Simple Summary
This study investigates how farmers’ social and demographic factors influence the spread of Foot and Mouth Disease (FMD) in livestock and its economic impact on smallholder farmers in Indonesia. The goal was to assess the effects of these factors on FMD infection and the financial strain it places on farmers. The findings revealed that FMD altered farmers’ behaviors regarding animal health and increased their treatment costs. This study concluded that factors like economic situation, decision-making roles, and cattle ownership heightened the risk of FMD. It emphasizes the importance of prioritizing high-risk farmers in FMD control efforts to reduce both social and economic burdens.
Abstract
Foot and Mouth Disease (FMD) poses significant challenges to livestock management and agricultural economies worldwide. This study examines the effect of farmers’ sociodemographic factors on livestock infected with Foot and Mouth Disease (FMD) and analyzes its socioeconomic impact on smallholder farmers in Indonesia. This study collected data from 992 households (202 infected and 790 non-infected) in the special region of Yogyakarta province. The research used propensity score matching (PSM) treatment effect analysis to assess the socioeconomic impact of FMD outbreaks on smallholder farmers. Our results demonstrated that FMD significantly increased (p < 0.01) smallholder farmers’ social behavior, including knowledge, attitude, and practice (KAP). Furthermore, farmers whose animals are already infected with FMD must spend an additional IDR 258,000 to IDR 270,000 on treatment compared to non-infected ones. This study provides empirical evidence that farmer characteristics, including women’s decision-making, income, farming group, and cattle ownership, determine the likelihood of FMD infection, which implies that farmers with specific characteristics may heighten the risk of FMD infection. We concluded that FMD has changed social behavior and accelerated economic loss for smallholder farmers. Hence, farmers with animals at risk of FMD infection are prioritized in FMD control programs.
1. Introduction
One of the most socially and economically devastating diseases in the world’s livestock industry is Foot and Mouth Disease (FMD). Southeast Asia (SEA) is one of the regions impacted by the FMD outbreak [1,2]. FMD is endemic to the continental SEA (Vietnam, Myanmar, Thailand, Laos, Peninsular Malaysia, and Cambodia); currently, SEA countries that are free from FMD without the need for vaccination include Brunei Darussalam, Indonesia, Singapore, and the Philippines (Figure 1) [3].
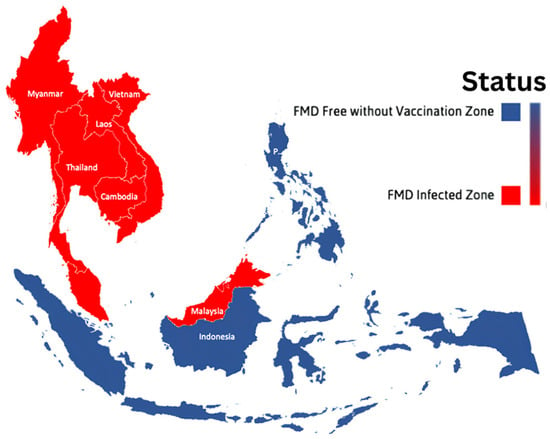
Figure 1.
The distribution of Foot and Mouth Disease (FMD) occurrences throughout southeast Asia.
Nevertheless, an FMD outbreak occurred in cattle on 12 April 2022, in East Java province, Indonesia, which was caused by an Aphthovirus species (family Picornaviridae). On 6 May 2022, the World Organization of Animal Health announced the confirmation of 3496 cases in the provinces of Aceh and East Java, suspending Indonesia’s FMD-free designation as of 12 April 2022 [1,4,5]. As of 3 November 2022, there have been 570,137 cases (morbidity rate = 1.04 percent) among 54,767,135 cattle, 9785 deaths (morbidity rate = 0.02 percent), 12,650 culls, and 5,199,595 vaccinations (Figure 2). FMD primarily affects large ruminants, particularly cattle [6,7]. Following data from [8], 94.27 percent of cattle were infected, including buffalo (4.56 percent), goats (0.80 percent), sheep (0.36 percent), and swine (0.02 percent). Large ruminants are the focus of intensive eradication since they have an enormous prevalence of FMD cases and the highest economic value.
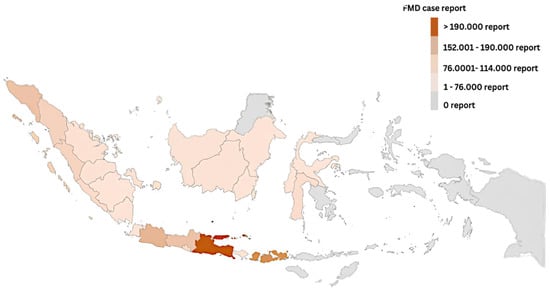
Figure 2.
A map of Indonesia’s FMD-affected areas as of 3 November 2022.
In addition to the re-emergence of FMD in Southeast Asia, the year 2025 marked a significant shift in the global epidemiological landscape of the disease, with confirmed outbreaks reported in several European countries. This development underscores the need for renewed international vigilance and coordinated control efforts, as FMD is no longer confined to traditionally endemic regions. The virus has been detected in areas of Europe typically free from FMD, with Germany recently reporting an outbreak, although it is now FMD-free. However, outbreaks continue in Hungary and Slovakia. In response, the UK has banned imports of meat and dairy products from affected European countries, including Austria, due to the outbreak in Hungary [9].
Historically, FMD was first identified in Indonesia in September 1887 (East Java, Malang), 1892 (Sumatra, East Java), 1902 (Sulawesi), 1906 (Madura, Kalimantan), 1907 (Sulawesi), 1911 (West Nusa Tenggara), 1913 (Madura), 1952, 1956–1958, and 1962 (Bali), 1972–1974, and last in 1983, according to the World Reference Laboratory for Foot and Mouth Disease website http://www.wrlfmd.org (accessed on 12 August 2024). The Indonesian livestock authorities conducted control programs for smallholder farmers to eradicate FMD. These programs included stamping out, controlling livestock movements, disinfecting vehicles, closing infected areas to prevent livestock movement, controlling abattoirs including meat distribution and slaughter, controlling quarantine stations, isolating as well as treating livestock, disinfecting livestock premises, mass vaccinating, and reporting cases. Thus, Indonesia had been declared FMD-free since 1986 [10,11].
Despite a period of almost forty years without a single outbreak of Foot and Mouth Disease, Indonesia was unable to avoid the occurrence of the ailment. The suspected recurrence of FMD can be attributed to (1) inadequate border surveillance. Indonesia, the most extensive archipelagic nation globally (Figure 2), possesses an exceptionally lengthy coastline [12] and (2) poor livestock management practices among smallholder farmers. Nevertheless, smallholder farmers in Indonesia provide more than 90% of the country’s domestic cattle production [13,14,15]; however, the behavior of smallholder farmers is influenced by obsolete knowledge and attitudes [16]. Poor disease knowledge and attitudes associated with disease prevalence can propel underreporting and awareness deficits [17]. Bandura [18] argued that the behaviors of people are shaped by the surrounding society. Therefore, the increase in FMD in Indonesia is presumed to lead to societal changes in farmer behavior, including knowledge, attitudes, and livestock management practices.
Concurrently, the veterinary sector incurs significant financial losses due to productivity declines caused by FMD. The effects refer to the direct negative consequences that hinder livestock production, including reduced weight gains, decreased fertility, and increased mortality, particularly in young animals. Additionally, there are associated costs related to addressing diseases or infections, such as expenses for treatments, vaccinations, movement restrictions, and eradication precautions [19]. Moreover, the primary infectious FMD poses a severe risk to ruminants. Although adult animal mortality is typically low, millions of cattle have been slaughtered in efforts to quickly control and eradicate FMD [20,21,22]. Recent research on the impact of FMD on smallholder farmers has been reported. According to studies conducted in Cambodia, an outbreak of FMD was followed by a loss of 54–92% of the value of animals and a 4.4–11.7% annual decline in household income [23,24]. It corresponds with the FMD-related loss of 22–30% in animal values observed in Laos [25].
Presently, there are insufficient data regarding the societal and financial ramifications of FMD in Indonesia. Moreover, the specific farmer factors that make their livestock more susceptible to FMD remain unidentified. Therefore, this work aspires to fill this gap and aims to examine the farmer characteristics that determine FMD prevalence and analyze the socioeconomic impact of FMD on smallholder cattle farmers. This study provides insights into the behavior alterations in livestock management programs that farmers conduct in response to FMD and estimates of the additional costs that farmers should incur during FMD outbreaks.
2. Materials and Methods
2.1. Data Collection
This study is a survey implementing a cross-sectional design. One-to-one interviews were conducted using a predesigned structured questionnaire. A stratified random sampling technique selected smallholder farmers from four DIY regencies (Gunungkidul, Bantul, Kulon Progo, and Sleman). A farmer who typically possesses three heads of cattle with less than 5000 m2 of land, alongside an average of four family members, is considered a smallholder [26]. All farmers involved in this study focus on production primarily for domestic consumption. The special region of Yogyakarta province on Java Island was chosen as the study area because more than half of the farmers were affected by the FMD outbreak.
Based on the higher cattle population, survey data on cattle farming were collected from each district of every regency. Therefore, areas with higher cattle numbers were surveyed with more respondents. However, there are fewer cases of FMD in the central population (Gunungkidul) due to the assumption that the type of cattle rearing is individual rather than communal (Sleman). Thus, in areas with more FMD cases, more samples were taken with fewer respondents, with 2 to 3 respondents per group of farmers. Figure 3 and Table 1 illustrate the respondents’ distribution by district for each regency. We ensured the representativeness of the sample by considering regional distribution and farmer characteristics. Despite these efforts, potential selection bias could still exist, as certain thresholds and requirements were applied in selecting participants. However, the high number of respondents in this study helped mitigate any such biases, enhancing the robustness of the findings.

Figure 3.
FMD cases and the location of the respondent sample.

Table 1.
Distribution of the respondent sample.
In addition, the Yogyakarta area serves as a livestock traffic area and gateway for cattle trade [27]. The survey was conducted between September and October 2022. A total of 992 respondents were surveyed for the main data who were then categorized into groups according to their exposure to the FMD outbreak (FMD-infected or non-infected animals). The response rate for this study was 99%, with 992 out of 1000 initially contacted farmers completing the survey. The data were grouped into infected and non-infected categories during the pandemic of FMD. The respondents possessed the required permission and were informed regarding the research objective. If the respondents agreed to be interviewed, they were requested to sign the consent letter. The respondents were assured that their information will be kept confidential and used only for research.
2.2. Variable Measurement
The distinct composite variables covered the farmers’ characteristics, farm-associated information, and farmers’ knowledge, attitudes, and practices (Table 2). This study used the KAP model that originates in learning theory [18], as adopted by [28,29]. Knowledge, attitudes, and practices are significant elements in models for behavioral change. Knowledge is the cognitive and non-symbolic experience of meaning that results from information understanding [30,31]. Attitude indicates a positive or negative assessment of an issue [32]. Practice refers to frequent behaviors impacted by broadly recognized social norms and values [33].

Table 2.
Definition of variables.
A knowledge assessment was conducted using a score system consisting of eleven questions related to the disease, prevention, and treatment of FMD. According to [35], the objective knowledge index was established by assigning a score of 1 to each true or false statement answered correctly by the respondent, and a score of 0 to erroneous responses. The social outcomes were then summed and divided into two categories: “less” has a total score of (1–5), and “good” (6–11). A total of six questions were included to assess farmers’ responses to FMD outbreaks, using a 6-point Likert scale to measure their attitudes. A farmer’s attitude was classified as “negative” if a rating (1–3) was given and “positive” otherwise (4–6). Furthermore, equivalent to knowledge, practice is measured using a scoring method with nine behavioral assertions of appropriate disease management programs. Farmers were marked as “less” if they accomplished fewer than four activities and as “good” if they conducted six to nine. The categories “less” and “negative” were then encoded as 0, whereas “good” and “positive” were encoded as 1. Hence, the total KAP ratings were calculated as a variable social impact.
2.3. Statistical Analysis
The statistical analysis in this study was conducted using STATA 14, with the “psmatch2” package. Continuous variables, including farmer characteristics, were compared using nonparametric Wilcoxon tests. To analyze the outcomes, we implemented an econometric model using the Average Treatment on the Treated (ATT) approach, based on the propensity score matching (PSM) method [36,37,38,39,40,41,42]. PSM is particularly advantageous in observational studies because it reduces confounding bias by balancing observed covariates between treatment and control groups, making them more comparable—similar to what randomization achieves in experimental studies [43].
First, propensity scores were calculated using a multivariable logistic regression model. The dependent variable in this model was the group classification (FMD-infected vs. non-infected), and the independent variables were baseline farmer characteristics that were unbalanced between groups, including age, education, household size, land size, women’s involvement in decision-making, income, farmer group, cattle ownership, farming system, and farming experience.
Second, to ensure the propensity scores were within the common support area, we excluded outliers based on the score distribution of both groups. A matching test was then performed to confirm that the averages of each covariate and the propensity score did not significantly differ between the treated and untreated groups [42]. As noted in prior research [26], this step prevents bias that could arise from linking distinct treatment groups (infected vs. non-infected) and ensures more accurate matching.
The third step involved matching farmers with FMD-infected animals to farmers with non-infected animals based on their propensity scores, which represent the likelihood of FMD infection given their characteristics [40,42]. Finally, the mean outcome difference between the two paired groups was calculated to estimate the social and economic impacts of FMD outbreaks on smallholder farmers. In addition, the ATT values derived through PSM also enabled us to assess behavioral changes in livestock management practices using the KAP (Knowledge, Attitude, and Practice) score and estimate additional cost differences between treated and untreated groups due to the FMD outbreak [40,42].
Rosenbaum and Rubin [40] first introduced the concept of the propensity score, defined as the likelihood of receiving treatment given the pre-treatment parameters. The treatment impact analysis based on PSM for FMD can be mathematically expressed as follows:
where D = {1, 0} is a binary treatment indicator, 1 represents farmers with FMD-infected animals (treated group), and 0 represents farmers without FMD-infected animals (control group). The variable X denotes the multidimensional vector of pre-treatment characteristics. If the treatment assignment is randomized within cells defined by X, then it is also randomized within cells defined by the propensity score p(X) [40]. Consequently, assuming a population of units represented by i, the average treatment effect on the treated (ATT) can be estimated as follows:
where Y1i represents the outcome if farmer iii’s animal was infected by FMD, and Y0i represents the outcome if their animal was not infected. The ATT measures the difference in outcomes between treated and control groups, calculated from the distribution of participants’ propensity scores, assuming conditional independence and sufficient overlap in p(Xi). To avoid sampling bias, we discard observations where the propensity scores fall outside the common support range, i.e., the highest propensity score in the treated group and the lowest propensity score in the control group. Matching estimates are only valid if matching tests confirm that the propensity score distributions of both groups are comparable and that the average of each covariate and propensity score does not significantly differ between groups. If the common support condition is met, matching can proceed without introducing bias [36].
To address the challenge of uncertainty in estimating the ATT with continuous propensity scores, several methodologies have been proposed in the literature. The most commonly used techniques include Nearest Neighbor Matching (NNM), Radius Matching (RM), Kernel Matching (KM), and Stratification Matching (SM). SM divides the range of the propensity score into intervals, ensuring that treatment and control units within each interval have similar propensity scores. However, SM may discard observations from blocks with no matching control units, whereas NNM ensures that each treated unit finds a match by comparing it to the nearest untreated unit in terms of propensity score. According to Becker and Ichino [37], NNM compares each treated subject to the untreated subject with the closest propensity score, providing robust estimations of the treatment effect.
3. Results
As demonstrated in Table 3, the farmers with animals infected with FMD and those with non-infected animals had distinct mean scores for various features. For instance, the formal education level of farmers with animals infected with FMD is much higher than that of farmers with non-infected animals. Additionally, those affected with FMD are more likely to have a smaller family size with subsidiary involvement from women. Considering other farm characteristics, farmers with animals afflicted with FMD have an IDR 570,642.230 higher household income and a TLU of cattle higher than 0.19 than farmers with non-infected animals. With communal cattle rearing, there were more cases of FMD than individual livestock sheds. Table 3 provides further details about the participants in this study. For example, the median age of the respondents was 54.10. The average formal education level was 8.53 years, exhibiting that the majority of farmers in Indonesia have not completed nine years of elementary education. Some responders have never been to a formal educational institution. The average farm household comprised 3.75 members, 1.27 TLU of cattle, 1 to 2 heads, and 513 m2 of land tenure. Most respondents are farmers with 20 years of experience in cattle fattening practices. It has been nearly two generations since the last incidence of FMD in Indonesia.

Table 3.
Descriptive statistics of farmer characteristics.
This study was conducted one month after the peak number of FMD cases (July 2022); therefore, most farmers have good knowledge, attitudes, and practices about FMD (Table 4). Animal healthcare workers have implemented disease management programs through disease socialization, vaccination, and cattle movement control. However, over half of all responders seem to have inadequate knowledge of FMD (Table 5). Most farmers are still not advised by officers. In contrast, most farmers have a positive attitude and value-based practices toward the FMD eradication program. Theoretically, this is because farmers have witnessed and suffered the losses caused by FMD. The losses incurred by FMD represent a significant portion of the rising costs associated with the prevention and control of FMD-infected cattle. According to Table 5, the average treatment cost per animal is IDR 316,548, while the average prevention cost is IDR 41,162. Prior to matching, the average prevention cost was IDR 41,778, while the treatment cost per animal remained unchanged. Furthermore, if the livestock cannot be rescued, the loss might escalate.

Table 4.
Frequency table for KAP score answers for smallholder farmers relating to FMD.

Table 5.
Cross-tabulation of farmers’ socioeconomic conditions by FMD prevalence.
Table 6 presents the estimation of propensity scores using the Logit model (Equation (1)) to predict the likelihood of FMD infection. The resulting propensity score distribution for both infected and non-infected groups shows a common support region between 0.03079 and 0.64891, with an average score of 0.209 (Std. Dev = 0.136). Observations with propensity scores outside this common support range were excluded from the analysis. The results indicate that certain characteristics of smallholder farmers, such as inadequate land for cattle raising, are closely associated with the prevalence of FMD infection. Specifically, farmers who use cattle sheds with limited space are more vulnerable to FMD exposure, as virus-carrying agents can more easily spread in confined environments [44].

Table 6.
Logit model results of sociodemographic determinants influencing the probability of FMD outbreaks.
Most smallholder agricultural laborers are family based, typically consisting of a husband, wife, and their children. In this setup, the husband primarily works on the farm, while the wife occasionally assists with livestock care. As a result, farmers who involve women in the livestock-raising process tend to have a lower risk of FMD infection. Additionally, income from livestock sales is closely linked to the number of cattle a farmer owns. However, farmers with larger herds are more vulnerable to FMD, as increased movement of people in and out of the pen, often related to the sale or purchase of livestock, heightens the risk of disease transmission. A similar risk is observed in farmer cooperatives with communal pens. Due to the lack of control over animal movement, these pens are more susceptible to FMD. If one animal contracts the disease, it can quickly spread to the others. This suggests that specific characteristics of farmers, such as herd size and involvement in communal pens, may increase the susceptibility of their animals to FMD infection. Therefore, farmers at higher risk should be prioritized in FMD control programs.
We conducted several analyses to assess the presence of common support in both groups, ensuring the compatibility of the balancing test with the Logit model and verifying the reliability of the common support [26]. The results of the balance assessment using the NNM technique, presented in Table 7, indicate no statistically significant differences in the mean values of the independent variables between the two groups. This suggests that characteristics such as age, education, household size, women’s involvement in decision-making, land size, income, cattle ownership, farmer group, farming system type, and farming experience are balanced between farmers with FMD-infected animals and farmers with non-infected animals (Table 7). After confirming the equal distribution of the uncontrolled and control groups based on these variables (additional information related to residual balances, S1, Figure S1; S2, Figure S2; S3, Table S1), we proceeded to assess the ATT using the PSM method (Table 8). This allowed us to estimate the social and economic impacts of FMD outbreaks, including changes in livestock management practices and additional costs. The impact was measured using several alternative estimators, including SM, NNM, RM, and KM. A t-test was used as a control to illustrate the effects of FMD outbreaks before and after matching.

Table 7.
Conformity evaluation of the common supports after matching.

Table 8.
The socioeconomic impact of FMD on smallholder farmers.
This study’s findings indicate that FMD outbreaks can alter farmers’ social behaviors, including knowledge, attitudes, and practice, and lead to extra expenses (Table 8). Farmers exposed to FMD compared with those without FMD exposure possess notable social and economic value differences. Farmers whose livestock are impacted by FMD are typically rated 1.4 to 1.9 points higher than those whose animals are unaffected. In addition, the costs borne by farmers affected by FMD exceed IDR 258,000 and IDR 270,000. This indicates that FMD has a social and economic impact on smallholder farmers. Additionally, a summary of the economic impact of FMD in selected countries is presented in Table 9.

Table 9.
The economic impact of FMD in selected countries.
4. Discussion
4.1. Livestock Management Practices in Response to FMD Prevalence
In general, farmers have limited knowledge of FMD (Table 5) in the studied area. As there had been no outbreaks of FMD for the past 36 years, farmers lack knowledge about the disease. This is further confirmed by the average livestock-raising experience of farmers, which is approximately 20.26 years (Table 4). Hence, farmer households lack experience in managing FMD outbreaks. This study was conducted during the FMD outbreak when the government incessantly conducted disease prevention socialization [52]. Therefore, some farmers may possess knowledge related to FMD outbreaks. Nevertheless, most farmer households are unaware of the risk of FMD transmission through the movement of farmers, animal products, and livestock equipment contaminated with disease outbreaks (Table 4).
Attitude is a readiness to respond positively or negatively to an object. The attitudes of farmer households are a response to the prevention, handling, and control of animal disease outbreaks [53]. Farmer households in this study demonstrated a positive attitude to FMD outbreak responses (Table 5). This attitude represents practicing proper biosecurity, getting vaccinated against diseases, seeking treatment, and consuming animal carcasses that have been afflicted by FMD outbreaks (Table 4). Practice is the implementation of the prevention, handling, and control of animal disease outbreaks. These practices include handling sick animals, products from infected animals, and carcasses of infected animals [54]. In this study, the farmers’ household practices were good overall. It can control FMD outbreaks and restrict widespread disease occurrence. Prevention practices are illustrated by many farmers participating in the government’s vaccination program and limiting access in and out of farms (Table 4). It is correlated to the farmers in Cambodia who will modify their disease management strategies by engaging in vaccination programs [55].
The average treatment costs per animal in the infected group were IDR 316,548, with the most considerable cost of treatment during infected livestock with an average of IDR 180,547. The cost of the treatment is not much different from farmers in Uganda, with a cost of USD 19 or around IDR 293,588 [50]. In contrast, the average treatment costs per animal in the non-infected group were IDR 41,162, with the most significant number for doctor’s examinations with an average of IDR 24,922.28.
4.2. Farmer Characteristics as Determinants of FMD Prevalence
The estimation results (Table 5) indicate that the socioeconomic characteristics of farmers are strongly related to the risk of infection from strategic infectious animal diseases. In particular, land size affects the livestock infection rate. This finding also confirms that small land size causes livestock population density, increasing the risk factor of livestock being infected with the disease. The land size also impacts the proximity of farmers’ cages to other breeders, which means that transmission can more easily occur [56]. The close proximity of farmers’ cages is due to farmers being members of livestock groups. As a result of high farm density and reliance on community grazing, smallholder systems are troublesome due to their multiple inter-farm connections.
Moreover, farmers may have fewer clear incentives to combat the disease. Vaccinating smallholdings has logistical challenges in achieving high coverage, particularly in countries with less proficient veterinary services that lack the capacity to enforce standards and provide compensation. Consequently, there is a requirement for FMD control in smallholder systems [19]. If achieving universal control is not feasible, farmers desiring to manage FMD should be supported through the provision of high-quality vaccinations (in case doubts arise about their effectiveness, vaccine acceptance may be limited) and by mitigating the harm caused by individuals not participating in FMD management. Governments in certain nations subsidize vaccinations for small farms. Although not yet a possibility, commodity-based commerce would give businesses access to affluent markets without the massive obstacle of establishing national or zonal independence [55].
The findings confirm women’s role in strategic infectious animal control in livestock businesses. The role of women contributes to the decision-making process related to livestock rearing and the costs of running a livestock business [57]. The role of women in the livestock industry is access to, control over, and advantages over available resources. In accessing information sources, they have less access than men. This is because women are restricted in mobility by their partners [58]. In the control aspect, the role of men dominates in the livestock business, but in its implementation, it still involves women as laborers and influences the livestock business that is practiced. Regarding benefits, the livestock business activities carried out benefit all family members [59]. The majority of smallholder farmers use their livestock as a source of income and a depository for their assets, which are frequently managed in conjunction with other means of subsistence [60]. Increased livestock ownership increases the likelihood of cattle infection, particularly infectious illnesses such as FMD. If cattle become contaminated with FMDV, non-susceptible animals pose a danger of FMD transmission. The risk of contamination is greater close to infected buildings. The presence of FMD on a property may be identified (“infected premises”) or yet, undetected [44].
4.3. Impact of FMD on Farmers’ Socioeconomic Conditions
The results (Table 8) demonstrate that the average impact of FMD on socioeconomics is almost identical and significant at one percent with the SM, NNM, RM, and KM methods. The outbreak of FMD can have social consequences that are evident at the individual, household, and community levels. The social impacts on farmer households have been demonstrated through the KAP approach (Table 3). FMD can significantly impact the social knowledge, attitudes, and practices of breeders. FMD is an extremely infectious viral illness that impacts animals with divided hooves, like cattle, resulting in substantial losses in livestock productivity and trade. As a result, breeders may experience economic losses, leading to changes in their attitudes toward breeding and animal husbandry practices. They may also be forced to adopt new measures to prevent the spread of FMD, such as increased biosecurity measures, leading to changes in their knowledge and practices. Moreover, FMD outbreaks may result in changes in public attitudes toward livestock farming and consumption, potentially affecting the breeders’ reputation and ability to sell their products [55].
The impact of FMD on the per capita expenditure of farmers for treatment costs ranges from IDR 258,000 to IDR 270,000 more than farmers with non-infected animals. In absolute terms, the SM method shows the most significant impact (IDR 270,000), and the RM method shows the most negligible impact (IDR 258,000) per capita expenditure. FMD can have a considerable impact on medical expenses. It can include medical expenses and biosecurity improvement costs. The economic losses caused by FMD outbreaks can also affect farmers’ ability to pay for medical expenses. Therefore, the control and prevention of FMD are essential to reduce medical expenses and maintain the health and productivity of farm animals. Since euthanasia of FMD-positive animals is not a mandatory policy, animals that survive FMD may still be sold, contingent upon a thorough veterinary assessment prior to slaughter. Therefore, the costs incurred by farmers for depopulation require government attention and support. The government, through its extension officers, can support farmers by providing education on disease management, assisting with the isolation of infected livestock, and offering compensation for animals that succumb to the disease [43,61,62,63]. The Government of Indonesia regulates this in ministerial decree no. 518/KPTS/PK.300/M/7/2022, which consists of instructions for the depopulation of healthy animals, sick animals, suspected illnesses, or animals carrying FMD [64]. These measures include regular veterinary inspections, vaccination campaigns, and quarantine protocols to prevent the spread of diseases. The government has stipulated the amount of assistance in an oral and nail disease emergency in decree no. 08048/KPTS/PK.300/F/07/2022, which states that farmers with cattle affected by FMD that die or are euthanized will be given cash of IDR 10,000,000. This assistance is limited to five animals per ownership [65].
Table 9 outlines the economic impact of Foot and Mouth Disease (FMD) across various countries, emphasizing the broader implications of the disease. In regions with endemic FMD, the disease significantly disrupts production, trade, and control measures, with the poorest populations suffering the most due to limited market access. In countries like Ethiopia, India, and Uganda, smallholder farmers experience heightened vulnerability, as the disease impacts milk production, livestock health, and market value, often leading to financial distress. The social and psychological effects are particularly severe in countries with intensive farming systems, such as India. While some nations, like Nigeria, benefit from effective control measures that provide a high return on investment, the overall economic burden remains substantial. In developed countries like Australia, the impact of FMD is largely due to trade disruptions and production losses, which can be exacerbated by trade sanctions. These findings underscore the need for targeted FMD control strategies, particularly for smallholders in developing regions, to mitigate economic losses and enhance food security.
The increasing production costs, particularly the treatment costs associated with FMD outbreaks, can have significant broader implications for cattle farmers’ household income and food security. FMD treatment typically involves direct costs for veterinary services, medications, and potentially the culling of infected animals, all of which place a financial strain on farmers, especially those with limited resources [66,67]. For smallholder cattle farmers, who often rely on livestock as their primary source of income, the economic impact of FMD can be severe. The direct financial burden of treatment can deplete savings or reduce farmers’ ability to invest in other necessary areas, such as improving farm infrastructure or purchasing additional livestock. Moreover, the loss of cattle, either due to disease or culling, directly impacts household income, reducing the farmer’s capacity to meet daily living expenses or reinvest in their farming operations [68].
In terms of food security, FMD outbreaks can disrupt the supply of meat, which is a crucial source of nutrition in rural areas. With the loss of livestock and the high costs associated with treating infected animals, farmers may be forced to sell remaining cattle at lower prices, further compromising their financial stability [69]. This can lead to a reduced ability to provide for the household’s nutritional needs, exacerbating food insecurity [70]. Thus, the increased treatment costs associated with FMD outbreaks not only threaten the financial stability of cattle farmers but also have long-term consequences for household income and food security, making it critical for policymakers to address both the economic and social dimensions of FMD prevention management.
Our findings have significant policy implications for Indonesia and globally, given that animals, equipment, transportation, and human movement can transmit the FMD virus. Implementing effective FMD mitigation strategies through comprehensive risk management is crucial. This approach involves several key actions, such as controlling animals as a source of disease (reservoir), identifying high-risk areas for virus outbreaks, and addressing risky practices that facilitate virus transmission [71,72,73]. Specifically, targeted control measures could involve providing additional resources and training to high-risk farmers, particularly those with large herds or in communal farming environments. These farmers could benefit from focused educational campaigns on biosecurity practices, vaccination protocols, and disease surveillance to minimize the risk of infection.
Further, the establishment of a robust monitoring system to track livestock movements and animal health status would help identify areas most vulnerable to outbreaks [71,73]. Closing affected areas to the movement of animals, products, and equipment is another practical measure to prevent the spread of the virus. In this context, local authorities could implement checkpoints for livestock transportation, ensuring that only disease-free animals are allowed to enter or leave designated outbreak zones. Moreover, previous studies have highlighted that cattle farmers in Yogyakarta are particularly susceptible to FMD risks [74]. To mitigate these risks, targeted support, such as financial assistance for subsidies for vaccines or veterinary services, could help high-risk farmers better manage the costs associated with FMD prevention.
Overall, our results suggest that FMD outbreaks have impacted the socioeconomic conditions of smallholder cattle farmers in Yogyakarta. After nearly four decades, the re-emergence of FMD cases in Indonesia has impacted the social and economic aspects of livestock farming [75,76,77,78]. The impact of FMD disease extends beyond the scope of animal health to include socioeconomic factors. Farmers impacted by FMD incur significant losses, including increased costs for livestock treatment, decreased livestock production, declining livestock prices, and high livestock mortality rates [78].
5. Conclusions
The findings of this study are highly relevant not only for Indonesia but also in a broader international context—particularly considering that Foot and Mouth Disease (FMD) will reappear in Europe in 2025. This unexpected resurgence in a region previously considered free from FMD highlights how transboundary animal diseases remain a global threat. It also emphasizes the importance of understanding how farmers in different parts of the world experience and respond to such outbreaks, especially those operating in vulnerable, small-scale farming systems.
In our work, we explored the key characteristics that influence a farmer’s likelihood of experiencing an FMD outbreak and assessed how the disease affects the social and economic realities of smallholder cattle farmers. We found that several farmer-specific factors, such as household income, participation in farming groups, the number of cattle owned, and particularly the involvement of women in decision-making are closely linked to the risk of FMD infection. This suggests that some farmers, due to their socioeconomic conditions, face a higher vulnerability to the disease. Beyond identifying these risk factors, our results show that FMD significantly changes farmers’ behaviors and increases their financial burden. Social behaviors, specifically knowledge, attitudes, and practices related to animal health, improved significantly among farmers with FMD-infected animals, likely as a reaction to the crisis. Economically, those affected by FMD had to spend between IDR 258,000 and IDR 270,000 more per animal on treatment compared to their non-affected peers. Based on these insights, we recommend several targeted policy measures: (1) FMD control and prevention strategies should be tailored for high-risk farmers, especially those with limited land, greater herd sizes, higher incomes, or those who practice communal cattle rearing. These strategies should include improved training, tailored vaccination plans, and access to subsidized disinfection resources. (2) Treatment-related financial pressures could be eased through compensation schemes that more accurately reflect real losses and through the creation of emergency funds or microinsurance products designed for smallholders. (3) More effective education programs are needed to address knowledge gaps. (4) We suggest that the positive role women play in reducing FMD risk should be encouraged and supported through gender-sensitive training, peer networks, and inclusive extension services. Together, these actions could not only strengthen Indonesia’s ability to manage FMD but also provide valuable guidance for other regions. Supporting the resilience of smallholder farmers is not just a local priority but a global necessity in the face of re-emerging animal health threats.
In addition, this study has several limitations that should be noted. The findings are based on a specific region, which may not fully represent other areas with different socioeconomic conditions. Additionally, this study focuses on short-term impacts, while the long-term effects on livelihoods and local economies remain underexplored. Future research should expand geographically, incorporate longitudinal data, and examine the broader socioeconomic consequences, including food security and community well-being. Moreover, while this study highlights the role of women in managing FMD risks, further research on gender-sensitive interventions and their effectiveness in diverse contexts is needed. This will contribute to a more comprehensive understanding of FMD’s long-term impacts and inform more effective policy and intervention strategies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/vetsci12060542/s1, S1. Figure S1: Covariate distributions for infected and non-infected groups before and after matching; S2. Figure S2: Propensity score distribution overlap for treated and control groups; S3, Table S1: Balance statistics for covariates before and after matching.
Author Contributions
Conceptualization, A.T., B.G., S.K. and R.A.; methodology, A.T. and B.G.; software, A.T.; validation, B.G.; formal analysis, A.T.; investigation, A.T.; resources, A.T.; data curation, B.G., P.S., M.A.U.M. and S.K.; writing—original draft preparation, A.T.; writing—review and editing, B.G., P.S., M.A.U.M., R.A. and S.K.; visualization, A.T.; supervision, B.G., P.S., M.A.U.M. and S.K.; project administration, A.T.; funding acquisition, A.T., B.G., R.A. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
The project received financial support from the Matching Fund Kedai Reka, Ministry of Education, Culture, Research, and Technology, Indonesia, in 2022. This funding facilitated the collaboration between the Faculty of Animal Science, Universitas Gadjah Mada, and Ternaknesia Farm Innovation Ltd. under Partnership Agreement (number 3257.1/J01.1.25/KS/2022 and 094/SRE-PKS/TN/VII/2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All participants gave their informed consent after receiving a thorough explanation of this study’s objectives and procedures.
Data Availability Statement
The data are contained within this article and Supplementary Materials.
Acknowledgments
The authors acknowledge the help and support of Nguyen Hoang Qui, Indra Wahyu Pratama, and students of the Department of Livestock Socio-economics for conducting data collection and providing insightful comments. This study is supported by the University of Debrecen Scientific Research Bridging Fund (DETKA) and the University of Debrecen Program for Scientific Publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Organization for Animal Health Official Disease Status. Available online: https://www.woah.org/en/what-we-do/animal-health-and-welfare/official-disease-status/ (accessed on 27 November 2022).
- González Gordon, L.; Porphyre, T.; Muhanguzi, D.; Muwonge, A.; Boden, L.; Bronsvoort, B.M.d.C. A Scoping Review of Foot-and-mouth Disease Risk, Based on Spatial and Spatio-temporal Analysis of Outbreaks in Endemic Settings. Transbound. Emerg. Dis. 2022, 69, 3198–3215. [Google Scholar] [CrossRef] [PubMed]
- Blacksell, S.D.; Siengsanan-Lamont, J.; Kamolsiripichaiporn, S.; Gleeson, L.J.; Windsor, P.A. A History of FMD Research and Control Programmes in Southeast Asia: Lessons from the Past Informing the Future. Epidemiol. Infect. 2019, 147, e171. [Google Scholar] [CrossRef] [PubMed]
- Nason, J. Foot and Mouth Disease Reported in Indonesia. Beef Central. Available online: https://www.beefcentral.com/news/foot-and-mouth-disease-outbreak-reported-in-indonesia/ (accessed on 27 November 2022).
- Chen, R.; Gardiner, E.; Quigley, A. Foot and Mouth Disease Outbreak in Indonesia: Summary and Implications. Glob. Biosecurity 2022, 4, 175. [Google Scholar] [CrossRef]
- Feng, B.; Gao, J. AnthraxKP: A Knowledge Graph-Based, Anthrax Knowledge Portal Mined from Biomedical Literature. Database 2022, 2022, baac037. [Google Scholar] [CrossRef]
- Sieng, S.; Patrick, I.W.; Walkden-Brown, S.W.; Sar, C. A Cost-benefit Analysis of Foot and Mouth Disease Control Program for Smallholder Cattle Farmers in Cambodia. Transbound. Emerg. Dis. 2022, 69, 2126–2139. [Google Scholar] [CrossRef]
- Ministry of Agriculture Republic Indonesia FMD Infected Map. Available online: https://crisiscenterpmk.ditjenpkh.pertanian.go.id/peta-terdampak-pmk/?l=en (accessed on 27 November 2022).
- United Nations FAO Calls for Action amid Foot-and-Mouth Disease Outbreaks. Available online: https://news.un.org/en/story/2025/05/1162896 (accessed on 17 May 2025).
- Soehadji, M.M.; Setyaningsih, H. The Experience of Indonesia in the Control and Eradication of Foot-and-Mouth Disease. In Proceedings of the Diagnosis and Epidemiology of Foot-and-Mouth Disease in Southeast Asia: Proceedings of an International Workshop, Lampang, Thailand, 6–9 September 1993; Australian Centre for International Agricultural Research Proceedings: Canberra, Australia, 1994. [Google Scholar]
- Windsor, P. Contribution of Australian Veterinarians to the Eradication and Control of FMD in Southeast Asia. In Proceedings of the Australian Veterinary History Record, Brisbane, Australia, 24–29 May 2015. [Google Scholar]
- Afriansyah, A.; Darmawan, A.R.; Pramudianto, A. Enforcing Law in Undelimited Maritime Areas: Indonesian Border Experience. Int. J. Mar. Coast. Law 2022, 37, 282–299. [Google Scholar] [CrossRef]
- Lestari, V.S.; Sirajuddin, S.N.; Asnawi, A. Biosecurity Adoption on Cattle Farms in Indonesia. Eur. J. Sustain. Dev. 2014, 3, 403–408. [Google Scholar] [CrossRef]
- Widiati, R. Developing Beef Cattle Industry at Smallholders to Support Beef Self-Sufficiency. Indones. Bull. Anim. Vet. Sci. 2015, 24, 191–200. [Google Scholar] [CrossRef]
- Iyai, D.A.; Nurhayati, D.; Pakage, S.; Widayati, I. Impact of Conventional Cattle Farming Systems on Farmer Awareness, Livestock Output and Household Income. J. Ilmu Produksi Dan Teknol. Has. Peternak. 2020, 8, 144–150. [Google Scholar] [CrossRef]
- Dernburg, A.R.; Fabre, J.; Philippe, S.; Sulpice, P.; Calavas, D. A Study of the Knowledge, Attitudes, and Behaviors of French Dairy Farmers Toward the Farm Register. J. Dairy Sci. 2007, 90, 1767–1774. [Google Scholar] [CrossRef]
- Govindaraj, G.; Nagalingam, M.; Nethrayini, K.; Shalini, R.; Shome, R.; Bambal, R.; Sairiwal, L.; Rahman, H. Assessment of Brucellosis Knowledge, Attitude and Practice among Veterinarians in India. J. Exp. Biol. Agric. Sci. 2016, 4, S83–S94. [Google Scholar] [CrossRef]
- Bandura, A. Social Learning Theories; Prentice Hall: Hoboken, NJ, USA, 1976. [Google Scholar]
- Knight-Jones, T.J.D.; Rushton, J. The Economic Impacts of Foot and Mouth Disease—What Are They, How Big Are They and Where Do They Occur? Prev. Vet. Med. 2013, 112, 161–173. [Google Scholar] [CrossRef]
- Rodriguez, L.L.; Gay, C.G. Development of Vaccines toward the Global Control and Eradication of Foot-and-Mouth Disease. Expert. Rev. Vaccines 2011, 10, 377–387. [Google Scholar] [CrossRef]
- Verma, A.K.; Pal, B.C.; Singh, C.P.; Jain, U.; Yadav, S.K.; Mahima, M. Studies of the Outbreaks of Foot and Mouth Disease in Uttar Pradesh, India, Between 2000 and 2006. Asian J. Epidemiol. 2010, 3, 141–147. [Google Scholar] [CrossRef]
- Onono, J.O.; Wieland, B.; Rushton, J. Constraints to Cattle Production in a Semiarid Pastoral System in Kenya. Trop. Anim. Health Prod. 2013, 45, 1415–1422. [Google Scholar] [CrossRef]
- Shankar, B.; Morzaria, S.; Fiorucci, A.; Hak, M. Animal Disease and Livestock-Keeper Livelihoods in Southern Cambodia. Int. Dev. Plan. Rev. 2012, 34, 39–63. [Google Scholar] [CrossRef]
- Young, J.R.; Suon, S.; Andrews, C.J.; Henry, L.A.; Windsor, P.A. Assessment of Financial Impact of Foot and Mouth Disease on Smallholder Cattle Farmers in Southern Cambodia. Transbound. Emerg. Dis. 2013, 60, 166–174. [Google Scholar] [CrossRef]
- Rast, L.; Windsor, P.A.; Khounsy, S. Limiting the Impacts of Foot and Mouth Disease in Large Ruminants in Northern Lao People’s Democratic Republic by Vaccination: A Case Study. Transbound. Emerg. Dis. 2010, 57, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.R.S.; Liu, Z.; Lund, M. The Impact of Biogas Technology Adoption for Farm Households—Empirical Evidence from Mixed Crop and Livestock Farming Systems in Indonesia. Renew. Sustain. Energy Rev. 2017, 74, 1371–1378. [Google Scholar] [CrossRef]
- Martindah, E. Risk Factors, Attitude and Knowledge of Farmers in Controlling Anthrax. Indones. Bull. Anim. Vet. Sci. 2018, 27, 135. [Google Scholar] [CrossRef][Green Version]
- Nguyen, T.P.L.; Seddaiu, G.; Roggero, P.P. Declarative or Procedural Knowledge? Knowledge for Enhancing Farmers’ Mitigation and Adaptation Behaviour to Climate Change. J. Rural. Stud. 2019, 67, 46–56. [Google Scholar] [CrossRef]
- Liao, X.; Nguyen, T.P.L.; Sasaki, N. Use of the Knowledge, Attitude, and Practice (KAP) Model to Examine Sustainable Agriculture in Thailand. Reg. Sustain. 2022, 3, 41–52. [Google Scholar] [CrossRef]
- Wessman, W.L. The Nature of Thought: Maturity of Mind; University Press of America: New York, NY, USA, 2006. [Google Scholar]
- Hulme, M. “Gaps” in Climate Change Knowledge. Environ. Humanit. 2018, 10, 330–337. [Google Scholar] [CrossRef]
- Ajzen, I.; Fishbein, M. Attitudes and the Attitude-Behavior Relation: Reasoned and Automatic Processes. Eur. Rev. Soc. Psychol. 2000, 11, 1–33. [Google Scholar] [CrossRef]
- Bourdieu, P. The Logic of Practice; Stanford University Press: Redwood City, CA, USA, 1990. [Google Scholar]
- Abera, A.; Yirgu, T.; Uncha, A. Determinants of Rural Livelihood Diversification Strategies among Chewaka Resettlers’ Communities of Southwestern Ethiopia. Agric. Food Secur. 2021, 10, 30. [Google Scholar] [CrossRef]
- House, R.J.; Hanges, P.J.; Javidan, M.; Dorfman, P.W.; Gupta, V. Culture, Leadership, and Organizations. The Globe Study of 62 Societies; Sage Publications: London, UK, 2004. [Google Scholar]
- Khandker, S.; Koolwal, G.B.; Samad, H. Handbook on Impact Evaluation; The World Bank: Washington, DC, USA, 2009; ISBN 978-0-8213-8028-4. [Google Scholar]
- Becker, S.O.; Ichino, A. Estimation of Average Treatment Effects Based on Propensity Scores. Stata J. 2002, 2, 358–377. [Google Scholar] [CrossRef]
- Caliendo, M.; Kopeinig, S. Some Practical Guidance for the Implementation of Propensity Score Matching. J. Econ. Surv. 2008, 22, 31–72. [Google Scholar] [CrossRef]
- Imbens, G.W.; Rubin, D.B. Causal Inference for Statistics, Social, and Biomedical Sciences; Cambridge University Press: Cambridge, UK, 2015; ISBN 9780521885881. [Google Scholar]
- Rosenbaum, P.R.; Rubin, D.B. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Stuart, E.A. Matching Methods for Causal Inference: A Review and a Look Forward. Stat. Sci. 2010, 25, 1. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Dehejia, R.H.; Wahba, S. Propensity Score-Matching Methods for Nonexperimental Causal Studies. Rev. Econ. Stat. 2002, 84, 151–161. [Google Scholar] [CrossRef]
- Auty, H.; Mellor, D.; Gunn, G.; Boden, L.A. The Risk of Foot and Mouth Disease Transmission Posed by Public Access to the Countryside During an Outbreak. Front. Vet. Sci. 2019, 6, 381. [Google Scholar] [CrossRef] [PubMed]
- Sougou, N.M.; Bassoum, O.; Faye, A.; Leye, M.M.M. Women’s Autonomy in Health Decision-Making and Its Effect on Access to Family Planning Services in Senegal in 2017: A Propensity Score Analysis. BMC Public Health 2020, 20, 872. [Google Scholar] [CrossRef]
- Rasmussen, P.; Shaw, A.P.; Jemberu, W.T.; Knight-Jones, T.; Conrady, B.; Apenteng, O.O.; Cheng, Y.; Muñoz, V.; Rushton, J.; Torgerson, P.R. Economic Losses Due to Foot-and-Mouth Disease (FMD) in Ethiopian Cattle. Prev. Vet. Med. 2024, 230, 106276. [Google Scholar] [CrossRef]
- Govindaraj, G.; Ganeshkumar, B.; Nethrayini, K.R.; Shalini, R.; Balamurugan, V.; Pattnaik, B.; Rahman, H. Farm Community Impacts of Foot-and-Mouth Disease Outbreaks in Cattle and Buffaloes in Karnataka State, India. Transbound. Emerg. Dis. 2017, 64, 849–860. [Google Scholar] [CrossRef]
- Alhaji, N.B.; Amin, J.; Aliyu, M.B.; Mohammad, B.; Babalobi, O.O.; Wungak, Y.; Odetokun, I.A. Economic Impact Assessment of Foot-and-Mouth Disease Burden and Control in Pastoral Local Dairy Cattle Production Systems in Northern Nigeria: A Cross-Sectional Survey. Prev. Vet. Med. 2020, 177, 104974. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, G. The Economic Impacts of a Hypothetical Foot and Mouth Disease Outbreak in Australia. Aust. J. Agric. Resour. Econ. 2024, 68, 23–43. [Google Scholar] [CrossRef]
- Baluka, S.A. Economic Effects of Foot and Mouth Disease Outbreaks along the Cattle Marketing Chain in Uganda. Vet. World 2016, 9, 544–553. [Google Scholar] [CrossRef]
- Mohamad, A.; Shaari, N.F.; Hamzah, H.Z. Impact of Foot and Mouth Disease on Cattle Production in Peninsular Malaysia. J. Sustain. Sci. Manag. 2023, 18, 143–150. [Google Scholar] [CrossRef]
- National Disaster Management Authority Penanganan PMK: Biosecurity Untuk Perlindungan Ternak Dan Kesehatan Masyarakat di Wilayah Yogyakarta. Available online: https://bnpb.go.id/berita/penanganan-pmk-biosecurity-untuk-perlindungan-ternak-dan-kesehatan-masyarakat-di-wilayah-yogyakarta (accessed on 6 February 2023).
- Dutta, P.K.; Biswas, H.; Ahmed, J.U.; Shakif-Ul-Azam, M.; Ahammed, B.M.J.; Dey, A.R. Knowledge, Attitude and Practices (KAP) towards Anthrax among Livestock Farmers in Selected Rural Areas of Bangladesh. Vet. Med. Sci. 2021, 7, 1648–1655. [Google Scholar] [CrossRef]
- Islam, M.S.; Hossain, M.J.; Mikolon, A.; Parveen, S.; Khan, M.S.U.; Haider, N.; Chakraborty, A.; Titu, A.M.N.; Rahman, M.W.; Sazzad, H.M.S.; et al. Risk Practices for Animal and Human Anthrax in Bangladesh: An Exploratory Study. Infect. Ecol. Epidemiol. 2013, 3, 21356. [Google Scholar] [CrossRef] [PubMed]
- Sieng, S.; Patrick, I.W.; Windsor, P.A.; Walkden-Brown, S.W.; Sar, C.; Smith, R.G.B.; Kong, R. Knowledge, Attitudes and Practices of Smallholder Farmers on Foot and Mouth Disease Control in Two Cambodian Provinces. Transbound. Emerg. Dis. 2022, 69, 1983–1998. [Google Scholar] [CrossRef] [PubMed]
- Sangrat, W.; Thanapongtharm, W.; Poolkhet, C. Identification of Risk Areas for Foot and Mouth Disease in Thailand Using a Geographic Information System-Based Multi-Criteria Decision Analysis. Prev. Vet. Med. 2020, 185, 105183. [Google Scholar] [CrossRef]
- Asnawi, A.; Nurlaelah, S.; Hastang; Abdullah, A. Constraints and Role of Women in Beef Cattle Farming to Access Financing in South Sulawesi, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2020, 492, 012145. [Google Scholar] [CrossRef]
- Saini, V.; Saini, R. Livestock Sector: A Tool for Women Empowerment. Pharma Innov. J. 2021, 10, 139–143. [Google Scholar]
- Waiswa, D.; Jolly, A. Implications of Gender Discrimination for Household Food Security among Small Holder Dairy Farmers in Nakaloke, Mbale District, Uganda. Res. J. Agric. For. Sci. Int. Sci. Community Assoc. 2021, 9, 1–11. [Google Scholar]
- Young, J.R.; Rast, L.; Suon, S.; Bush, R.D.; Henry, L.A.; Windsor, P.A. The Impact of Best Practice Health and Husbandry Interventions on Smallholder Cattle Productivity in Southern Cambodia. Anim. Prod. Sci. 2014, 54, 629. [Google Scholar] [CrossRef]
- Wolf, C. Producer Livestock Disease Management Incentives and Decisions. Int. Food Agribus. Manag. Rev. 2005, 8, 46–61. [Google Scholar]
- Guntoro, B.; Qui, N.H.; Triatmojo, A. Challenges and Roles of Extension Workers on Cyber Extension as Information Media. KnE Life Sci. 2022, 2022, 547–555. [Google Scholar] [CrossRef]
- Sulastri, E.; Triatmojo, A.; A’yun, A.Q.; Tatipikalawan, J.M. Effect of Extension Program on Improving Farmers’ Knowledge in the Narrowing Coastal Area of Segara Anakan Lagoon, Indonesia. J. Ilmu Ternak Dan Vet. 2024, 29, 161–171. [Google Scholar] [CrossRef]
- Ministry of Agriculture Republic Indonesia. Decision of the Minister of Agriculture of the Republic of Indonesia Number 518/KPTS/PK.300/M/7/2022 on the Provision of Compensation and Assistance in Certain Emergency Conditions of Foot and Mouth Disease; Decree of the Ministry of Agriculture Republic Indonesia: Jakarta, Indoneisa, 2022; pp. 1–19. [Google Scholar]
- Directorate General of Livestock And Animal Health, Ministry of Agriculture Republic Indonesia. Petunjuk Teknis Pemberian Bantuan Dalam Keadaan Tertentu Darurat Penyakit Mulut Dan Kuku; Decree of the Directorate General of Livestock And Animal Health, Ministry of Agriculture Republic Indonesia: Jakarta, Indonesia, 2022; pp. 1–22. [Google Scholar]
- Do, H.; Nguyen, H.-T.-M.; Van Ha, P.; Dang Van, K. A Cost-Benefit Analysis of Vietnam’s 2006–2010 Foot-and-Mouth Disease Control Program. Prev. Vet. Med. 2022, 206, 105703. [Google Scholar] [CrossRef] [PubMed]
- Lyons, N.A.; Afzal, M.; Toirov, F.; Irshad, A.; Bartels, C.J.M.; Rushton, J. Economic Considerations for Advancement Through the Progressive Control Pathway: Cost–Benefit Analysis of an FMD Disease-Free Zone in Punjab Province, Pakistan. Front. Vet. Sci. 2021, 8, 703473. [Google Scholar] [CrossRef]
- Nampanya, S.; Khounsy, S.; Abila, R.; Dy, C.; Windsor, P.A. Household Financial Status and Gender Perspectives in Determining the Financial Impact of Foot and Mouth Disease in Lao PDR. Transbound. Emerg. Dis. 2016, 63, 398–407. [Google Scholar] [CrossRef]
- Kerfua, S.D.; Railey, A.F.; Marsh, T.L. Household Production and Consumption Impacts of Foot and Mouth Disease at the Uganda-Tanzania Border. Front. Vet. Sci. 2023, 10, 1156458. [Google Scholar] [CrossRef]
- Casey-Bryars, M.; Reeve, R.; Bastola, U.; Knowles, N.J.; Auty, H.; Bachanek-Bankowska, K.; Fowler, V.L.; Fyumagwa, R.; Kazwala, R.; Kibona, T.; et al. Waves of Endemic Foot-and-Mouth Disease in Eastern Africa Suggest Feasibility of Proactive Vaccination Approaches. Nat. Ecol. Evol. 2018, 2, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Iriarte, M.V.; Gonzáles, J.L.; Gil, A.D.; de Jong, M.C.M. Animal Movements and FMDV Transmission during the High-Risk Period of the 2001 FMD Epidemic in Uruguay. Transbound. Emerg. Dis. 2023, 2023, 8883502. [Google Scholar] [CrossRef]
- Moreno, F.; Galvis, J.; Gómez, F. Correction: A Foot and Mouth Disease Ranking of Risk Using Cattle Transportation. PLoS ONE 2023, 18, e0294615. [Google Scholar] [CrossRef]
- Pomeroy, L.W.; Moritz, M.; Garabed, R. Network Analyses of Transhumance Movements and Simulations of Foot-and-Mouth Disease Virus Transmission among Mobile Livestock in Cameroon. Epidemics 2019, 28, 100334. [Google Scholar] [CrossRef] [PubMed]
- Guntoro, B.; Triatmojo, A.; Ariyadi, B.; Hoang Qui, N. Risk Analysis in Cattle Farmers’ Prevention Practices of Anthrax and Foot and Mouth Disease in Yogyakarta Province, Indonesia. Adv. Anim. Vet. Sci. 2022, 11, 987–997. [Google Scholar] [CrossRef]
- Wiratsudakul, A.; Sekiguchi, S. The Implementation of Cattle Market Closure Strategies to Mitigate the Foot-and-Mouth Disease Epidemics: A Contact Modeling Approach. Res. Vet. Sci. 2018, 121, 76–84. [Google Scholar] [CrossRef]
- de Menezes, T.C.; Luna, I.; de Miranda, S.H.G. Network Analysis of Cattle Movement in Mato Grosso Do Sul (Brazil) and Implications for Foot-and-Mouth Disease. Front. Vet. Sci. 2020, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Compston, P.; Limon, G.; Häsler, B. A Systematic Review of the Methods Used to Analyze the Economic Impact of Endemic Foot-and-mouth Disease. Transbound. Emerg. Dis. 2022, 69, e2249–e2260. [Google Scholar] [CrossRef] [PubMed]
- Sutawi, S.; Wahyudi, A.; Malik, A.; Suyatno, S.; Hidayati, A.; Rahayu, I.D.; Hartatie, E.S. Re-Emergence of Foot and Mouth Disease Outbreak in Indonesia: A Review. Adv. Anim. Vet. Sci. 2023, 11, 264–271. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).