk/guidance/mtg71 (accessed on 27 May 2025).
- Winston, J.A.; Suchodolski, J.S.; Gaschen, F.; Busch, K.; Marsilio, S.; Costa, M.C.; Chaitman, J.; Coffey, E.L.; Dandrieux, J.R.S.; Gal, A.; et al. Clinical Guidelines for Fecal Microbiota Transplantation in Companion Animals. Adv. Small Anim. Care 2024, 5, 79–107. [Google Scholar] [CrossRef]
- Toresson, L.; Spillmann, T.; Pilla, R.; Ludvigsson, U.; Hellgren, J.; Olmedal, G.; Suchodolski, J.S. Clinical Effects of Faecal Microbiota Transplantation as Adjunctive Therapy in Dogs with Chronic Enteropathies—A Retrospective Case Series of 41 Dogs. Vet. Sci. 2023, 10, 271. [Google Scholar] [CrossRef]
- Niina, A.; Kibe, R.; Suzuki, R.; Yuchi, Y.; Teshima, T.; Matsumoto, H.; Kataoka, Y.; Koyama, H. Fecal microbiota transplantation as a new treatment for canine inflammatory bowel disease. Biosci. Microbiota Food Health 2021, 40, 98–104. [Google Scholar] [CrossRef]
- Chaitman, J.; Ziese, A.L.; Pilla, R.; Minamoto, Y.; Blake, A.B.; Guard, B.C.; Isaiah, A.; Lidbury, J.A.; Steiner, J.M.; Unterer, S.; et al. Fecal Microbial and Metabolic Profiles in Dogs With Acute Diarrhea Receiving Either Fecal Microbiota Transplantation or Oral Metronidazole. Front. Vet. Sci. 2020, 7, 192. [Google Scholar] [CrossRef]
- Alves, J.C.; Santos, A.; Jorge, P.; Pitães, Â. Faecal microbiome transplantation improves clinical signs of chronic idiopathic large bowel diarrhoea in working dogs. Vet. Rec. 2023, 193, e3052. [Google Scholar] [CrossRef] [PubMed]
- Gal, A.; Barko, P.C.; Biggs, P.J.; Gedye, K.R.; Midwinter, A.C.; Williams, D.A.; Burchell, R.K.; Pazzi, P. One dog’s waste is another dog’s wealth: A pilot study of fecal microbiota transplantation in dogs with acute hemorrhagic diarrhea syndrome. PLoS ONE 2021, 16, e0250344. [Google Scholar] [CrossRef] [PubMed]
- Jugan, M.C.; KuKanich, K.; Freilich, L. Clinical response in dogs with acute hemorrhagic diarrhea syndrome following randomized probiotic treatment or fecal microbiota transplant. Front. Vet. Sci. 2023, 10, 1050538. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.; Colville, A. Adverse events in faecal microbiota transplant: A review of the literature. J. Hosp. Infect. 2016, 92, 117–127. [Google Scholar] [CrossRef]
- Khoruts, A.; Sadowsky, M.J. Understanding the mechanisms of faecal microbiota transplantation. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 508–516. [Google Scholar] [CrossRef]
- Collier, A.J.; Gomez, D.E.; Monteith, G.; Plattner, B.L.; Verbrugghe, A.; Webb, J.; Weese, J.S.; Blois, S.L. Investigating fecal microbial transplant as a novel therapy in dogs with inflammatory bowel disease: A preliminary study. PLoS ONE 2022, 17, e0276295. [Google Scholar] [CrossRef]
- Karimi, M.; Shirsalimi, N.; Hashempour, Z.; Salehi Omran, H.; Sedighi, E.; Beigi, F.; Mortezazadeh, M. Safety and efficacy of fecal microbiota transplantation (FMT) as a modern adjuvant therapy in various diseases and disorders: A comprehensive literature review. Front. Immunol. 2024, 15, 1439176. [Google Scholar] [CrossRef]
- Hong, A.S.; Tun, K.M.; Hong, J.M.; Batra, K.; Ohning, G. Fecal Microbiota Transplantation in Decompensated Cirrhosis: A Systematic Review on Safety and Efficacy. Antibiotics 2022, 11, 838. [Google Scholar] [CrossRef]
- Sokol, H.; Landman, C.; Seksik, P.; Berard, L.; Montil, M.; Nion-Larmurier, I.; Bourrier, A.; Le Gall, G.; Lalande, V.; De Rougemont, A.; et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: A pilot randomized controlled study. Microbiome 2020, 8, 12. [Google Scholar] [CrossRef]
- Kedia, S.; Virmani, S.; Vuyyuru, S.K.; Kumar, P.; Kante, B.; Sahu, P.; Kaushal, K.; Farooqui, M.; Singh, M.; Verma, M.; et al. Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: A randomised controlled trial. Gut 2022, 71, 2401–2413. [Google Scholar] [CrossRef]
- Rebello, D.; Wang, E.; Yen, E.; Lio, P.A.; Kelly, C.R. Hair Growth in Two Alopecia Patients after Fecal Microbiota Transplant. ACG Case Rep. J. 2017, 4, e107. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Xu, H.M.; Liu, Y.D.; Shou, D.W.; Nie, Y.Q.; Chen, H.T.; Zhou, Y.J. Fecal microbiota transplantation as a novel approach for the treatment of atopic dermatitis. J. Dermatol. 2021, 48, e574–e576. [Google Scholar] [CrossRef] [PubMed]
- Mashiah, J.; Karady, T.; Fliss-Isakov, N.; Sprecher, E.; Slodownik, D.; Artzi, O.; Samuelov, L.; Ellenbogen, E.; Godneva, A.; Segal, E.; et al. Clinical efficacy of fecal microbial transplantation treatment in adults with moderate-to-severe atopic dermatitis. Immun. Inflamm. Dis. 2022, 10, e570. [Google Scholar] [CrossRef] [PubMed]
- Baek, O.D.; Hjermitslev, C.K.; Dyreborg, L.; Baunwall, S.M.D.; Høyer, K.L.; Rågård, N.; Hammeken, L.H.; Povlsen, J.V.; Ehlers, L.H.; Hvas, C.L. Early Economic Assessment of Faecal Microbiota Transplantation for Patients with Urinary Tract Infections Caused by Multidrug-Resistant Organisms. Infect. Dis. Ther. 2023, 12, 1429–1436. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C.; Tang, C.; He, Q.; Zhao, X.; Li, N.; Li, J. Successful treatment of severe sepsis and diarrhea after vagotomy utilizing fecal microbiota transplantation: A case report. Crit. Care 2015, 19, 37. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, J.; Wang, J.; Yang, Y.; Huang, J.; Gong, H.; Cui, H.; Chen, D. Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Crit. Care 2016, 20, 332. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Kassam, Z.; Fagan, A.; Gavis, E.A.; Liu, E.; Cox, I.J.; Kheradman, R.; Heuman, D.; Wang, J.; Gurry, T.; et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology 2017, 66, 1727–1738. [Google Scholar] [CrossRef]
- Mehta, R.; Kabrawala, M.; Nandwani, S.; Kalra, P.; Patel, C.; Desai, P.; Parekh, K. Preliminary experience with single fecal microbiota transplant for treatment of recurrent overt hepatic encephalopathy-A case series. Indian. J. Gastroenterol. 2018, 37, 559–562. [Google Scholar] [CrossRef]
- He, Z.; Cui, B.T.; Zhang, T.; Li, P.; Long, C.Y.; Ji, G.Z.; Zhang, F.M. Fecal microbiota transplantation cured epilepsy in a case with Crohn’s disease: The first report. World J. Gastroenterol. 2017, 23, 3565–3568. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Zhou, Y.; Qian, C.; Zou, J.; Chen, R. Efficacy and safety of washed microbiota transplantation in the treatment of brittle diabetes. Chin. J. Diabetes Mellit. 2020, 12, 962–967. [Google Scholar]
- de Groot, P.; Nikolic, T.; Pellegrini, S.; Sordi, V.; Imangaliyev, S.; Rampanelli, E.; Hanssen, N.; Attaye, I.; Bakker, G.; Duinkerken, G.; et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut 2021, 70, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yi, P.; Zhu, M.; Zhou, W.; Zhang, B.; Yi, X.; Long, H.; Zhang, G.; Wu, H.; Tsokos, G.C.; et al. Safety and efficacy of fecal microbiota transplantation for treatment of systemic lupus erythematosus: An EXPLORER trial. J. Autoimmun. 2022, 130, 102844. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Muller, G.Y.; Flores-Treviño, S.; Bocanegra-Ibarias, P.; Robles-Espino, D.; Garza-González, E.; Fabela-Valdez, G.C.; Camacho-Ortiz, A. Changes in the Progression of Chronic Kidney Disease in Patients Undergoing Fecal Microbiota Transplantation. Nutrients 2024, 16, 1109. [Google Scholar] [CrossRef]
- Sugita, K.; Shima, A.; Takahashi, K.; Ishihara, G.; Kawano, K.; Ohmori, K. Pilot evaluation of a single oral fecal microbiota transplantation for canine atopic dermatitis. Sci. Rep. 2023, 13, 8824. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, R.; Cheng, M.; Wang, L.; Chao, J.; Li, J.; Zheng, P.; Xie, P.; Zhang, Z.; Yao, H. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome 2019, 7, 116. [Google Scholar] [CrossRef]
- Schmidt, E.K.; Torres-Espin, A.; Raposo, P.J.; Madsen, K.L.; Kigerl, K.A.; Popovich, P.G.; Fenrich, K.K.; Fouad, K. Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. PLoS ONE 2020, 15, e0226128. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Brown, K.; Godovannyi, A.; Ma, C.; Zhang, Y.; Ahmadi-Vand, Z.; Dai, C.; Gorzelak, M.A.; Chan, Y.; Chan, J.M.; Lochner, A. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. ISME J. 2016, 10, 321–332. [Google Scholar] [CrossRef]
- Thomsen, M.; Künstner, A.; Wohlers, I.; Olbrich, M.; Lenfers, T.; Osumi, T.; Shimazaki, Y.; Nishifuji, K.; Ibrahim, S.M.; Watson, A.; et al. A comprehensive analysis of gut and skin microbiota in canine atopic dermatitis in Shiba Inu dogs. Microbiome 2023, 11, 232. [Google Scholar] [CrossRef]
- Paschall, R.E.; Quimby, J.M.; Lourenço, B.N.; Summers, S.C.; Schmiedt, C.W. The Effect of Renaltec on Serum Uremic Toxins in Cats with Experimentally Induced Chronic Kidney Disease. Vet. Sci. 2024, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.C.; Quimby, J.M.; Isaiah, A.; Suchodolski, J.S.; Lunghofer, P.J.; Gustafson, D.L. The fecal microbiome and serum concentrations of indoxyl sulfate and p-cresol sulfate in cats with chronic kidney disease. J. Vet. Intern. Med. 2019, 33, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Squire, N.; Lux, C.; Tolbert, K.; Lidbury, J.; Sun, X.; Suchodolski, J.S. Characterization of the Fecal Microbiome in Dogs Receiving Medical Management for Congenital Portosystemic Shunts. Front. Vet. Sci. 2022, 9, 897760. [Google Scholar] [CrossRef] [PubMed]
- Habermaass, V.; Olivero, D.; Gori, E.; Mariti, C.; Longhi, E.; Marchetti, V. Intestinal Microbiome in Dogs with Chronic Hepatobiliary Disease: Can We Talk about the Gut–Liver Axis? Animals 2023, 13, 3174. [Google Scholar] [CrossRef]
- Liu, P.Y.; Xia, D.; McGonigle, K.; Carroll, A.B.; Chiango, J.; Scavello, H.; Martins, R.; Mehta, S.; Krespan, E.; Lunde, E.; et al. Immune-mediated hematological disease in dogs is associated with alterations of the fecal microbiota: A pilot study. Anim. Microbiome 2023, 5, 46. [Google Scholar] [CrossRef]
- Hullar, M.A.J.; Lampe, J.W.; Torok-Storb, B.J.; Harkey, M.A. The canine gut microbiome is associated with higher risk of gastric dilatation-volvulus and high risk genetic variants of the immune system. PLoS ONE 2018, 13, e0197686. [Google Scholar] [CrossRef]
- Drechsler, Y.; Dong, C.; Clark, D.E.; Kaur, G. Canine Atopic Dermatitis: Prevalence, Impact, and Management Strategies. Vet. Med. 2024, 15, 15–29. [Google Scholar] [CrossRef]
- Amitay, E.L.; Werner, S.; Vital, M.; Pieper, D.H.; Höfler, D.; Gierse, I.J.; Butt, J.; Balavarca, Y.; Cuk, K.; Brenner, H. Fusobacterium and colorectal cancer: Causal factor or passenger? Results from a large colorectal cancer screening study. Carcinogenesis 2017, 38, 781–788. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Camacho, J.; Steiner, J.M. Analysis of bacterial diversity in the canine duodenum, jejunum, ileum, and colon by comparative 16S rRNA gene analysis. FEMS Microbiol. Ecol. 2008, 66, 567–578. [Google Scholar] [CrossRef]
- Minamoto, Y.; Minamoto, T.; Isaiah, A.; Sattasathuchana, P.; Buono, A.; Rangachari, V.R.; McNeely, I.H.; Lidbury, J.; Steiner, J.M.; Suchodolski, J.S. Fecal short-chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J. Vet. Intern. Med. 2019, 33, 1608–1618. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Tavoukjian, V. Faecal microbiota transplantation for the decolonization of antibiotic-resistant bacteria in the gut: A systematic review and meta-analysis. J. Hosp. Infect. 2019, 102, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Manges, A.R.; Steiner, T.S.; Wright, A.J. Fecal microbiota transplantation for the intestinal decolonization of extensively antimicrobial-resistant opportunistic pathogens: A review. Infect. Dis. 2016, 48, 587–592. [Google Scholar] [CrossRef]
- Dubberke, E.R.; Mullane, K.M.; Gerding, D.N.; Lee, C.H.; Louie, T.J.; Guthertz, H.; Jones, C. Clearance of Vancomycin-Resistant Enterococcus Concomitant With Administration of a Microbiota-Based Drug Targeted at Recurrent Clostridium difficile Infection. Open Forum Infect. Dis. 2016, 3, ofw133. [Google Scholar] [CrossRef]
- Gopalsamy, S.N.; Sherman, A.; Woodworth, M.H.; Lutgring, J.D.; Kraft, C.S. Fecal Microbiota Transplant for Multidrug-Resistant Organism Decolonization Administered During Septic Shock. Infect. Control Hosp. Epidemiol. 2018, 39, 490–492. [Google Scholar] [CrossRef]
- Woodworth, M.H.; Hayden, M.K.; Young, V.B.; Kwon, J.H. The Role of Fecal Microbiota Transplantation in Reducing Intestinal Colonization With Antibiotic-Resistant Organisms: The Current Landscape and Future Directions. Open Forum Infect. Dis. 2019, 6, ofz288. [Google Scholar] [CrossRef]
- Nooij, S.; Vendrik, K.E.W.; Zwittink, R.D.; Ducarmon, Q.R.; Keller, J.J.; Kuijper, E.J.; Terveer, E.M. Long-term beneficial effect of faecal microbiota transplantation on colonisation of multidrug-resistant bacteria and resistome abundance in patients with recurrent Clostridioides difficile infection. Genome Med. 2024, 16, 37. [Google Scholar] [CrossRef]
- Woodworth, M.H.; Conrad, R.E.; Haldopoulos, M.; Pouch, S.M.; Babiker, A.; Mehta, A.K.; Sitchenko, K.L.; Wang, C.H.; Strudwick, A.; Ingersoll, J.M.; et al. Fecal microbiota transplantation promotes reduction of antimicrobial resistance by strain replacement. Sci. Transl. Med. 2023, 15, eabo2750. [Google Scholar] [CrossRef]
- Huttner, B.D.; de Lastours, V.; Wassenberg, M.; Maharshak, N.; Mauris, A.; Galperine, T.; Zanichelli, V.; Kapel, N.; Bellanger, A.; Olearo, F.; et al. A 5-day course of oral antibiotics followed by faecal transplantation to eradicate carriage of multidrug-resistant Enterobacteriaceae: A randomized clinical trial. Clin. Microbiol. Infect. 2019, 25, 830–838. [Google Scholar] [CrossRef]
- Hyun, J.; Lee, S.K.; Cheon, J.H.; Yong, D.E.; Koh, H.; Kang, Y.K.; Kim, M.H.; Sohn, Y.; Cho, Y.; Baek, Y.J.; et al. Faecal microbiota transplantation reduces amounts of antibiotic resistance genes in patients with multidrug-resistant organisms. Antimicrob. Resist. Infect. Control 2022, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Chopyk, D.M.; Grakoui, A. Contribution of the Intestinal Microbiome and Gut Barrier to Hepatic Disorders. Gastroenterology 2020, 159, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Kaul, E.; Hartmann, K.; Reese, S.; Dorsch, R. Recurrence rate and long-term course of cats with feline lower urinary tract disease. J. Feline Med. Surg. 2020, 22, 544–556. [Google Scholar] [CrossRef] [PubMed]
- WHO. Prevention and Control of Antimicrobial Resistance in the Food Chain: Guidance for Food Safety Authorities in Europe; WHO: Geneva, Switzerland, 2024; p. 68. [Google Scholar]
- Kao, D.; Roach, B.; Park, H.; Hotte, N.; Madsen, K.; Bain, V.; Tandon, P. Fecal microbiota transplantation in the management of hepatic encephalopathy. Hepatology 2016, 63, 339–340. [Google Scholar] [CrossRef]
- Craven, L.; Rahman, A.; Nair Parvathy, S.; Beaton, M.; Silverman, J.; Qumosani, K.; Hramiak, I.; Hegele, R.; Joy, T.; Meddings, J.; et al. Allogenic Fecal Microbiota Transplantation in Patients With Nonalcoholic Fatty Liver Disease Improves Abnormal Small Intestinal Permeability: A Randomized Control Trial. Am. J. Gastroenterol. 2020, 115, 1055–1065. [Google Scholar] [CrossRef]
- Witjes, J.J.; Smits, L.P.; Pekmez, C.T.; Prodan, A.; Meijnikman, A.S.; Troelstra, M.A.; Bouter, K.E.C.; Herrema, H.; Levin, E.; Holleboom, A.G.; et al. Donor Fecal Microbiota Transplantation Alters Gut Microbiota and Metabolites in Obese Individuals With Steatohepatitis. Hepatol. Commun. 2020, 4, 1578–1590. [Google Scholar] [CrossRef]
- Dhiman, R.K.; Roy, A.; Premkumar, M.; De, A.; Verma, N.; Duseja, A.K. Single session fecal microbiota transplantation in decompensated cirrhosis: An initial experience of clinical endpoints. In The Liver Meeting Digital Experience™; AASLD: Alexandria, VA, USA, 2020. [Google Scholar]
- Woodhouse, C.; Edwards, L.; Mullish, B.H.; Kronsten, V.; Tranah, T.; Zamalloa, A.; Flach, C.; Douiri, A.; Marchesi, J.; Patel, V. Results of the PROFIT trial, a PROspective randomised placebo-controlled feasibility trial of faecal microbiota transplantation in advanced cirrhosis. J. Hepatol. 2020, 73, S77–S78. [Google Scholar] [CrossRef]
- Ren, Y.D.; Ye, Z.S.; Yang, L.Z.; Jin, L.X.; Wei, W.J.; Deng, Y.Y.; Chen, X.X.; Xiao, C.X.; Yu, X.F.; Xu, H.Z.; et al. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology 2017, 65, 1765–1768. [Google Scholar] [CrossRef]
- Ahmad, J.; Kumar, M.; Sarin, S.K.; Sharma, S.; Choudhury, A.; Jindal, A.; Trehanpati, N.; Kulkarni, A.; Choudhury, S.; Verma, A. Faecal microbiota transplantation with tenofovir is superior to tenofovir alone in improving clinical outcomes in acute-on-chronic liver failure due to hepatitis B: An open label randomized controlled trial (NCT02689245). J. Hepatol. 2019, 70, e102. [Google Scholar] [CrossRef]
- Konturek, P.C.; Harsch, I.A.; Konturek, K.; Schink, M.; Konturek, T.; Neurath, M.F.; Zopf, Y. Gut–Liver Axis: How Do Gut Bacteria Influence the Liver? Med. Sci. 2018, 6, 79. [Google Scholar] [CrossRef]
- Cullen, J.M. Summary of the World Small Animal Veterinary Association standardization committee guide to classification of liver disease in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2009, 39, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, U.; Bulanda, E.; Wypych, T.P. Bile acids in immunity: Bidirectional mediators between the host and the microbiota. Front. Immunol. 2022, 13, 949033. [Google Scholar] [CrossRef] [PubMed]
- Valtolina, C.; Favier, R.P. Feline Hepatic Lipidosis. Vet. Clin. North. Am. Small Anim. Pract. 2017, 47, 683–702. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.T.; Kleiner, D.E. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism 2016, 65, 1080–1086. [Google Scholar] [CrossRef]
- Webb, C.B. Hepatic lipidosis: Clinical review drawn from collective effort. J. Feline Med. Surg. 2018, 20, 217–227. [Google Scholar] [CrossRef]
- Han, H.; Wang, M.; Zhong, R.; Yi, B.; Schroyen, M.; Zhang, H. Depletion of gut microbiota inhibits hepatic lipid accumulation in high-fat diet-fed mice. Int. J. Mol. Sci. 2022, 23, 9350. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Shen, F.; Cao, H.-X.; Ding, W.-J.; Chen, Y.-W.; Fan, J.-G. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci. Rep. 2017, 7, 1529. [Google Scholar] [CrossRef]
- Yang, F.; Wang, L.-K.; Li, X.; Wang, L.-W.; Han, X.-Q.; Gong, Z.-J. Sodium butyrate protects against toxin-induced acute liver failure in rats. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 309–315. [Google Scholar] [CrossRef]
- Chinna Meyyappan, A.; Forth, E.; Wallace, C.J.K.; Milev, R. Effect of fecal microbiota transplant on symptoms of psychiatric disorders: A systematic review. BMC Psychiatry 2020, 20, 299. [Google Scholar] [CrossRef]
- Lahtinen, P.; Jalanka, J.; Hartikainen, A.; Mattila, E.; Hillilä, M.; Punkkinen, J.; Koskenpato, J.; Anttila, V.-J.; Tillonen, J.; Satokari, R.; et al. Randomised clinical trial: Faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2020, 51, 1321–1331. [Google Scholar] [CrossRef]
- Kilinçarslan, S.; Evrensel, A. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with inflammatory bowel disease: An experimental study. Actas Esp. Psiquiatr. 2020, 48, 1–7. [Google Scholar] [PubMed]
- Kurokawa, S.; Kishimoto, T.; Mizuno, S.; Masaoka, T.; Naganuma, M.; Liang, K.-C.; Kitazawa, M.; Nakashima, M.; Shindo, C.; Suda, W. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: An open-label observational study. J. Affect. Disord. 2018, 235, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Hitchon, C.A.; Walld, R.; Bolton, J.M.; Sareen, J.; Walker, J.R.; Graff, L.A.; Patten, S.B.; Singer, A.; Lix, L.M.; et al. Increased Burden of Psychiatric Disorders in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 25, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Nguyen, G.C.; Benchimol, E.I.; Bernstein, C.N.; Bitton, A.; Kaplan, G.G.; Murthy, S.K.; Lee, K.; Cooke-Lauder, J.; Otley, A.R. The Impact of Inflammatory Bowel Disease in Canada 2018: Quality of Life. J. Can. Assoc. Gastroenterol. 2018, 2 (Suppl. 1), S42–S48. [Google Scholar] [CrossRef]
- Alcocer-Gómez, E.; de Miguel, M.; Casas-Barquero, N.; Núñez-Vasco, J.; Sánchez-Alcazar, J.A.; Fernández-Rodríguez, A.; Cordero, M.D. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav. Immun. 2014, 36, 111–117. [Google Scholar] [CrossRef]
- Olby, N.J.; Moore, S.A.; Brisson, B.; Fenn, J.; Flegel, T.; Kortz, G.; Lewis, M.; Tipold, A. ACVIM consensus statement on diagnosis and management of acute canine thoracolumbar intervertebral disc extrusion. J. Vet. Intern. Med. 2022, 36, 1570–1596. [Google Scholar] [CrossRef]
- Gunaratne, A.W.; Clancy, A.; Borody, T. S3437 Antibiotic Therapy Followed by Faecal Microbiota Transplantation Alleviates Epilepsy–A Case Report. Off. J. Am. Coll. Gastroenterol. ACG 2020, 115, S1783. [Google Scholar] [CrossRef]
- Cai, T.T.; Ye, X.L.; Yong, H.J.; Song, B.; Zheng, X.L.; Cui, B.T.; Zhang, F.M.; Lu, Y.B.; Miao, H.; Ding, D.F. Fecal microbiota transplantation relieve painful diabetic neuropathy: A case report. Medicine 2018, 97, e13543. [Google Scholar] [CrossRef]
- Muenyi, V.; Kerman, D.H. Changes in the body mass index (BMI) of patients treated with fecal microbiota transplant (FMT) for recurrent C. difficile infection. Gastroenterology 2017, 152, S820–S821. [Google Scholar] [CrossRef]
- Trevelline, B.K.; Kohl, K.D. The gut microbiome influences host diet selection behavior. Proc. Natl. Acad. Sci. USA 2022, 119, e2117537119. [Google Scholar] [CrossRef]
- Swartz, T.D.; Duca, F.A.; de Wouters, T.; Sakar, Y.; Covasa, M. Up-regulation of intestinal type 1 taste receptor 3 and sodium glucose luminal transporter-1 expression and increased sucrose intake in mice lacking gut microbiota. Br. J. Nutr. 2012, 107, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Schalla, M.A.; Stengel, A. Effects of microbiome changes on endocrine ghrelin signaling—A systematic review. Peptides 2020, 133, 170388. [Google Scholar] [CrossRef] [PubMed]
- Handl, S.; German, A.J.; Holden, S.L.; Dowd, S.E.; Steiner, J.M.; Heilmann, R.M.; Grant, R.W.; Swanson, K.S.; Suchodolski, J.S. Faecal microbiota in lean and obese dogs. FEMS Microbiol. Ecol. 2013, 84, 332–343. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.E.; Kim, H.B.; Isaacson, R.; Seo, K.W.; Song, K.H. Association of obesity with serum leptin, adiponectin, and serotonin and gut microflora in beagle dogs. J. Vet. Intern. Med. 2015, 29, 43–50. [Google Scholar] [CrossRef]
- Kieler, I.N.; Shamzir Kamal, S.; Vitger, A.D.; Nielsen, D.S.; Lauridsen, C.; Bjornvad, C.R. Gut microbiota composition may relate to weight loss rate in obese pet dogs. Vet. Med. Sci. 2017, 3, 252–262. [Google Scholar] [CrossRef]
- Fischer, M.M.; Kessler, A.M.; Kieffer, D.A.; Knotts, T.A.; Kim, K.; Wei, A.; Ramsey, J.J.; Fascetti, A.J. Effects of obesity, energy restriction and neutering on the faecal microbiota of cats. Br. J. Nutr. 2017, 118, 513–524. [Google Scholar] [CrossRef]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE 2011, 6, e25792. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Gonzalez, A.; Krieg, R.; Massey, H.D.; Carl, D.; Ghosh, S.; Gehr, T.W.B.; Ghosh, S.S. Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression. Nephrol. Dial. Transplant. 2018, 34, 783–794. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, T.; Wu, J.; Zhao, L.; Li, Q.; Varghese, Z.; Moorhead, J.F.; Powis, S.H.; Chen, Y.; Ruan, X.Z. Chronic inflammation exacerbates glucose metabolism disorders in C57BL/6J mice fed with high-fat diet. J. Endocrinol. 2013, 219, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Genser, L.; Aguanno, D.; Soula, H.A.; Dong, L.; Trystram, L.; Assmann, K.; Salem, J.E.; Vaillant, J.C.; Oppert, J.M.; Laugerette, F.; et al. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J. Pathol. 2018, 246, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Mokkala, K.; Pellonperä, O.; Röytiö, H.; Pussinen, P.; Rönnemaa, T.; Laitinen, K. Increased intestinal permeability, measured by serum zonulin, is associated with metabolic risk markers in overweight pregnant women. Metabolism 2017, 69, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.P.; Wang, B.; Jain, S.; Ding, J.; Rejeski, J.; Furdui, C.M.; Kitzman, D.W.; Taraphder, S.; Brechot, C.; Kumar, A.; et al. A mechanism by which gut microbiota elevates permeability and inflammation in obese/diabetic mice and human gut. Gut 2023, 72, 1848–1865. [Google Scholar] [CrossRef]
- Laia, N.L.; Barko, P.C.; Sullivan, D.R.; McMichael, M.A.; Williams, D.A.; Reinhart, J.M. Longitudinal analysis of the rectal microbiome in dogs with diabetes mellitus after initiation of insulin therapy. PLoS ONE 2022, 17, e0273792. [Google Scholar] [CrossRef]
- Kwong, T.C.; Chau, E.C.T.; Mak, M.C.H.; Choy, C.T.; Chan, L.T.; Pang, C.K.; Zhou, J.; Poon, P.H.C.; Guan, Y.; Tsui, S.K.W.; et al. Characterization of the Gut Microbiome in Healthy Dogs and Dogs with Diabetes Mellitus. Animals 2023, 13, 2479. [Google Scholar] [CrossRef]
- Frías Ordoñez, J.S.; Otero Regino, W. Chronic diarrhea in the diabetic. A review of the literature. Rev. Gastroenterol. Peru. 2016, 36, 340–349. [Google Scholar]
- Minkoff, N.Z.; Aslam, S.; Medina, M.; Tanner-Smith, E.E.; Zackular, J.P.; Acra, S.; Nicholson, M.R.; Imdad, A. Fecal microbiota transplantation for the treatment of recurrent Clostridioides difficile (Clostridium difficile). Cochrane Database Syst. Rev. 2023, 4, Cd013871. [Google Scholar] [CrossRef]
- Niessen, S.J.M.; Hazuchova, K.; Powney, S.L.; Guitian, J.; Niessen, A.P.M.; Pion, P.D.; Shaw, J.A.; Church, D.B. The Big Pet Diabetes Survey: Perceived Frequency and Triggers for Euthanasia. Vet. Sci. 2017, 4, 27. [Google Scholar] [CrossRef]
- Benedict, S.L.; Mahony, O.M.; McKee, T.S.; Bergman, P.J. Evaluation of bexagliflozin in cats with poorly regulated diabetes mellitus. Can. J. Vet. Res. 2022, 86, 52–58. [Google Scholar]
- Zain, N.M.M.; Ter Linden, D.; Lilley, A.K.; Royall, P.G.; Tsoka, S.; Bruce, K.D.; Mason, A.J.; Hatton, G.B.; Allen, E.; Goldenberg, S.D.; et al. Design and manufacture of a lyophilised faecal microbiota capsule formulation to GMP standards. J. Control Release 2022, 350, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Herstad, K.M.V.; Moen, A.E.F.; Gaby, J.C.; Moe, L.; Skancke, E. Characterization of the fecal and mucosa-associated microbiota in dogs with colorectal epithelial tumors. PLoS ONE 2018, 13, e0198342. [Google Scholar] [CrossRef] [PubMed]
- Mahiddine, F.Y.; You, I.; Park, H.; Kim, M.J. Microbiome Profile of Dogs with Stage IV Multicentric Lymphoma: A Pilot Study. Vet. Sci. 2022, 9, 409. [Google Scholar] [CrossRef]
- Zheng, H.-H.; Du, C.-T.; Yu, C.; Tang, X.-Y.; Huang, R.-L.; Zhang, Y.-Z.; Gao, W.; Xie, G.-H. The Relationship of Tumor Microbiome and Oral Bacteria and Intestinal Dysbiosis in Canine Mammary Tumor. Int. J. Mol. Sci. 2022, 23, 10928. [Google Scholar] [CrossRef] [PubMed]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Regan, D.; Guth, A.; Coy, J.; Dow, S. Cancer immunotherapy in veterinary medicine: Current options and new developments. Vet. J. 2016, 207, 20–28. [Google Scholar] [CrossRef]
- Dias, J.N.R.; André, A.S.; Aguiar, S.I.; Gil, S.; Tavares, L.; Aires-da-Silva, F. Immunotherapeutic Strategies for Canine Lymphoma: Changing the Odds Against Non-Hodgkin Lymphoma. Front. Vet. Sci. 2021, 8, 621758. [Google Scholar] [CrossRef]
- Breczko, W.J.; Bubak, J.; Miszczak, M. The Importance of Intestinal Microbiota and Dysbiosis in the Context of the Development of Intestinal Lymphoma in Dogs and Cats. Cancers 2024, 16, 2255. [Google Scholar] [CrossRef]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef]
- Chen, D.; Wu, J.; Jin, D.; Wang, B.; Cao, H. Fecal microbiota transplantation in cancer management: Current status and perspectives. Int. J. Cancer 2019, 145, 2021–2031. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Yue, J. Radiotherapy and the gut microbiome: Facts and fiction. Radiat. Oncol. 2021, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Madkour, M.A.; Altaf, R.A.; Sayed, Z.S.; Yasen, N.S.; Elbary, H.A.; Elsayed, R.A.; Mohamed, E.N.; Toema, M.; Wadan, A.-H.S.; Nafady, M.H.; et al. The Role of Gut Microbiota in Modulating Cancer Therapy Efficacy. Adv. Gut Microbiome Res. 2024, 2024, 9919868. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, Y.; Wu, Z. Programming of metabolic and autoimmune diseases in canine and feline: Linkage to the gut microbiome. Microb. Pathog. 2023, 185, 106436. [Google Scholar] [CrossRef] [PubMed]
- Cintio, M.; Scarsella, E.; Sgorlon, S.; Sandri, M.; Stefanon, B. Gut microbiome of healthy and arthritic dogs. Vet. Sci. 2020, 7, 92. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Q.; Zhao, Y.; Zou, Y.; Chen, M.; Zhou, S.; Wang, Z. The relationship of Megamonas species with nonalcoholic fatty liver disease in children and adolescents revealed by metagenomics of gut microbiota. Sci. Rep. 2022, 12, 22001. [Google Scholar] [CrossRef]
- Dai, L.; Tang, Y.; Zhou, W.; Dang, Y.; Sun, Q.; Tang, Z.; Zhu, M.; Ji, G. Gut microbiota and related metabolites were disturbed in ulcerative colitis and partly restored after mesalamine treatment. Front. Pharmacol. 2021, 11, 620724. [Google Scholar] [CrossRef]
- Vientós-Plotts, A.I.; Ericsson, A.C.; McAdams, Z.L.; Rindt, H.; Reinero, C.R. Temporal changes of the respiratory microbiota as cats transition from health to experimental acute and chronic allergic asthma. Front. Vet. Sci. 2022, 9, 983375. [Google Scholar] [CrossRef]
- Mou, Z.; Yang, Y.; Hall, A.B.; Jiang, X. The taxonomic distribution of histamine-secreting bacteria in the human gut microbiome. BMC Genom. 2021, 22, 1–11. [Google Scholar] [CrossRef]
- Li, L.-L.; Wang, Y.-T.; Zhu, L.-M.; Liu, Z.-Y.; Ye, C.-Q.; Qin, S. Inulin with different degrees of polymerization protects against diet-induced endotoxemia and inflammation in association with gut microbiota regulation in mice. Sci. Rep. 2020, 10, 978. [Google Scholar] [CrossRef]
- Borody, T.; Campbell, J.; Torres, M.; Nowak, A.; Leis, S. Reversal of idiopathic thrombocytopenic purpura [ITP] with fecal microbiota transplantation [FMT]: 941. Off. J. Am. Coll. Gastroenterol. ACG 2011, 106, S352. [Google Scholar] [CrossRef]
- Glickman, L.T.; Glickman, N.W.; Schellenberg, D.B.; Raghavan, M.; Lee, T.L. Incidence of and breed-related risk factors for gastric dilatation-volvulus in dogs. J. Am. Vet. Med. Assoc. 2000, 216, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zeng, J.; Peng, L.; Wang, L.; Zheng, W.; Di, W.; Yang, Y. Fecal microbiota transplantation for membranous nephropathy. CEN Case Rep. 2021, 10, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bai, M.; Yang, X.; Wang, Y.; Li, R.; Sun, S. Alleviation of refractory IgA nephropathy by intensive fecal microbiota transplantation: The first case reports. Ren. Fail. 2021, 43, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Marino, C.L.; Lascelles, B.D.; Vaden, S.L.; Gruen, M.E.; Marks, S.L. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J. Feline Med. Surg. 2014, 16, 465–472. [Google Scholar] [CrossRef]
- Graboski, A.L.; Redinbo, M.R. Gut-Derived Protein-Bound Uremic Toxins. Toxins 2020, 12, 590. [Google Scholar] [CrossRef]
- Li, X.J.; Shan, Q.Y.; Wu, X.; Miao, H.; Zhao, Y.Y. Gut microbiota regulates oxidative stress and inflammation: A double-edged sword in renal fibrosis. Cell Mol. Life Sci. 2024, 81, 480. [Google Scholar] [CrossRef]
- Caggiano, G.; Stasi, A.; Franzin, R.; Fiorentino, M.; Cimmarusti, M.T.; Deleonardis, A.; Palieri, R.; Pontrelli, P.; Gesualdo, L. Fecal Microbiota Transplantation in Reducing Uremic Toxins Accumulation in Kidney Disease: Current Understanding and Future Perspectives. Toxins 2023, 15, 115. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Papadopoulou, I.; Bantouna, D.; De Lastic, A.L.; Rodi, M.; Mouzaki, A.; Gogos, C.A.; Zolota, V.; Maroulis, I. Fecal Microbiota Transplantation and Hydrocortisone Ameliorate Intestinal Barrier Dysfunction and Improve Survival in a Rat Model of Cecal Ligation and Puncture-Induced Sepsis. Shock 2021, 55, 666–675. [Google Scholar] [CrossRef]
| Study | Study Design | Disease/Disorder | Participants | Protocol | Clinical Effects | Proposed Translation to Companion Animals | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Case report | Alopecia | 2 people | N/A | Incidental sustained hair regrowth following FMT for Clostridioides difficile infection | Canine and feline alopecia | [22] |
| 2 | Case report | Atopic dermatitis | 1 person | Endoscopic delivery of FMT into duodenum. Three FMTs administered every other day. Stool from one donor. | Incidental resolution of rash associated with atopic dermatitis, with sustained recovery on follow-up examination | Canine atopic dermatitis | [23] |
| 3 | Case report | Atopic dermatitis | 9 people | Two doses of 15 frozen, oral capsulized FMTs. Administered over 2 consecutive days. Stool from one donor. | Significant reduction in SCORAD scores following FMT, no significant change with placebo treatment. | Canine atopic dermatitis | [24] |
| 4 | Single-centre cohort study | Urinary tract infection caused by MDROs | 5 people | At least one FMT, administered either by colonoscopy or oral capsules. Stool from one donor. | FMT application was associated with both the reduced number of MDRO-related urinary tract infections and hospital admission days. | Cainne and feline UTI | [25] |
| 5 | Case report | Sepsis and Diarrhoea | 1 person | Single fresh FMT delivered via nasogastric tube. Stool from one donor. | Successful treatment of a patient with sepsis and severe diarrhoea after a vagotomy. | Sepsis | [26] |
| 6 | Case report | MODS following sepsis | 2 people | Single fresh FMT delivered via nasogastric tube. Stool from one donor. | MODS and severe diarrhoea were alleviated in both patients. | Sepsis | [27] |
| 7 | Randomized clinical trial | Hepatic encephalopathy | 20 people | Single frozen-thawed FMT instilled by rectal enema, retained for 30 min. Stool from one donor. | Significant improvement in PHES and EncephalApp Stroop in the FMT group compared to baseline. No improvement in pre-and post-FMT values among the standard of care arm. | Canine portosystemic shunt, chronic hepatobiliary disease, drug/toxin induced liver injury, feline hepatic lipidosis. | [28] |
| 8 | Case series | Recurrent hepatic encephalopathy | 10 people | Single FMT instilled via colonoscopy. Stool from one donor. | Sustained clinical response in 6 patients as defined by a significant reduction in arterial ammonia level, and improvement in CTP and MELD scores. | Canine portosystemic shunt, chronic hepatobiliary disease, drug/toxin induced liver injury, feline hepatic lipidosis. | [29] |
| 9 | Case report | Epilepsy | 1 person | Multiple fresh FMTs administered by gastroscope. Stool from microbiota bank. | Incidental cure of epilepsy in a patient who received FMT for Crohn’s disease, with maintained response 20-months after withdrawing antiepileptic drugs. | Canine epilepsy. | [30] |
| 10 | Prospective clinical trial | Unstable diabetes mellitus | 14 people | Three washed microbiota transplantations, from frozen-thawed faeces, over 3 days. Instilled into proximal jejunum via gastroendoscopy. Multiple stool donors. | Daily insulin dose and glucose excursions markedly dropped. Glycaemic variability indices significantly improved up to 1 month. | Canine and feline diabetes mellitus. | [31] |
| 11 | Randomised controlled trial | T1DM | 10 people | Three autologous or allogenic faecal transplantations by nasoduodenal tube using freshly produced faeces at 0, 2, and 4 months. | FMT halted the decline in endogenous insulin production in those recently diagnosed with T1DM. | Canine and feline diabetes mellitus. | [32] |
| 12 | Clinical trial | Melanoma | 15 people | Single FMT administered via colonoscopy. Stool from one donor. | FMT overcame primary resistance to anti-PD-1 therapy in a subset of patients with advanced melanoma. | Canine and feline refractory melanoma. | [33] |
| 13 | Single-arm pilot clinical trial | Systemic lupus erythematosus | 20 people | Multiple frozen encapsulated FMTs administered orally for three consecutive weeks (30 capsules per week). | Following FMT there were significant reductions in SLEDAI-2K scores and levels of serum anti-dsDNA antibody. | Canine systemic lupus erythematosus. | [34] |
| 14 | Single-centre, double-blind, randomised, placebo-controlled clinical trial | Chronic Kidney Disease | 28 people | Multiple frozen encapsulated FMTs administered orally for 30 days. 15 capsules every 12 h four times on day 0, 10, and 30 (total 180 capsules). | More patients in the placebo group progressed to chronic kidney disease than the FMT group. The FMT group maintained stable renal function parameters. | Canine and feline chronic kidney disease. | [35] |
| Author | Study Design | Disease/Disorder | Participants | Frequency of FMT | Protocol | Clinical Effects | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Pilot study, single-arm, open-label clinical trial | CAD | 12 dogs | Single | Single fresh FMT delivered orally. | Significant decrease in CADESI scores and PVAS scores following FMT. | [36] |
| 2 | Experimental animal study | Depressive behaviour following chronic unpredictable stress | Mice | Single | Fresh FMTs pooled from wild type and NLRP3 KO donor mice, respectively. Antibiotic-treated mice were orally administered FMTs by oral gavage on 3 consecutive days. | Transplantation of gut microbiota from NLRP3 knock-out mice ameliorated chronic unpredictable stress induced depressive-like behaviours. | [37] |
| 3 | Experimental animal study | Acute SCI | Mice | Multiple | Fresh FMT from healthy uninjured rats administered by oral gavage once a day on the day of injury and for 2 days after. | FMT prevented SCI induced dysbiosis and anxiety-like behaviour. | [38] |
| 4 | Experimental animal study | Obesity | Mice | Single | Frozen FMT from twin mice discordant for obesity. Each FMT was introduced, via a single oral gavage, into a group of 8–9-week-old adult male germ-free C57BL/6J mice. | Increase in total body fat and mass in mice administered FMT from obese donors. | [39] |
| 5 | Experimental animal study | T1DM | Mice | Multiple | Multiple fresh FMTs administered via oral gavage into recipient mice four times over 12 days. | FMT from non-obese diabetic mice induced insulitis in non-obese resistant mice. | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishigaki, A.; Marchesi, J.R.; Previdelli, R.L. Faecal Microbiota Transplantation as an Adjuvant Treatment for Extraintestinal Disorders: Translating Insights from Human Medicine to Veterinary Practice. Vet. Sci. 2025, 12, 541. https://doi.org/10.3390/vetsci12060541
Nishigaki A, Marchesi JR, Previdelli RL. Faecal Microbiota Transplantation as an Adjuvant Treatment for Extraintestinal Disorders: Translating Insights from Human Medicine to Veterinary Practice. Veterinary Sciences. 2025; 12(6):541. https://doi.org/10.3390/vetsci12060541
Chicago/Turabian StyleNishigaki, Alice, Julian R. Marchesi, and Renato L. Previdelli. 2025. "Faecal Microbiota Transplantation as an Adjuvant Treatment for Extraintestinal Disorders: Translating Insights from Human Medicine to Veterinary Practice" Veterinary Sciences 12, no. 6: 541. https://doi.org/10.3390/vetsci12060541
APA StyleNishigaki, A., Marchesi, J. R., & Previdelli, R. L. (2025). Faecal Microbiota Transplantation as an Adjuvant Treatment for Extraintestinal Disorders: Translating Insights from Human Medicine to Veterinary Practice. Veterinary Sciences, 12(6), 541. https://doi.org/10.3390/vetsci12060541






