Exploring Antimicrobial Resistance in Bacteria from Fecal Samples of Insectivorous Bats: A Preliminary Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Bacterial Isolation and Identification
2.3. Antimicrobial Susceptibility Tests
3. Results
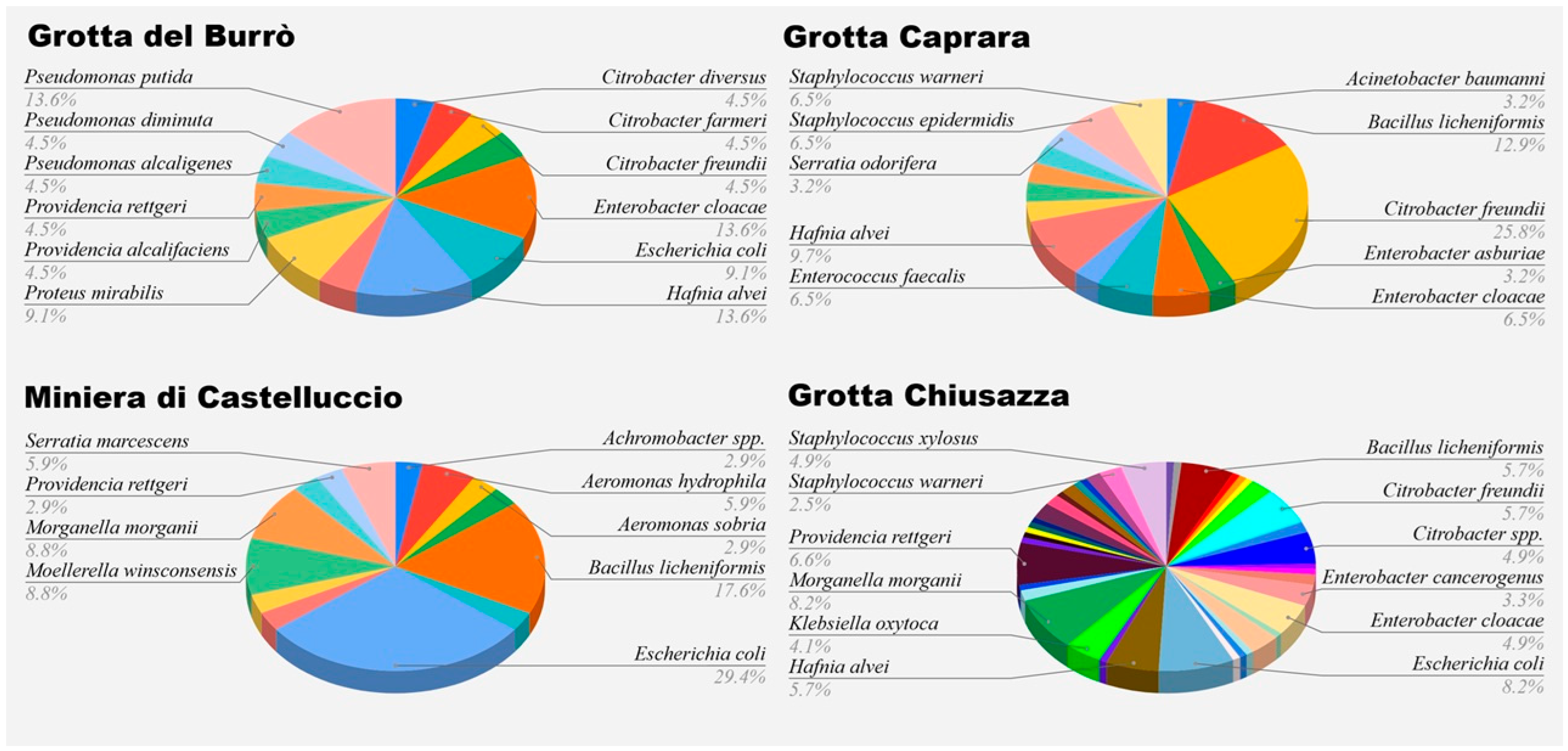
3.1. Sampling
3.2. Bacterial Strain Collection
3.3. Antimicrobial Susceptibility Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance. |
| MDR | Multidrug-resistant. |
| ARB | Antimicrobial-resistant bacteria. |
References
- Burgin, C.J.; Colella, J.P.; Kahn, P.L.; Upham, N.S. How Many Species of Mammals Are There? J. Mammal. 2018, 99, 1–14. [Google Scholar] [CrossRef]
- Massaad, M.; Da Silveira Bueno, R.; Bentaleb, I.; La Mantia, T. Bats of Sicily: Historical Evidence, Current Knowledge, Research Biases and Trends. Nat. Hist. Sci. 2023, 10, 45–58. [Google Scholar] [CrossRef]
- Szentivanyi, T.; McKee, C.; Jones, G.; Foster, J.T. Trends in Bacterial Pathogens of Bats: Global Distribution and Knowledge Gaps. Transbound. Emerg. Dis. 2023, 9285855. [Google Scholar] [CrossRef] [PubMed]
- Federici, L.; Masulli, M.; De Laurenzi, V.; Allocati, N. An Overview of Bats Microbiota and Its Implication in Transmissible Diseases. Front. Microbiol. 2022, 13, 1012189. [Google Scholar] [CrossRef]
- Laborda, P.; Sanz-García, F.; Ochoa-Sánchez, L.E.; Gil-Gil, T.; Hernando-Amado, S.; Martínez, J.L. Wildlife and Antibiotic Resistance. Front. Cell. Infect. Microbiol. 2022, 12, 873989. [Google Scholar] [CrossRef] [PubMed]
- Gwenzi, W.; Musiyiwa, K.; Mangori, L. Sources, Behaviour and Health Risks of Antimicrobial Resistance Genes in Wastewaters: A Hotspot Reservoir. J. Environ. Chem. Eng. 2020, 8, 102220. [Google Scholar] [CrossRef]
- Foti, M.; Grasso, R.; Fisichella, V.; Mascetti, A.; Colnaghi, M.; Grasso, M.; Spena, M.T. Antimicrobial Resistance in Physiological and Potentially Pathogenic Bacteria Isolated in Southern Italian Bats. Animals 2023, 13, 966. [Google Scholar] [CrossRef]
- Singh, R.; Kim, K. Environmental Occurrence of Antibiotic Resistance, Control Measures and Challenges in Finding Therapeutic Management. Emerg. Contam. 2025, 11, 100440. [Google Scholar] [CrossRef]
- Adams, R.J.; Kim, S.S.; Mollenkopf, D.F.; Mathys, D.A.; Schuenemann, G.M.; Daniels, J.B.; Wittum, T.E. Antimicrobial-Resistant Enterobacteriaceae Recovered from Companion Animal and Livestock Environments. Zoonoses Public Health 2018, 65, 519–527. [Google Scholar] [CrossRef]
- Devnath, P.; Karah, N.; Graham, J.P.; Rose, E.S.; Asaduzzaman, M. Evidence of Antimicrobial Resistance in Bats and Its Planetary Health Impact for Surveillance of Zoonotic Spillover Events: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 20, 243. [Google Scholar] [CrossRef]
- Soto-López, J.D.; Diego-del Olmo, M.; Fernández-Soto, P.; Muro, A. Bats as an Important Source of Antimicrobial-Resistant Bacteria: A Systematic Review. Antibiotics 2025, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Mickleburgh, S.B.; Sechrest, W.; Walsh, A.L. Global Overview of the Conservation of Island Bats: Importance, Challenges, and Opportunities. In Island Bats. Ecology, Evolution, and Conservation; Fleming, T.H., Racey, P.A., Eds.; University of Chicago Press: Chicago, IL, USA, 2009. [Google Scholar]
- Conenna, I.; Rocha, R.; Russo, D.; Cabeza, M. Insular Bats and Research Effort: A Review of Global Patterns and Priorities. Mammal. Rev. 2017, 47, 169–182. [Google Scholar] [CrossRef]
- Caruso, D.; Costa, G. Ricerche Faunistiche Ed Ecologiche Sulle Grotte Di Sicilia. VI—Fauna Cavernicola Di Sicilia (Catalogo ragionato). Animalia 1978, 5, 423–513. [Google Scholar]
- Caruso, D. Il Popolamento Cavernicolo Della Sicilia. Biogeographia 1982, 7, 1–29. [Google Scholar] [CrossRef]
- Zava, B.; Corrao, A.; Catalano, E. Chirotteri Cavernicoli Di Sicilia. In Proceedings of the Atti del IX° Concreso Internacional de Espeleologia, Barcellona, Spain, 1–7 August 1986; Volume II, pp. 187–189. [Google Scholar]
- Caruso, D. L’attuale Stato Delle Conoscenze Sulla Fauna Delle Grotte Di Sicilia (Ricerche Faunistiche Ed Ecologiche Sulla Grotte Di Sicilia. VIII). In Proceedings of the Atti I Convegno Regionale di Speleologia della Sicilia, Ragusa, Italy, 14–16 December 1990; 1990; Volume II, pp. 346–378. [Google Scholar]
- Caruso, D.; Grasso, R. La Fauna Delle Grotte. In Proceedings of the Atti del Convegno su “La fauna degli Iblei”; Ragonese, B., Ed.; Ente Fauna Siciliana: Noto, Italy, 1996; pp. 201–281. [Google Scholar]
- Di Bella, S.; Giacchino, I.; Blanda, V.; Gucciardi, F.; Scibetta, S.; La Russa, F.; Lastra, A.; Purpari, G.; Grasso, R.; Spena, M.T.; et al. Zoonotic Bacteria and Vector-Borne Protozoa in Troglophilus Bat Colonies in Sicily (Southern Italy): A Biomolecular Survey. Animals 2025, 15, 488. [Google Scholar] [CrossRef]
- Mira, F.; Gucciardi, F.; Schiró, G.; Grasso, R.; Spena, M.T.; Kemenesi, G.; Vaiana, C.; Anzá, D.; Di Paola, L.; Di Bella, S.; et al. Molecular Surveillance for Potential Zoonotic Pathogens in Troglophilus Bats: Detection and Molecular Characterization of Bat Coronaviruses in Southern Italy. Pathogens 2025, 14, 457. [Google Scholar] [CrossRef]
- Foti, M.; Spena, M.T.; Fisichella, V.; Mascetti, A.; Colnaghi, M.; Grasso, M.; Piraino, C.; Sciurba, F.; Grasso, R. Cultivable Bacteria Associated with the Microbiota of Troglophile Bats. Animals 2022, 12, 2684. [Google Scholar] [CrossRef]
- Agnelli, P.; Martinoli, A.; Patriarca, E.; Scaravelli, D.; Genovesi, P. Linee Guida per Il Monitoraggio Dei Chirotteri: Indicazioni Metodologiche per Lo Studio e La Conservazione Dei Pipistrelli in Italia. In Quaderni di Conservazione della Natura; Ministero dell’Ambiente—Istituto Nazionale Fauna Selvatica: Roma, Italy, 2004. [Google Scholar]
- Carter, G.R.; Cole, J.R., Jr. Diagnostic Procedure in Veterinary Bacteriology and Mycology.; Elsevier Science: Saint Louis, MO, USA, 1990; ISBN 978-0-323-13818-5. [Google Scholar]
- Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Boone, D.R., Castenholz, R.W., Garrity, G.M., Eds.; Springer: New York, NY, USA, 2001; ISBN 978-0-387-98771-2. [Google Scholar]
- Grimont, P.A.D.; Weill, F.-X. Antigenic Formulae of the Salmonella Serovars. WHO Collab. Cent. Ref. Res. Salmonella 2007, 9, 1–166. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; Supplement M100; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2023; ISBN 978-1-68440-171-0. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Rastall, R.A. Bacteria in the Gut: Friends and Foes and How to Alter the Balance. J. Nutr. 2004, 134, 2022S–2026S. [Google Scholar] [CrossRef]
- Whitaker, J.O., Jr.; Dannelly, H.K.; Prentice, D.A. Chitinase in Insectivorous Bats. J. Mammal. 2004, 85, 15–18. [Google Scholar] [CrossRef]
- Selvin, J.; Lanong, S.; Syiem, D.; De Mandal, S.; Kayang, H.; Kumar, N.S.; Kiran, G.S. Culture-Dependent and Metagenomic Analysis of Lesser Horseshoe Bats’ Gut Microbiome Revealing Unique Bacterial Diversity and Signatures of Potential Human Pathogens. Microb. Pathog. 2019, 137, 103675. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.M.; Leech, J.; Puechmaille, S.J.; Lopez, J.V.; Teeling, E.C. Is There a Link between Aging and Microbiome Diversity in Exceptional Mammalian Longevity? PeerJ 2018, 6, e4174. [Google Scholar] [CrossRef]
- Mühldorfer, K. Bats and Bacterial Pathogens: A Review. Zoonoses Public Health 2013, 60, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Dhivahar, J.; Parthasarathy, A.; Krishnan, K.; Kovi, B.S.; Pandian, G.N. Bat-Associated Microbes: Opportunities and Perils, an Overview. Heliyon 2023, 9, e22351. [Google Scholar] [CrossRef]
- Edenborough, K.M.; Mu, A.; Mühldorfer, K.; Lechner, J.; Lander, A.; Bokelmann, M.; Couacy-Hymann, E.; Radonic, A.; Kurth, A. Microbiomes in the Insectivorous Bat Species Mops Condylurus Rapidly Converge in Captivity. PLoS ONE 2020, 15, e0223629. [Google Scholar] [CrossRef]
- Vengust, M.; Knapic, T.; Weese, J.S. The Fecal Bacterial Microbiota of Bats; Slovenia. PLoS ONE 2018, 13, e0196728. [Google Scholar] [CrossRef]
- Wolkers-Rooijackers, J.C.M.; Rebmann, K.; Bosch, T.; Hazeleger, W.C. Fecal Bacterial Communities in Insectivorous Bats from the Netherlands and Their Role as a Possible Vector for Foodborne Diseases. Acta 2019, 20, 475–483. [Google Scholar] [CrossRef]
- Dimkić, I.; Fira, D.; Janakiev, T.; Kabić, J.; Stupar, M.; Nenadić, M.; Unković, N.; Grbić, M.L. The Microbiome of Bat Guano: For What Is This Knowledge Important? Appl. Microbiol. Biotechnol. 2021, 105, 1407–1419. [Google Scholar] [CrossRef]
- Corduneanu, A.; Mihalca, A.D.; Sándor, A.D.; Hornok, S.; Malmberg, M.; Viso, N.P.; Bongcam-Rudloff, E. The Heart Microbiome of Insectivorous Bats from Central and South Eastern Europe. Comp. Immunol. Microbiol. Infect. Dis. 2021, 75, 101605. [Google Scholar] [CrossRef]
- Gerbáčová, K.; Maliničová, L.; Kisková, J.; Maslišová, V.; Uhrin, M.; Pristaš, P. The Faecal Microbiome of Building-Dwelling Insectivorous Bats (Myotis Myotis and Rhinolophus Hipposideros) Also Contains Antibiotic-Resistant Bacterial Representatives. Curr. Microbiol. 2020, 77, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Mulec, J.; Kogovšek, B.; Skok, S.; Tomazin, R.; Šturm, S.; Ambrožič Avguštin, J. Antimicrobial Resistant Escherichia Coli from Karst Waters, Surfaces and Bat Guano in Slovenian Caves. Acta Carsol. 2020, 49, 265–279. [Google Scholar] [CrossRef]
- Garcês, A.; Correia, S.; Silva, V.; Pereira, J.E.; Amorim, F.; Igrejas, G.; Poeta, P. Detection of Antimicrobial Resistance in Faecal Escherichia Coli from European Free-Tailed Bats (Tadarida teniotis) in Portugal. Acta Chiropterol. 2020, 21, 403. [Google Scholar] [CrossRef]
- Garcês, A.; Correia, S.; Amorim, F.; Pereira, J.E.; Igrejas, G.; Poeta, P. First Report on Extended-Spectrum Beta-Lactamase (ESBL) Producing Escherichia coli from European Free-Tailed Bats (Tadarida teniotis) in Portugal: A One-Health Approach of a Hidden Contamination Problem. J. Hazard. Mater. 2019, 370, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Allocati, N.; Petrucci, A.G.; Di Giovanni, P.; Masulli, M.; Di Ilio, C.; De Laurenzi, V. Bat–Man Disease Transmission: Zoonotic Pathogens from Wildlife Reservoirs to Human Populations. Cell Death Discov. 2016, 2, 16048. [Google Scholar] [CrossRef]
- Rojas-Sereno, Z.E.; Streicker, D.G.; Suarez-Yana, T.; Lineros, M.; Yung, V.; Godreuil, S.; Benavides, J.A. Detection of Antimicrobial-Resistant Enterobacterales in Insectivorous Bats from Chile. R. Soc. Open Sci. 2023, 10, 231177. [Google Scholar] [CrossRef]
| Antimicrobials Classes | Molecules | |
|---|---|---|
| Gram-negative Panel | Aminoglycosides | Gentamicin |
| Streptomycin | ||
| Tobramycin | ||
| Carbapenems | Imipenem | |
| Meropenem | ||
| Cephalosporins | Cefotaxime | |
| Ceftazidime | ||
| Ceftazidime + clavulanic acid | ||
| Quinolones | Nalidixic acid | |
| Chloramphenicol | Chloramphenicol | |
| Fluoroquinolones | Ciprofloxacin | |
| Enrofloxacin | ||
| Monobactams | Aztreonam | |
| Penicillins | Amoxicillin | |
| Amoxicillin + clavulanic acid | ||
| Ampicillin | ||
| Sulphonamides | Cotrimoxazole | |
| Tetracyclines | Tetracycline | |
| Doxycyclin | ||
| Gram-positive Panel | Aminoglycosides | Gentamicin |
| Tobramycin | ||
| Carbapenems | Imipenem | |
| Meropenem | ||
| Cephalosporins | Ceftazidime | |
| Cefepime | ||
| Ceftaroline | ||
| Fluoroquinolones | Enrofloxacin | |
| Glycopeptides | Vancomycin | |
| Lincosamides | Lincomycin | |
| Macrolides | Erythromycin | |
| Penicillins | Amoxicillin | |
| Ampicillin | ||
| Oxacillin | ||
| Amoxicillin + clavulanic acid | ||
| Ampicillin + sulbactam | ||
| Ticarcillin + clavulanic acid | ||
| Tetracyclines | Minocycline | |
| Tetracycline |
| Classes | Molecules | Number of Isolates (%) | ||
|---|---|---|---|---|
| R | I | S | ||
| Aminoglycosides | Gentamicin | 51 (34.2) | 1 (0.7) | 97 (65.1) |
| Streptomycin | 91 (61.1) | 6 (4) | 52 (34.9) | |
| Tobramycin | 57 (38.3) | 3 (2) | 89 (59.7) | |
| Carbapenems | Imipenem | 65 (43.6) | 5 (3.4) | 79 (53) |
| Meropenem | 9 (6) | 3 (2) | 137 (91.9) | |
| Cephalosporins | Cefotaxime | 34 (22.8) | 5 (3.4) | 110 (73.8) |
| Ceftazidime | 26 (17.4) | 3 (2) | 120 (80.5) | |
| Ceftazidime + clavulanic acid | 16 (10.7) | 6 (4) | 127 (85.2) | |
| Quinolones | Nalidixic acid | 18 (12.1) | 9 (6) | 122 (81.9) |
| Chloramphenicol | Chloramphenicol | 12 (8.1) | 3 (29) | 134 (89.9) |
| Fluoroquinolones | Ciprofloxacin | 6 (4) | 0 | 143 (96) |
| Enrofloxacin | 0 | 2 (1.3) | 147 (98.7) | |
| Monobactams | Aztreonam | 21 (14.1) | 1 (0.7) | 127 (85.2) |
| Penicillins | Amoxicillin | 98 (65.8) | 1 (0.7) | 50 (33.6) |
| Amoxicillin + clavulanic acid | 44 (29.5) | 4 (2.7) | 101(67.8) | |
| Ampicillin | 38 (25.5) | 3 (2) | 108 (72.5) | |
| Sulphonamides | Trimethoprim-Sulfamethoxazole | 10 (6.7) | 1 (0.7) | 138 (92.6) |
| Tetracyclines | Tetracycline | 38 (25.5) | 2 (1.3) | 109 (73.2) |
| Doxycycline | 44 (29.5) | 11 (7.4) | 94 (63.1) | |
| Classes | Molecules | Number of Isolates (%) | ||
|---|---|---|---|---|
| R | I | S | ||
| Aminoglycosides | Gentamicin | 20 (46.5) | 0 | 23 (53.5) |
| Tobramycin | 23 (53.5) | 0 | 20 (46.5) | |
| Carbapenems | Imipenem | 14 (32.6) | 3 (7) | 26 (60.5) |
| Meropenem | 11 (25.6) | 2 (4.7) | 30 69.8) | |
| Cephalosporins | Ceftazidime | 14 (32.6) | 0 | 29 (67.4) |
| Cefepime | 14 (32.6) | 1 (2.3) | 28 (65.1) | |
| Ceftaroline | 14 (32.6) | 1 (2.3) | 28 (65.1) | |
| Fluoroquinolones | Enrofloxacin | 8 (18.6) | 0 | 35 (81.4) |
| Glycopeptides | Vancomycin | 22 (51.2) | 1 (2.3) | 20 (46.5) |
| Lincosamides | Lincomycin | 31 (72.1) | 2 (4.7) | 10 (23.3) |
| Macrolides | Erythromycin | 19 (44.2) | 3 (7) | 21 (48.8) |
| Penicillins | Amoxicillin | 16 (37.2) | 0 | 27 (62.8) |
| Ampicillin | 21 (48.8) | 0 | 22 (51.2) | |
| Oxacillin | 32 (74.4) | 0 | 11 (25.6) | |
| Amoxicillin + clavulanic acid | 12 (27.9) | 0 | 31 (72.1) | |
| Ampicillin + sulbactam | 1 (2.3) | 0 | 42 (97.7) | |
| Ticarcillin + clavulanic acid | 7 (16.3) | 1 (2.3) | 35 (81.4) | |
| Tetracyclines | Minocycline | 1 (2.3) | 2 (4.7) | 40 (93) |
| Tetracycline | 2 (4.7) | 0 | 41 (95.3) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bella, S.; Gambino, D.; Foti, M.; Orlandella, B.M.; Fisichella, V.; Gucciardi, F.; Mira, F.; Grasso, R.; Spena, M.T.; Purpari, G.; et al. Exploring Antimicrobial Resistance in Bacteria from Fecal Samples of Insectivorous Bats: A Preliminary Study. Vet. Sci. 2025, 12, 516. https://doi.org/10.3390/vetsci12060516
Di Bella S, Gambino D, Foti M, Orlandella BM, Fisichella V, Gucciardi F, Mira F, Grasso R, Spena MT, Purpari G, et al. Exploring Antimicrobial Resistance in Bacteria from Fecal Samples of Insectivorous Bats: A Preliminary Study. Veterinary Sciences. 2025; 12(6):516. https://doi.org/10.3390/vetsci12060516
Chicago/Turabian StyleDi Bella, Santina, Delia Gambino, Maria Foti, Bianca Maria Orlandella, Vittorio Fisichella, Francesca Gucciardi, Francesco Mira, Rosario Grasso, Maria Teresa Spena, Giuseppa Purpari, and et al. 2025. "Exploring Antimicrobial Resistance in Bacteria from Fecal Samples of Insectivorous Bats: A Preliminary Study" Veterinary Sciences 12, no. 6: 516. https://doi.org/10.3390/vetsci12060516
APA StyleDi Bella, S., Gambino, D., Foti, M., Orlandella, B. M., Fisichella, V., Gucciardi, F., Mira, F., Grasso, R., Spena, M. T., Purpari, G., & Guercio, A. (2025). Exploring Antimicrobial Resistance in Bacteria from Fecal Samples of Insectivorous Bats: A Preliminary Study. Veterinary Sciences, 12(6), 516. https://doi.org/10.3390/vetsci12060516






